Open Access Article
Open Access ArticleSequential cycloaddition and ring expansion reaction of arynes and methylenebenzothiopheneones: synthesis of a benzo-fused eight-membered ring via sulfonium ylides†
Peng Xiaoa,
Shikuan Sua,
Wei Wanga,
Weiguo Caoa,
Jie Chena,
Jian Li *a and
Yali Chen
*a and
Yali Chen *ab
*ab
aDepartment of Chemistry, Innovative Drug Research Center, College of Sciences & Institute for Sustainable Energy, Shanghai University, 99 Shangda Road, Shanghai 200444, P. R. China. E-mail: ylchen@staff.shu.edu.cn; lijian@shu.edu.cn
bKey Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200444, P. R. China
First published on 29th November 2019
Abstract
A sequential cycloaddition and ring expansion of arynes and methylenebenzothiopheneones has been disclosed. This strategy proceeds through a sulfonium ylides intermediate and allows for the efficient synthesis of a sulfur-containing benzo-fused eight-membered ring.
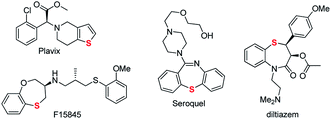
As leading biologically relevant structural components, organosulfur compounds are widely present in many pharmaceuticals and bioactive compounds.1 Accordingly, sulfur-containing compounds have found their medical applications including as antibacterials, anti-inflammatories, dermatologics, and cancer treatments. A survey of the structures of compounds among the top 200 brand name drugs by U.S. retail sales (RS) in 2011 revealed that 24.8% of the drugs contain sulfur. The list included the famous Plavix (blood thinner, no. 2) and Seroquel (antipsychotic drug, no. 6).2 In addition, the benzothiazepine-containing drug diltiazem (calcium channel blocker) is used in the treatment of hypertension and angina (Scheme 1).3 On the other hand, Et743 as a sulfur-containing marine natural product was also used as an effective drug for the treatment of advanced soft tissue sarcoma.4 As a consequence, many efforts have been devoted to the synthesis of such skeletons.
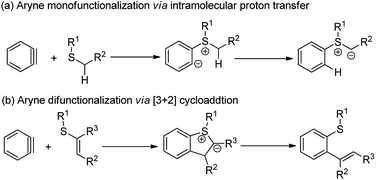
Arynes are recognized as one of the most reactive intermediates in organic synthesis. The past years have witnessed a great progress with respect to the carbon–carbon and carbon-heteroatom bond-forming reactions involving arynes.5,6 A careful literature screening revealed that reactions involving the insertion of aryne into C–C, C–N, C–O, C–S, and other bonds have been well documented.7–9 Furthermore, the aryne-based Diels–Alder reaction, [2 + 5], nucleophilic addition and annulation reactions.8,9 Among [2 + 8] and many other pericyclic reactions have been intensively investigated for the construction of various benzo-fused frameworks.10 Remarkably, the reactions between arynes and organosulfur compounds have been widely investigated. As such, arynes can react with a variety of organosulfur compounds including S(II), S(IV), and S(IV) valence state, thus allows for the diversity-oriented synthesis of aromatic organosulfurs that are difficult to access by conventional methods.11,12 Among them, we are particularly interested in those reactions involves sulfonium ylides, a neutral 1,2-dipolar species consisting of electron-rich carbon atom and an adjacent electro-positive sulfur atom. For example, nucleophilic attack of alkyl thioether towards aryne followed by intramolecular 1,4-proton shift contribute an elegant strategy for the formation of sulfonium ylides (Scheme 2a), which can be further applied to various conversions.13 Remarkably, this strategy has been established from various modes of trapping of HDDA-generated benzynes with sulfides by Hoye and co-workers.14 Recently, Studer and co-workers have disclosed that vinyl thioethers lacking α-CH protons reacted with benzyne through direct [3 + 2] cycloaddition to give cyclic sulfonium ylides. This methods eventually enables the preparation of corresponding tetrasubstituted alkenes with high stereoselectivity (Scheme 2b).15 In the past years, we have paid much attention to the aryne chemistry and the heterocycle synthesis.16,17 As a continuation, herein we report that the unprecedented reaction of arynes and methylenebenzothiopheneones enables the synthesis of structurally unusual benzo-fused eight-membered ring.
