Open Access Article
Open Access ArticleEffects of storage condition on the physicochemical characteristics of sunflower seed oil†
Wei Xua,
Juan Yaoa,
Yang Yi *a,
Hong-Xun Wang
*a,
Hong-Xun Wang b and
Li-Mei Wangb
b and
Li-Mei Wangb
aCollege of Food Science & Engineering, Wuhan Polytechnic University, Wuhan 430023, PR China. E-mail: yiy86@whpu.edu.cn; xuwei1216@163.com; Yaoj1995@163.com; Tel: +86 13886152207
bCollege of Biology and Pharmaceutical Engineering, Wuhan Polytechnic University, Wuhan 430023, PR China. E-mail: wanghongxun7736@163.com; wanglimeiyx@163.com
First published on 20th December 2019
Abstract
The effects of storage condition on the physicochemical characteristics of sunflower seed oil (SSO) were investigated, to understand the required conditions and the typical indicators for its quality control. The changes of SSO in peroxide value (PV), acid value (AV), fatty acid (FA) composition, Fourier transform infrared (FTIR) spectrum and volatile compound (VC) during 11 month storage under seven different conditions, were analyzed. The PVs and AVs of the seven groups all increased with time, but the PVs fluctuated strongly during the last 4 months. The between-group differences in PV and AV indicated that light-exposure and high-temperature (≥40 °C) both accelerated the production and degradation of primary oxidation products of FA. However, the FA composition of SSO did not obviously change regardless of storage condition and time, as well as its FTIR characteristics. By contrast, its VC composition was significantly changed by light-exposure and high-temperature (≥55 °C). 3-Methyl-2,5-furandione, acetic acid/1-phenylethyl ester, 2-pentyl-furan and limonene might be the main VCs related to the desirable flavor, in which 3-methyl-2,5-furandione in all the groups showed a significantly decreased percentage of VC composition during storage. Light-exposure and high-temperature enhanced the accumulation of aldehydes, especially hexanal and (E)-2-heptenal, which principally contributed to the undesirable flavor of SSO. 3-Methyl-2,5-furandione, hexanal and (E)-2-heptenal were proposed to be marker compounds for its quality control. A low-temperature and dark condition is necessary for SSO to remain a desirable flavor.
1. Introduction
Sunflower seed oil (SSO) mostly produced in the Russian Federation, Ukraine, Argentina and Turkey is one of the most consumed edible oils after soybean oil, rapeseed oil and cotton seed oil, with an annual consumption of about 8.6 million tons.1,2 SSO has been recognized as a healthy choice due to its desired composition of fatty acids (FAs), especially containing high contents of polyunsaturated fatty acids (PUFAs).3,4 However, its high PUFA content is also associated with a high risk of oxidative deterioration, which involves the formation of primary peroxides (hydroperoxides) in the presence of oxygen, the degradation of peroxides and the formation of secondary products such as aldehydes, ketones, alcohols, lactones and tertiary oxidation products, resulting in undesirable flavors, quality losses and shelf life reduction.5 Of greater concern, those products of oxidative deterioration can cause physiological disorders such as aging, atherosclerosis and carcinogenesis.6 Accordingly, more and more efforts have been paid for minimizing the oxidative changes of SSO before consumption.In recent years, the major concerns on the quality control of SSO during storage were related to the addition of natural extracts for improving the quality and oxidative stability.2,7,8 By contrast, the effects of storage condition, involving in many complicated factors affecting the quality of vegetable oils,5,6,9,10 have been rarely investigated on SSO. The limited evidences about the quality effects of light exposure, temperature and container on SSO were obtained under moderate conditions in a short storage period or a long period with few samplings,6,10,11 which were insufficient to understand its required storage conditions.
The development of international transportation contributed to the world-wide consumption of SSO, especially in China, which annually had an imported amount more than 0.43 million tons since 2013.12 Ocean container transportation has been deemed as a critical element of any global supply chains.13 The SSO products transported in the traditional container might suffer the environment temperature as high as 72 °C,14 and the transportation from European countries to China by sea might last for about 35 days (refer to the data of Mckinley Logistics Co. Ltd., Tianjin, China). Therefore, the quality effects of long-time exposure at high temperatures, which may occur in the storage and international transportation of SSO, are necessary to be verified.
Moreover, the quality changes of SSO during storage were mostly indicated by some concrete parameters, such as peroxide value (PV), acid value (AV) and p-anisidine value,6,10,11 instead of comprehensive profiles. In combination with chemometrics methods, FTIR spectroscopy allows the qualitative and semi-quantitative profiling of organic compounds in oils, exhibiting the potential of rapid analysis on adulteration, deterioration and authentication.15–17 Similarly, volatile compounds (VCs) formed in the course of hydroperoxide decomposition were closely related to the oxidative changes of SSO during storage.18 However, the effects of storage condition on the FTIR and volatile profiles of SSO were unavailable.
The present work aimed to investigate the effects of storage condition on the physicochemical characteristics of SSO. In combination with light exposure or light elimination, storage temperatures ranging from 20 °C (close to mean room temperature) to 70 °C (extreme high-temperature) were adopted for an 11 month storage. Except the commonly used parameters for the quality inspection of vegetable oils (i.e. PV, AV and FA composition), the characteristic profiles of functional group and VC were respectively analyzed by FTIR spectroscopy and gas chromatography-mass spectrometry (GC-MS) combined with chemometrics methods.
2. Materials and methods
2.1. SSO products and their storage conditions
The SSO products from Aceites Abril (Ourense, Spain), which contained 45.95 mg vitamin E/100 g, were purchased. They were all produced on 24 August 2017 (2 years of shelf life) in a 0.5 L PET bottle. Seven groups (I–VII), each containing 13 products, were respectively kept under different conditions for 11 months (from 01 February 2018 to 01 January 2019), as shown in Table 1. The light exposure at room temperature was obtained naturally (without direct solar illumination), and that at 70 °C was carried out with artificial daylight in a 12/12 h light/dark cycle. On the 1st day of each month, one bottle from each group was sampled for tests.| Group | Storage temperature (°C) | Light exposure | Storage time (month) |
|---|---|---|---|
| a The lowest and highest room temperatures were recorded daily during storage, and their mean values of each month were in the ranges of 3–25 °C and 10–33 °C, respectively.b The SSO products in group VI and VII were placed in a 70 °C thermotank for a month, and were then kept at room temperature for 10 months. | |||
| I | Room temperaturea | No | 11 |
| II | Room temperature | Yes | 11 |
| III | 25 °C | No | 11 |
| IV | 40 °C | No | 11 |
| V | 55 °C | No | 11 |
| VIb | 70 °C/room temperature | No | 1/10 |
| VIIb | 70 °C/room temperature | Yes | 1/10 |
2.2. Measurement of PV, AV and FA
The PV and AV of sample were analyzed by the titration methods respectively described in the national standard GB5009.227 (ref. 19) and GB5009.229 (ref. 20) of China. The PV (mmol kg−1) was expressed as the molar amount of active oxygen per 1 kg sample, and the AV (mg KOH per g) were expressed as the mass of potassium hydroxide used to neutralize 1 g sample. The methyl-esterification of FA and the measurement of FA methyl ester were carried out by the method of Yao et al.,21 using an Agilent 7890A GC system (Agilent, Santa Clara, CA, USA). All the measurements were implemented with three replications for each sample.2.3. FTIR analysis
SSO was analyzed by using a Thermo Nicolet Nexus 670 FTIR spectrometer (Nicolet Instrument Corporation, Madison, USA) in the range of 4000–600 cm−1 with a resolution of 4 cm−1. Fifty microliter sample was scanned by a deuterated triglycine sulfate detector with the signal cumulative frequency of 16.2.4. Determination of VCs
The VCs of SSO were detected by a GC-MS method combined with the pretreatment of headspace-solid phase microextraction (HS-SPME). The detection was performed on an Agilent 7890A GC system coupled with a 5975C mass spectrometer (Agilent, USA). An Agilent HP-5MS capillary column (30 m length, 0.25 mm inner diameter and 0.25 μm thickness) was used with the following temperature programming: 35 °C (hold for 5 min); 35 °C → 60 °C (6 °C min−1); 60 °C → 70 °C (4 °C min−1); 70 °C → 150 °C (5 °C min−1); 150 °C → 220 °C (10 °C min−1); 220 °C (hold for 5 min). Moreover, N2 was used as carrier gas at a flow rate of 1.0 mL min−1, and the temperature of flame ionization detector was set at 250 °C. A SPME fiber (75 μm CAR/PDMS, Supelco, Bellefonte, USA) was preheated at the injection port (250 °C, 2 h) for adsorption. A bottle (20 mL) containing 7.0 g oil was heated in a 60 °C water bath for 20 min. The pretreated fiber was then exposed to the sample headspace for 60 min, followed by desorption (250 °C, 10 min) for detection. The MS detection conditions were as follows: electron ionization; ionization voltage 70 eV; ion source temperature 230 °C; MS Quad temperature 150 °C; transfer line temperature 280 °C; scan range 50–500 m/z; solvent delay 0.00 min. The peaks in the total ion chromatogram was identified by comparing their MS spectra to the standard spectra from the NIST11.L database.2.5. Data analysis