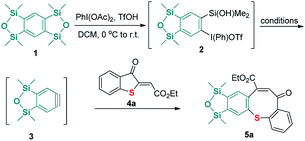
We commenced our investigation using benzyne precursor 1 and 2-methylenebenzothiophene-3-ones 4a as the model substrates. As shown in Table 1, heating the mixture of 1 and 4a in CH3CN with excessive CsF as fluorine source essentially afforded a new product 5a in 48% yield (Table 1, Entry 1). After that, reactions with other solvents including THF, toluene, 1,4-dioxane and DCM were also performed to enhance the yield of 5a. Pleasingly, the experimental outcome revealed that the employment of DCM as the solvent significantly increased the yield of 5a (Table 1, entries 2–5). Encouraged by these results, we then examined the present reactions with different reaction temperature, equivalence ratios and fluorine sources. The best performance was obtained when reaction was conducted under reflux and 1.5 equiv. of 1 (Table 1, entries 5–9). Of the fluorine sources tested, KF/18-C-6 and TBAF could not bring further improvement (Table 1, entries 10–12).
| Entry | F source | Equiv. of 1 | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1.0 mmol 2-methylenebenzothiophene-3-one 4a, fluorine source, in 3 mL solvent.b Isolated yield.c Bp of DCM: 39.8 °C.d Sealing reaction temperature. | |||||
| 1 | CsF | 1.5 | CH3CN | 40 | 48 |
| 2 | CsF | 1.5 | THF | 40 | 63 |
| 3 | CsF | 1.5 | Toluene | 40 | 62 |
| 4 | CsF | 1.5 | Dioxane | 40 | 59 |
| 5 | CsF | 1.5 | DCM | Refluxc | 87 |
| 6 | CsF | 1.5 | DCM | rt | 65 |
| 7 | CsF | 1.5 | DCM | 60d | 79 |
| 8 | CsF | 2.0 | DCM | Reflux | 82 |
| 9 | CsF | 1.0 | DCM | Reflux | 75 |
| 10 | TBAF | 1.5 | DCM | Reflux | 47 |
| 11 | KF/18-c-6 | 1.5 | DCM | Reflux | 67 |
| 12 | KF/18-c-6 | 1.5 | THF | Reflux | 71 |
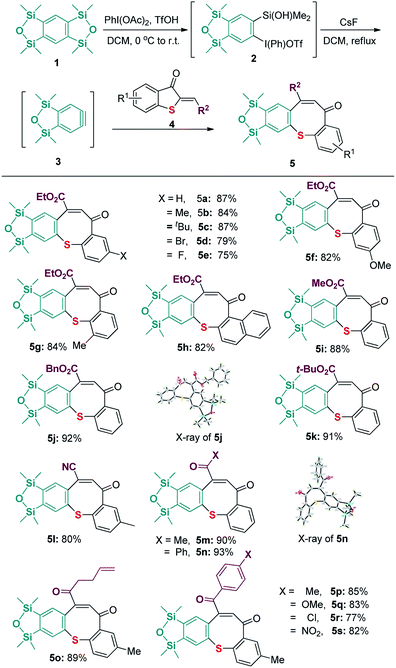
After the best conditions were obtained in hand, the feasibility of substituted 2-methylenebenzothiophene-3-ones 4 was next evaluated. As shown in Scheme 3, a variety of 2-methylenebenzothiophene-3-ones 4 having substituents including methyl, tert-butyl, halo, and methoxyl groups at the position 7, 6, 5 of the aromatic ring were used to react with benzyne precursor 1. All reactions worked well to produce the desired products (5a–5h). Additionally, when the ester moiety in substrate 4 was changed from OEt to OMe, OBn, or OtBu, the corresponding products 5i–5k were afforded in high performance. It was also worthy to note that when the electron-withdrawing groups such as cyano, and substituted carbonyl groups were employed instead of ester unit, the corresponding products 5l–5s were isolated in satisfactory yields. Furthermore, the structure of compounds 5j and 5n was unambiguously characterized by single-crystal X-ray analysis.18
After a broad substrate scope with benzyne precursor was established, reactions of naphthyne precursor 6 and substituted 2-methylenebenzothiophene-3-ones were subsequently tested. As shown in Scheme 4, reactions with 2-methylenebenzothiophene-3-ones 4 bearing different electron-withdrawing groups proceeded smoothly to produce products 9a–9e in satisfactory yield.
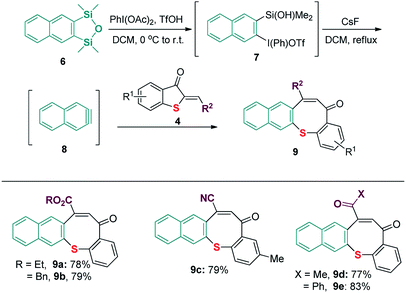 | ||
| Scheme 4 Reaction conditions: 1.5 mmol naphthyne precursor 6, 1.0 mmol 2-methylenebenzothiophene-3-one 4, CsF (3.0 mmol), in 3 mL DCM. Isolated yields are reported. | ||
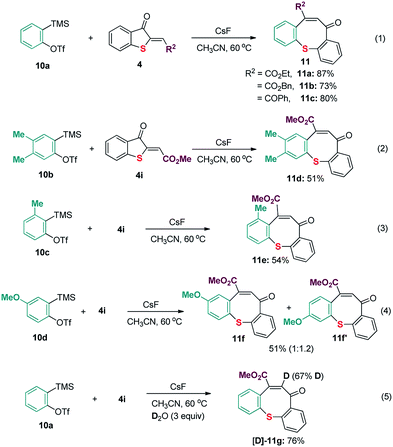
To gain more insight into the present reaction, several control experiments and preliminary mechanistic experiments were next conducted. As shown in Scheme 5, benzyne precursor ortho-(trimethylsilyl)aryltriflate 10a and 10b were proven to be compatible reaction partners to react with 2-methylenebenzothiophene-3-ones 4 (Scheme 5, eqn (1) and (2)). After that, reactions with unsymmetrical benzyne precursor 10c and 10d were also examined (Scheme 5, eqn (3) and (4)). Pleasingly, only one isomer was detected when 3-methyl-2-(trimethylsilyl)phenyl trifluoromethanesulfonate 10c was used. The isotope-labelling experiment with 10a and 4i also revealed that deuterium was incorporated in the product to produce [D]-11e (Scheme 5, eqn (5)). This result suggested that water was also involved in present transformation.
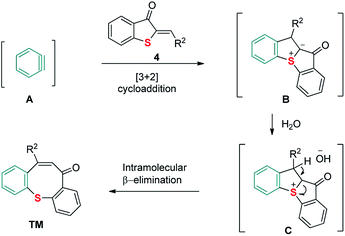
Based on the aforementioned results and previous reports, a plausible reaction mechanism is described in Scheme 6. Firstly, the [3 + 2] cycloaddition between 2-methylenebenzothiophene-3-ones 4 and aryne yields a sulfonium ylide intermediate B. In the presence of water, B is further protonated to deliver sulfonium salt C. After that, the in situ generated hydroxide ion serves as a base to facilitate the following intramolecular β-elimination, thus providing a quick access to the product.
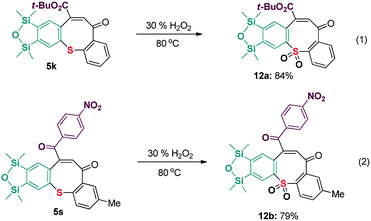
To further explore the application of present reaction, an oxidation reaction of the products was also attempted. In the presence of H2O2,19 the resultant sulfide products 5k and 5s experienced oxidation to corresponding functionalized sulfones 12a and 12b in an efficient and environmentally friendly manner (Scheme 7, eqn (1) and (2)).
In conclusion, we have described an unprecedented reaction from in situ generated aryne and 2-methylenebenzothiophene-3-ones. This strategy allows for a new approach to benzo-fused eight-membered ring containing sulfur, which was difficult to be synthesized by traditional methods. Mechanistically, this reaction proceeds through cycloaddition, proton shift, intramolecular β-elimination, and the formation of sulfonium ylide. Further study and application of the present reaction including the biological detection are still underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21272146) and the State Key Laboratory of Applied Organic Chemistry, Lanzhou University for financial support. This work is also sponsored by Natural Science Foundation of Shanghai (18ZR1413900).Notes and references
- (a) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832 CrossRef CAS PubMed; (b) Sulfur Compounds: Advances in Research and Application, ed. A. Q. Acton, Scholarly Editions, Atlanta, GA, 2012 Search PubMed.
- Please find the data reflecting the top 200 rankings from http://www.pharmacytimes.com/publications/issue/2012/July2012/Top-200-Drugs-of-2011, 2012.
- (a) K. Asano and S. Matsubara, ACS Catal., 2018, 8, 6273 CrossRef CAS; (b) H. Kugita, H. Inoue, M. Ikezaki, M. Konda and S. Takeo, Chem. Pharm. Bull., 1970, 18, 2284 CrossRef CAS.
- (a) W. He, Z. Zhang and D. Ma, Angew. Chem., Int. Ed., 2019, 58, 3972 CrossRef CAS PubMed; (b) J. Chen, X. Chen, M. Bois-Choussy and J. Zhu, J. Am. Chem. Soc., 2006, 128, 87 CrossRef CAS PubMed.