The significant difference (P < 0.05) between groups was analyzed by one-way analysis of variance (Student–Newman–Keuls test) using the SPSS Statistics 19 software (IBM, Armonk, NY, USA). The between-group correlation was assessed by Pearson's correlation test. The curvilinear integrating, common model fitting, similarity evaluation and multivariate statistical analysis of FTIR spectra and GC chromatograms were conducted on the ChemPattern software (Advanced Chemometric Solution 2017) of Chemmind Technologies (Beijing) Co., Ltd. (Beijing, China).3. Results and discussion
3.1. Effects of storage condition on the PV and AV of SSO
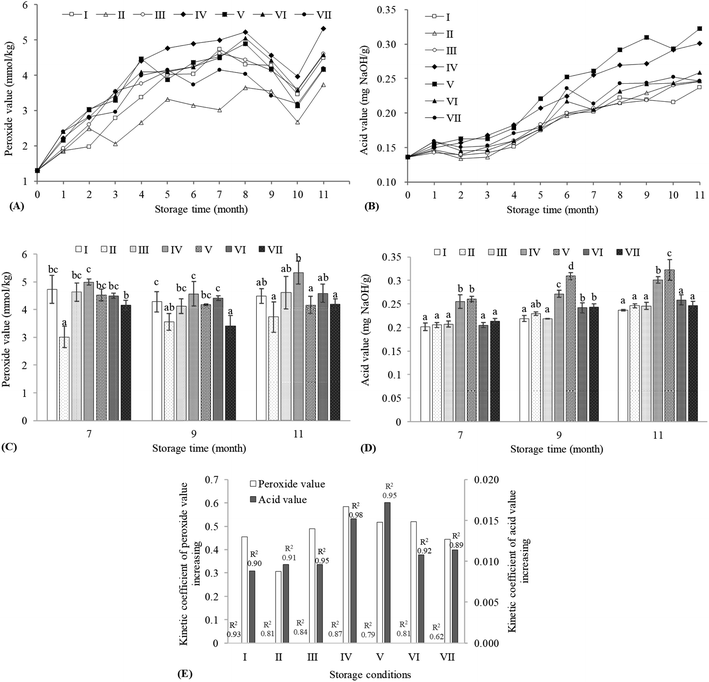
The primary oxidation of SSO was quantified by the level of hydroperoxides, namely PV. As seen in Fig. 1A, the PVs of seven groups obviously increased with time during the first 8 months of storage, decreased in the following 2 months, and rose again at the end of storage. The depletion of oxygen, the permeability of bottle to oxygen and the loss of antioxidants might jointly cause this fluctuation of PV.11,22,23 The initial oxygen in the bottle and the permeated oxygen through PET material might contribute to the increase of PV, which would weaken due to the consumption of oxygen. The break-up of peroxides into secondary products, which were responsible for the deterioration of flavor,23 was then a leading factor resulting in the decrease of PV. The α-tocopherol level fell by around 90% in olive oil after 9 month storage at 20 °C.23 It was suggested that the antioxidants in SSO were almost depleted after 10 month storage, and the elimination of oxidation-inhibition by antioxidants caused the rise of PV. In addition, SSO might alter the PET's barrier and mechanical properties by penetrating with a plasticizing effect, leading to the change of oxygen transmission rate at the later stage of storage (8–11 months).24 The AVs of seven groups steadily increased during storage, and the increases could be obviously observed after 3 months (Fig. 1B). It might be associated with the production of free FA via the hydrolysis of triacylglycerols and the formation of acids via the decomposition of peroxides. Those products could be served as catalysts for the further hydrolysis reaction, resulting in the increase of susceptibility to hydrolytic rancidity.23The statistical differences (P < 0.05) among seven groups were analyzed to explore the effects of storage condition on PV and AV (Fig. 1C and D), as well as their kinetic coefficients of value increasing during the storage (Fig. 1E). It was reported that light exposure could accelerate the production of primary oxidation products in edible oils and the consumption of oxygen in their containers.25,26 Compared to the vegetable oils exposed to light, the oils kept in dark had lesser oxidative alterations possibly due to the elimination of photo-oxidation and the maintenance of natural antioxidants such as tocopherol, pigments and phenols.5,11 The differences between light-exposed group (II and VII) and its light-eliminated counterpart (I and VI) indicated that light exposure contributed to the lower PVs, which might be related to the degradation of hydroperoxides. Moreover, a previous study indicated that a high storage temperature could speed up the auto-oxidation of SSO.6 However, groups I, III, IV and V showed no significant difference in the PV on the 7th and 9th month (P > 0.05). On the 11th month, the PV of group V was significantly lower than that of group IV (P < 0.05). It might be related to the effect of temperature on the oxygen transmission rate of PET bottle. For PET bottles containing olive oil, a higher storage temperature did not result in a larger oxygen transmission rate.24 Light exposure did not significantly change the AV of rice bran oil during storage.25 It was found that light exposure was not the main factor affecting the AV of SSO, but storage temperature was. The increase of AV during storage and its kinetic coefficient both had positive correlations with storage temperature.
3.2. Effect of storage condition on the FA composition of SSO
The SSO products used in this work were composed of (0.05 ± 0.01)% myristic acid, (2.54 ± 0.01)% palmitic acid, (0.13 ± 0.01)% palmitoleic acid, (1.96 ± 0.01)% stearic acid, (16.33 ± 0.01)% oleic acid, (0.81 ± 0.01)% elaidic acid, (0.24 ± 0.01)% linolelaidic acid, (76.96 ± 0.01)% linoleic acid, (0.14 ± 0.01)% arachidic acid, (0.10 ± 0.01)% eicosenoic acid, (0.19 ± 0.01)% linolenic acid, (0.41 ± 0.01)% behenic acid and (0.15 ± 0.01)% eicosapentaenoic acid (ESI Tables†). As the main FAs in SSO, linoleic acid and oleic acid showed the content variations less than 1.0% and 3.5% during storage, respectively. For saturated FAs, the contents of palmitic acid in seven groups all showed an increase of about 8% after storage, and those of stearic acid were almost constant (ESI Tables†). The FA composition did not significantly change during storage regardless of storage conditions, which was consistent with two previous reports. The FA composition of SSO and its chia oil blends (with and without the addition of antioxidants) did not vary significantly during 4 °C and 20 °C storage (P < 0.05).6 The FA change of SSO after 180 days storage at room temperature was also measured previously. Without the addition of natural antioxidative extracts and synthetic antioxidants, its linoleic acid content significantly decreased, but its FA composition did not change.8 For olive oils, the similar conclusion could be drawn. The FA compositions of olive oils in 5 different containers all remained more or less constant through 6 months investigation.5 Li et al. indicated that the FA composition of olive oil remained fairly constant during the storage regardless of the storage conditions due to the existence of antioxidants.233.3. Effect of storage condition on the FTIR characteristic of SSO
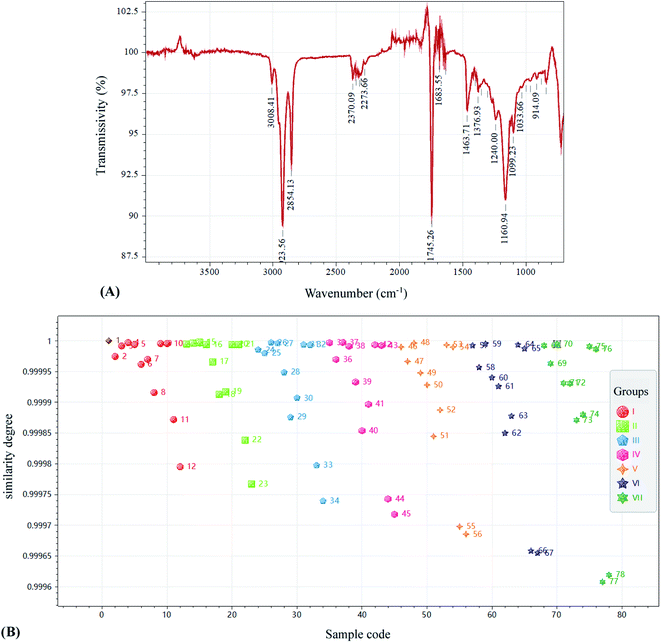
The FTIR spectra of SSO products under different storage conditions were recorded in the wavenumber range of 4000–600 cm−1. As shown in Fig. 2A, SSO before storage exhibited the characteristic bands as reported previously:27,28 the stretching vibration (SV) of![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H (cis), 3008.41 cm−1; the symmetric SV of –C–H (CH3), 2923.56 cm−1; the asymmetric SV of –C–H (CH2), 2854.13 cm−1; the SV of –C
C–H (cis), 3008.41 cm−1; the symmetric SV of –C–H (CH3), 2923.56 cm−1; the asymmetric SV of –C–H (CH2), 2854.13 cm−1; the SV of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester), 1745.26 cm−1; the SV of –C
O (ester), 1745.26 cm−1; the SV of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (acid), 1683.55 cm−1; the bending vibration (BV) of –C–H (CH2), 1463.71 cm−1; the symmetric BV of –C–H (CH3), 1376.93 cm−1; the SV of –C–O–, 1240.00 cm−1; the SV of –C–O, 1160.94, 1099.23 and 1033.66 cm−1; the BV (out of plane) of –HC
O (acid), 1683.55 cm−1; the bending vibration (BV) of –C–H (CH2), 1463.71 cm−1; the symmetric BV of –C–H (CH3), 1376.93 cm−1; the SV of –C–O–, 1240.00 cm−1; the SV of –C–O, 1160.94, 1099.23 and 1033.66 cm−1; the BV (out of plane) of –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–(cis), 914.09 cm−1. According to the correlation coefficient-based similarity analysis (Fig. 2B), it was found that the FTIR spectra of SSO products were highly similar (similarity degree > 0.9996) regardless of their storage conditions and times.
CH–(cis), 914.09 cm−1. According to the correlation coefficient-based similarity analysis (Fig. 2B), it was found that the FTIR spectra of SSO products were highly similar (similarity degree > 0.9996) regardless of their storage conditions and times.