- For selected reviews on aryne chemistry, see: (a) H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Chem. Soc. Rev., 2018, 47, 8030 RSC; (b) T. Roy and A. T. Biju, Chem. Commun., 2018, 54, 2580 RSC; (c) J. Shi, Y. Li and Y. Li, Chem. Soc. Rev., 2017, 46, 1707 RSC; (d) S. Yoshida and T. Hosoya, Chem. Lett., 2015, 44, 1450 CrossRef CAS; (e) D. Pérez, D. Peña and E. Guitián, Eur. J. Org. Chem., 2013, 5981 CrossRef; (f) C. M. Gampe and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3766 CrossRef CAS PubMed; (g) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (h) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC.
- For selected examples, see: (a) Y. Nakamura, Y. Miyata, K. Uchida, S. Yoshida and T. Hosoya, Org. Lett., 2019, 21, 5252 CrossRef CAS PubMed; (b) S. Bhattacharjee, A. Guin, R. N. Gaykar and A. T. Biju, Org. Lett., 2019, 21, 4383 CrossRef CAS PubMed; (c) J. Zhao, H. Li, P. Li and L. Wang, J. Org. Chem., 2019, 84, 9007 CrossRef CAS PubMed; (d) H. Xu, J. He, J. Shi, L. Tan, D. Qiu, X. Luo and Y. Li, J. Am. Chem. Soc., 2018, 140, 3555 CrossRef CAS PubMed; (e) S. Yoshida, T. Yano, Y. Misawa, Y. Sugimura, K. Igawa, S. Shimizu, K. Tomooka and T. Hosoya, J. Am. Chem. Soc., 2015, 137, 14071 CrossRef CAS PubMed; (f) Y. Nagashima, R. Takita, K. Yoshida, K. Hirano and M. Uchiyama, J. Am. Chem. Soc., 2013, 135, 18730 CrossRef CAS PubMed; (g) T. Kitamura, Z. Meng and Y. Fujiwara, Tetrahedron, 2000, 41, 6611 CrossRef CAS.
- (a) U. K. Tambar and B. M. Stoltz, J. Am. Chem. Soc., 2005, 127, 5340 CrossRef CAS PubMed; (b) U. K. Tambar, D. C. Ebner and B. M. Stoltz, J. Am. Chem. Soc., 2006, 128, 11752 CrossRef CAS PubMed; (c) R. Samineni, C. R. C. Bandi, P. Srihari and G. Mehta, Org. Lett., 2016, 18, 6184 CrossRef CAS PubMed; (d) P. Gouthami, R. Chegondi and S. Chandrasekhar, Org. Lett., 2016, 18, 2044 CrossRef CAS PubMed.
- (a) J. Schwan, M. Kleoff, B. Hartmayer, P. Heretsch and M. Christmann, Org. Lett., 2018, 20, 7661 CrossRef CAS PubMed; (b) A. C. Wright, C. K. Haley, G. Lapointe and B. M. Stoltz, Org. Lett., 2016, 18, 2793 CrossRef CAS PubMed; (c) Z. Liu and R. C. Larock, J. Am. Chem. Soc., 2005, 127, 13112 CrossRef CAS PubMed; (d) H. Yoshida, E. Shirakawa, Y. Honda and T. Hiyama, Angew. Chem., Int. Ed., 2002, 41, 3247 CrossRef CAS; (e) N. Saito, K. Nakamura, S. Shibano, S. Ide, M. Minami and Y. Sato, Org. Lett., 2013, 15, 386 CrossRef CAS PubMed.
- (a) R. N. Gaykar, S. Bhattacharjee and A. T. Biju, Org. Lett., 2019, 21, 737 CrossRef CAS PubMed; (b) S. J. Li, Y. Wang, J. K. Xu, D. Xie, S. K. Tian and Z. X. Yu, Org. Lett., 2018, 20, 4545 CrossRef CAS PubMed; (c) J. A. García-López, M. Çetin and M. F. Greaney, Angew. Chem., Int. Ed., 2015, 54, 2156 CrossRef PubMed; (d) T. Matsuzawa, K. Uchida, S. Yoshida and T. Hosoya, Org. Lett., 2017, 19, 5521 CrossRef CAS PubMed; (e) S. Yoshida, Y. Nakamura, K. Uchida, Y. Hazama and T. Hosoya, Org. Lett., 2016, 18, 6212 CrossRef CAS PubMed; (f) H. Yoshida, J. Ikadai, M. Shudo, J. Ohshita and A. Kunai, J. Am. Chem. Soc., 2013, 125, 6638 CrossRef PubMed; (g) K. Z. Łączkowski, D. García, D. Peña, A. Cobas, D. Pérez and E. Guitián, Org. Lett., 2011, 13, 960 CrossRef PubMed.
- (a) S. S. Bhojgude, A. Bhunia and A. T. Biju, Acc. Chem. Res., 2016, 49, 1658 CrossRef CAS PubMed; (b) V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2013, 11, 191 RSC; (c) Z. Wang, Y. Addepalli and Y. He, Org. Lett., 2018, 20, 644 CrossRef CAS PubMed; (d) D. Xu, Y. Zhao, D. Song, Z. Zhong, S. Feng, X. Xie, X. Wang and X. She, Org. Lett., 2017, 19, 3600 CrossRef CAS.
- For a recent review on aryne reactions with organosulfurcompounds, see: T. Matsuzawa, S. Yoshida and T. Hosoya, Tetrahedron Lett., 2018, 59, 4197 CrossRef CAS.
- For selected examples, see: (a) P. Garg and A. Singh, Org. Lett., 2018, 20, 1320 CrossRef CAS PubMed; (b) T. Zheng, J. Tan, R. Fan, S. Su, B. Liu, C. Tan and K. Xu, Chem. Commun., 2018, 54, 1303 RSC; (c) J. Shi, D. Qiu, J. Wang, H. Xu and Y. Li, J. Am. Chem. Soc., 2015, 137, 5670 CrossRef CAS PubMed; (d) S. Yoshida, K. Uchida, K. Igawa, T. Tomooka and T. Hosoya, Chem. Commun., 2014, 50, 15059 RSC; (e) F. L. Liu, J. R. Chen, Y. Q. Zou, Q. Wei and W. J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed; (f) S. K. Aithagani, S. Dara, G. Munagala, H. Aruri, M. Yadav, S. Sharma, R. A. Vishwakarma and P. P. Singh, Org. Lett., 2015, 17, 5547 CrossRef CAS PubMed; (g) J. Nakayama, T. Fujita and M. Hoshino, Chem. Lett., 1983, 249 CrossRef CAS.
- H. D. Xu, M. Q. Cai, W. J. He, W. H. Hu and M. H. Shen, RSC Adv., 2014, 4, 7623 RSC.
- J. Chen, V. Palani and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 4318 CrossRef CAS PubMed.
- Y. Li, C. Mgck-Lichtenfeld and A. Studer, Angew. Chem., Int. Ed., 2016, 55, 14435 CrossRef CAS PubMed.
- (a) S. Su, J. Li, M. Sun, H. Zhao, Y. Chen and J. Li, Chem. Commun., 2018, 54, 9611 RSC; (b) K. Kong, J. Zhang, P. Zhao, H. Lu, Z. Chen, W. Cao, J. Chen and Y. Chen, Tetrahedron, 2017, 73, 6742 CrossRef CAS; (c) J. Li, N. Wang, C. Li and X. Jia, Org. Lett., 2012, 14, 4994 CrossRef CAS PubMed; (d) Y. Chen, C. Hau, H. Wang, H. He, M. Wong and A. Lee, J. Org. Chem., 2006, 71, 3512 CrossRef CAS PubMed; (e) Y. Chen, J. Sun, X. Wei, W. Wong and A. Lee, J. Org. Chem., 2004, 69, 7190 CrossRef CAS PubMed.
- (a) B. Xue, S. Su, Y. Cui, Y. Fei, X. Jia, J. Li and J. Fang, Chem. Commun., 2019, 55, 12180 RSC; (b) J. Hu, Y. Fei, H. Zhao, Z. Wang, C. Li and J. Li, Chem. Commun., 2019, 55, 8394 RSC; (c) H. Yuan, C. Tang, S. Su, L. Cui, X. Jia, C. Li and J. Li, Chem. Commun., 2019, 55, 7231 RSC; (d) T. Jin, Z. Tang, J. Hu, H. Yuan, Y. Chen, C. Li, X. Jia and J. Li, Org. Lett., 2018, 20, 413 CrossRef CAS PubMed.
- CCDC 1915323, 1915320 for compound 5j, 5n contain the supplementary crystallographic data for this paper..
- M. Jereb, Green Chem., 2012, 14, 3047 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, HRMS (ESI) and X-ray crystal structure data (CIF) for compound 5j, 5n. CCDC 1915323 for compound 5j; 1915320 for compound 5n. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra09332j |
| This journal is © The Royal Society of Chemistry 2019 |