3.4. Effect of storage condition on the VC composition of SSO
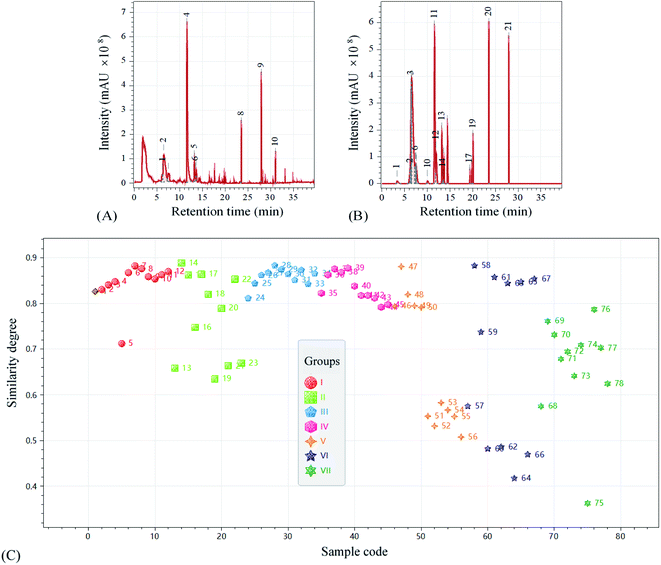
The VCs of SSO products were analyzed by HP-SPEM sampling, GC separation and MS detection. About one hundred chromatographic peaks were recorded in the retention time (RT) range of 6.163–39.386 min. The VC chromatogram of SSO before storage was shown in Fig. 3A. To explore the main changes related to different storage conditions, the common compounds with the peak area percentage of at least 1% in the chromatogram were selected. Accordingly, the common chromatogram model of all the 78 SSO samples was formed with 21 common peaks, as seen in Fig. 3B. Some of them belonged to the silicon compounds from sampling bottle and extraction column, and some with close RTs could be assigned to a same VC. Finally, 12 main VCs from SSO were confirmed and identified: 1-octene (6.16 min), hexanal (6.45 min), heptanal (10.01 min), 3-methyl-2,5-furandione (11.58 min), (E)-2-heptenal (11.93 min), 2-pentyl-furan (13.20 min), decane (13.49 min), octanal (13.64 min), D-limonene (14.42 min), heptanoic acid/2-propenyl ester (19.35 min), acetic acid/1-phenylethyl ester (19.76 min) and decanal (20.07 min). All of them were reported previously,18,29 except 3-methyl-2,5-furandione. As one of the main VCs in SSO, 3-methyl-2,5-furandione might accumulate via two ways: (1) it could be produced by the thermal decomposition of citric acid which was widely used as a degumming agent in the processing of vegetable oils; (2) as a specific secondary metabolite in fruits,30 it might also exist in sunflowerseed and could be transferred to oil during processing. The aldehydes detected were the main products of peroxide decomposition and the indicators of rancidity, resulting in an undesirable flavor.18 1-Octene might act as a petrol-like odor. In addition, acetic acid/1-phenylethyl ester (pineapple- or brandy-like odor), 2-pentyl-furan (butter- or green bean-like odor) and limonene (lemon- or orange-like odor) might contribute to the pleasant flavor of SSO.29To evaluate the effect of storage condition on the VC composition of SSO during the long-time storage, the similarity degrees of 78 chromatograms compared to the common model were analyzed by the correlation coefficient method (Fig. 3C). The VC composition of SSO would significantly change during storage under certain conditions. For groups I, III and IV, the values of similarity degree were almost in the range of 0.8–0.9. These relatively high values indicated that the VC composition of SSO did not significantly change during storage at a low temperature (≤40 °C) in dark. The comparison between group I and II confirmed that light exposure induced changes in the VC composition. In addition, the VC composition of SSO obviously changed after 5 month storage at a relatively high temperature (55 °C). A higher temperature (70 °C) lasting for one month brought great variations in the VC composition. It was consistent with a previous conclusion that light exposure and high temperature both accelerated the flavor deterioration of SSO and shortened its shelf-life.10 Li et al.23 indicated that cold storage condition (4.5 and −27 °C) was successful at retarding the oxidation and hydrolysis level during olive oil storage with no significant change in flavor aspect over 18 week storage.
The changes of SSO in the VC composition under different storage conditions were detailed in Table 2. As a dominating VC of aldehydes and a major product from the decomposition of linoleate hydroperoxide,31 hexanal showed an increasing percentage of composition during storage. Obviously, light exposure and high temperature would positively contribute to the accumulation of aldehydes, especially hexanal and (E)-2-heptenal. (E)-2-Heptenal could not be detected initially, and it was undetectable over the whole storage period of groups I and III. In other groups, by contrast, (E)-2-heptenal was detectable mostly during the 11 month storage. Moreover, the percentage of 3-methyl-2,5-furandione in the total VCs decreased largely during storage, and the decrease were obviously accelerated by high temperature and light exposure. It was suggested that hexanal, (E)-2-heptenal and 3-methyl-2,5-furandione were the important VCs associated with storage conditions and might play a key role in the quality control of SSO.
| Sample code | Groups | Storage time (month) | Relative percentage of peak area of volatile component (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.16 min | 6.45 min | 10.01 min | 11.58 min | 11.93 min | 13.20 min | 13.49 min | 13.64 min | 14.42 min | 19.35 min | 19.76 min | 20.07 min | |||
| 1 | — | 0 | 3.05 | 6.42 | 0.31 | 76.39 | — | 6.66 | 2.00 | 2.90 | 0.38 | 0.41 | 0.86 | 0.60 |
| 2 | I | 1 | 1.81 | 5.01 | 0.28 | 64.29 | — | 4.48 | 1.13 | 4.82 | 10.16 | 1.25 | 2.74 | 4.02 |
| 3 | I | 2 | 2.37 | 6.58 | 0.20 | 64.10 | — | 7.50 | 1.23 | 4.72 | 7.75 | 0.90 | 1.67 | 2.98 |
| 4 | I | 3 | 2.74 | 8.18 | 1.55 | 69.26 | — | 5.70 | 1.62 | 4.34 | 5.43 | 0.17 | 0.34 | 0.67 |
| 5 | I | 4 | 0.86 | 4.31 | 0.12 | 27.48 | — | 3.63 | 0.82 | 7.85 | 31.69 | 4.07 | 4.98 | 14.18 |
| 6 | I | 5 | 1.67 | 11.93 | 0.27 | 47.40 | — | 6.04 | 1.26 | 5.94 | 10.62 | 2.45 | 3.39 | 9.05 |
| 7 | I | 6 | 1.85 | 13.05 | 0.41 | 60.38 | — | 5.39 | 1.33 | 4.34 | 6.04 | 1.17 | 1.97 | 4.07 |
| 8 | I | 7 | 1.80 | 13.53 | 1.83 | 66.83 | — | 6.76 | 1.64 | 3.70 | 1.00 | 0.82 | 0.86 | 1.24 |
| 9 | I | 8 | 2.09 | 48.58 | 1.16 | 39.17 | — | 3.34 | 0.89 | 2.33 | 2.24 | 0.01 | 0.10 | 0.09 |
| 10 | I | 9 | 2.15 | 17.27 | 1.67 | 63.90 | — | 6.35 | 1.77 | 3.38 | 2.45 | 0.13 | 0.46 | 0.46 |
| 11 | I | 10 | 2.59 | 16.53 | 1.48 | 70.40 | — | 3.64 | 1.13 | 3.36 | 0.65 | — | 0.14 | 0.09 |
| 12 | I | 11 | 1.21 | 42.83 | 1.08 | 44.86 | — | 3.37 | 0.81 | 2.32 | 2.50 | 0.13 | 0.39 | 0.52 |
| 13 | II | 1 | 2.88 | 7.80 | 0.26 | 71.58 | — | 4.92 | 1.49 | 3.36 | 3.74 | 0.67 | 1.36 | 1.95 |
| 14 | II | 2 | 2.89 | 10.24 | 1.54 | 49.10 | — | 8.48 | 1.54 | 4.84 | 9.79 | 1.77 | 3.53 | 6.26 |
| 15 | II | 3 | 2.35 | 30.24 | 1.17 | 29.92 | 4.65 | 5.98 | 1.16 | 4.93 | 14.82 | 0.76 | 1.28 | 2.73 |
| 16 | II | 4 | 1.21 | 11.19 | 1.21 | 19.80 | 4.78 | 6.43 | 0.75 | 6.95 | 23.25 | 4.53 | 4.74 | 15.16 |
| 17 | II | 5 | 2.41 | 14.69 | 2.29 | 35.93 | 8.93 | 14.00 | 1.64 | 4.58 | 6.11 | 1.30 | 2.06 | 6.04 |
| 18 | II | 6 | 2.64 | 48.29 | 1.85 | 22.43 | 6.75 | 11.23 | 1.47 | 1.98 | 1.78 | 0.26 | 0.44 | 0.86 |
| 19 | II | 7 | 4.11 | 17.28 | — | 35.66 | 13.44 | 19.56 | 2.07 | 3.25 | 2.01 | 0.81 | 0.95 | 0.85 |
| 20 | II | 8 | 3.90 | 24.44 | 2.40 | 28.65 | 10.54 | 15.57 | 1.88 | 3.18 | 9.01 | — | 0.26 | 0.17 |
| 21 | II | 9 | 2.80 | 52.57 | — | 23.67 | 6.30 | 10.59 | 1.12 | 1.44 | 1.03 | 0.05 | 0.21 | 0.23 |
| 22 | II | 10 | 3.01 | 28.08 | — | 40.16 | 8.58 | 15.32 | 1.69 | 3.07 | — | — | 0.03 | 0.06 |
| 23 | II | 11 | 2.67 | 19.83 | — | 39.74 | 9.32 | 16.53 | 2.07 | 2.97 | 3.99 | 0.37 | 0.95 | 1.55 |
| 24 | III | 1 | 1.88 | 6.16 | 0.34 | 78.06 | — | 3.75 | 1.95 | 2.16 | 0.97 | 0.23 | 0.47 | 4.04 |
| 25 | III | 2 | 1.40 | 24.46 | 0.33 | 55.75 | — | 5.51 | 1.36 | 3.67 | 3.24 | 0.61 | 1.06 | 2.61 |
| 26 | III | 3 | 2.42 | 30.18 | 0.28 | 52.99 | — | 3.75 | 1.26 | 3.47 | 4.47 | 0.15 | 0.45 | 0.58 |
| 27 | III | 4 | 2.02 | 6.99 | 0.42 | 42.26 | — | 4.28 | 0.75 | 5.09 | 13.24 | 2.94 | 4.21 | 17.79 |
| 28 | III | 5 | 1.82 | 9.57 | 0.23 | 46.77 | — | 5.00 | 1.29 | 5.74 | 12.62 | 2.24 | 3.45 | 11.26 |
| 29 | III | 6 | 1.85 | 39.39 | 1.16 | 45.23 | — | 4.08 | 1.10 | 1.78 | 1.08 | 0.34 | 0.44 | 3.54 |
| 30 | III | 7 | 1.68 | 21.09 | 1.88 | 59.49 | — | 6.26 | 1.68 | 2.87 | 2.04 | 0.71 | 0.83 | 1.45 |
| 31 | III | 8 | 1.85 | 47.16 | 0.18 | 32.74 | — | 2.82 | 0.81 | 2.31 | 11.71 | — | 0.31 | 0.11 |
| 32 | III | 9 | 1.80 | 17.07 | 1.58 | 65.65 | — | 6.46 | 1.75 | 2.66 | 2.27 | 0.07 | 0.29 | 0.40 |
| 33 | III | 10 | 1.98 | 18.76 | 1.46 | 68.61 | — | 5.11 | 1.43 | 2.53 | — | — | 0.02 | 0.10 |
| 34 | III | 11 | 1.59 | 45.92 | 0.98 | 42.78 | — | 3.51 | 0.98 | 1.63 | 1.55 | 0.06 | 0.41 | 0.59 |
| 35 | IV | 1 | 0.71 | 17.57 | 0.16 | 52.42 | — | 2.88 | 0.66 | 4.01 | 10.38 | 1.84 | 3.10 | 6.26 |
| 36 | IV | 2 | 2.29 | 13.31 | 0.28 | 62.68 | — | 4.99 | 1.51 | 3.27 | 5.80 | 0.93 | 1.88 | 3.07 |
| 37 | IV | 3 | 2.44 | 11.91 | 0.26 | 46.00 | 5.93 | 4.35 | 0.71 | 5.81 | 18.60 | 0.53 | 1.18 | 2.28 |
| 38 | IV | 4 | 0.93 | 12.45 | 1.28 | 40.40 | — | 5.61 | 1.08 | 5.70 | 19.72 | 2.23 | 2.61 | 8.01 |
| 39 | IV | 5 | 1.07 | 36.88 | 1.01 | 33.25 | — | 4.07 | 0.74 | 3.99 | 9.24 | 1.57 | 2.14 | 6.04 |
| 40 | IV | 6 | 1.24 | 50.98 | 1.15 | 36.85 | — | 4.58 | 1.18 | 1.94 | 1.02 | 0.29 | 0.39 | 0.38 |
| 41 | IV | 7 | 1.68 | 52.84 | 1.13 | 30.12 | 5.31 | 4.77 | 0.94 | 1.51 | 0.70 | 0.22 | 0.43 | 0.37 |
| 42 | IV | 8 | 1.24 | 55.25 | 0.88 | 27.95 | 3.61 | 3.86 | 0.57 | 1.45 | 4.97 | — | 0.14 | 0.09 |
| 43 | IV | 9 | 2.55 | 22.19 | 1.78 | 50.50 | 9.22 | 7.44 | 1.57 | 2.05 | 2.08 | 0.05 | 0.30 | 0.27 |
| 44 | IV | 10 | 1.00 | 60.33 | 0.86 | 27.58 | 4.65 | 3.38 | 0.60 | 1.16 | 0.32 | 0.04 | 0.05 | 0.02 |
| 45 | IV | 11 | 0.83 | 57.15 | 0.92 | 26.82 | 4.97 | 4.54 | 0.68 | 1.05 | 2.55 | 0.04 | 0.21 | 0.22 |
| 46 | V | 1 | 2.43 | 9.97 | 1.34 | 65.96 | 8.84 | 4.52 | 0.97 | 2.01 | 0.57 | 0.56 | 1.21 | 1.63 |
| 47 | V | 2 | 1.75 | 19.49 | 1.54 | 52.10 | — | 8.22 | 1.05 | 3.18 | 5.02 | 1.09 | 2.45 | 4.11 |
| 48 | V | 3 | 1.76 | 21.12 | 1.94 | 38.28 | 8.62 | 9.45 | 1.19 | 3.37 | 5.69 | 1.07 | 2.48 | 5.03 |
| 49 | V | 4 | 0.91 | 47.06 | 0.21 | 17.92 | 5.89 | 8.10 | 0.88 | 3.06 | 8.54 | 1.19 | 1.78 | 4.45 |
| 50 | V | 5 | 1.82 | 23.81 | 2.43 | 22.27 | 11.35 | 15.17 | — | 3.54 | 7.84 | 1.90 | 2.50 | 7.38 |
| 51 | V | 6 | 2.48 | 37.38 | 2.55 | 19.84 | 16.40 | 17.45 | 1.38 | 1.34 | 0.52 | 0.15 | 0.28 | 0.23 |
| 52 | V | 7 | 2.99 | 30.60 | 3.01 | 18.55 | 17.70 | 25.56 | 0.56 | — | 0.13 | 0.24 | 0.37 | 0.29 |
| 53 | V | 8 | 2.20 | 35.82 | 2.55 | 17.49 | 16.70 | 21.00 | — | 1.79 | 2.20 | — | 0.18 | 0.09 |
| 54 | V | 9 | 1.85 | 40.90 | 2.71 | 15.53 | 11.36 | 23.44 | 1.92 | 1.45 | 0.43 | 0.03 | 0.24 | 0.14 |
| 55 | V | 10 | 3.35 | 35.22 | 2.63 | 12.48 | 22.15 | 21.93 | 1.38 | 0.44 | 0.28 | — | 0.13 | 0.03 |
| 56 | V | 11 | 1.50 | 67.11 | 2.33 | 5.01 | 9.61 | 13.03 | — | — | 0.98 | 0.04 | 0.21 | 0.17 |
| 57 | VI | 1 | 0.42 | 5.03 | 0.14 | 22.26 | — | 2.45 | 0.28 | 9.85 | 46.60 | 2.14 | 3.01 | 7.81 |
| 58 | VI | 2 | 1.74 | 14.16 | 1.69 | 57.71 | — | 7.79 | 1.40 | 3.49 | 4.52 | 1.29 | 2.24 | 3.97 |
| 59 | VI | 3 | 2.05 | 15.09 | 1.98 | 48.31 | 12.73 | 7.29 | 1.45 | 4.08 | 5.47 | 0.16 | 0.58 | 0.83 |
| 60 | VI | 4 | 0.51 | 29.79 | 1.14 | 16.51 | 3.53 | 5.50 | 0.51 | 5.77 | 19.24 | 2.96 | 3.97 | 10.56 |
| 61 | VI | 5 | 3.07 | 18.10 | 0.32 | 49.11 | 10.46 | 6.70 | 1.57 | 3.58 | 2.81 | 0.75 | 1.01 | 2.54 |
| 62 | VI | 6 | 1.94 | 23.30 | 0.24 | 48.64 | 11.07 | 7.93 | 1.66 | 2.55 | 1.59 | 0.32 | 0.36 | 0.39 |
| 63 | VI | 7 | 2.23 | 20.24 | 2.20 | 51.60 | 7.64 | 9.23 | 1.78 | 2.82 | 0.17 | 0.61 | 0.70 | 0.78 |
| 64 | VI | 8 | 1.49 | 55.33 | 1.28 | 27.40 | 4.68 | 5.97 | 0.76 | 2.62 | 0.41 | — | 0.03 | 0.03 |
| 65 | VI | 9 | 1.26 | 47.30 | 1.29 | 35.74 | 4.26 | 5.31 | 1.03 | 1.74 | 1.22 | 0.09 | 0.38 | 0.38 |
| 66 | VI | 10 | 1.72 | 51.39 | 1.08 | 31.44 | 6.17 | 4.05 | 0.75 | 1.96 | 1.29 | — | 0.09 | 0.07 |
| 67 | VI | 11 | 1.54 | 48.69 | 0.34 | 34.69 | 3.89 | 5.20 | 0.85 | 1.68 | 2.53 | 0.06 | 0.26 | 0.26 |
| 68 | VII | 1 | 0.42 | 5.03 | 0.14 | 22.26 | — | 2.45 | 0.28 | 9.85 | 46.60 | 2.14 | 3.01 | 7.81 |
| 69 | VII | 2 | 2.17 | 12.22 | 1.68 | 44.52 | 10.37 | 6.98 | 0.93 | 3.98 | 7.87 | 1.34 | 3.00 | 4.95 |
| 70 | VII | 3 | 2.00 | 15.19 | 1.77 | 38.62 | 8.62 | 6.74 | 0.86 | 5.59 | 14.94 | 0.96 | 1.24 | 3.47 |
| 71 | VII | 4 | 1.09 | 27.25 | 0.96 | 17.49 | 6.20 | 4.19 | 0.46 | 5.68 | 20.08 | 2.81 | 3.96 | 9.82 |
| 72 | VII | 5 | 1.55 | 13.34 | 1.45 | 24.79 | 8.43 | 7.82 | 0.87 | 5.26 | 17.94 | 3.20 | 3.63 | 11.73 |
| 73 | VII | 6 | 2.47 | 24.78 | 2.34 | 36.90 | 11.67 | 13.88 | 1.85 | 2.97 | 1.68 | 0.41 | 0.47 | 0.58 |
| 74 | VII | 7 | 2.71 | 23.56 | 2.33 | 33.46 | 11.07 | 14.56 | 1.76 | 3.13 | 1.88 | 0.99 | 1.63 | 2.92 |
| 75 | VII | 8 | 2.34 | 27.28 | 2.48 | 32.32 | 10.82 | 16.67 | 1.58 | 2.80 | 3.35 | 0.02 | 0.22 | 0.12 |
| 76 | VII | 9 | 2.98 | 21.72 | — | 43.19 | 7.07 | 18.46 | 2.10 | 2.56 | 1.18 | 0.13 | 0.22 | 0.38 |
| 77 | VII | 10 | 2.78 | 27.31 | 2.15 | 37.02 | 11.05 | 12.84 | 1.72 | 2.76 | 2.15 | — | 0.13 | 0.10 |
| 78 | VII | 11 | 2.03 | 57.32 | — | 20.56 | 7.74 | 8.66 | 1.11 | 1.27 | 0.92 | 0.03 | 0.16 | 0.21 |
The Pearson's relationship between VC and quality parameter was further investigated (data not shown). The percentage of aldehydes in the total VCs had positive correlations with both PV and AV (P < 0.01), and the main contributor should be hexanal which had the coefficients close to those of total aldehydes. For other aldehydes, heptanal also had positive correlations with PV and AV (P < 0.05), but both octanal and decanal showed negative correlations with them. Except that, PV showed negative correlations with 1-octene, 3-methyl-2,5-furandione, D-limonene and acetic acid/1-phenylethyl ester (P < 0.01 or P < 0.05), and AV exhibited negative correlations with 3-methyl-2,5-furandione, 2-pentyl-furan, D-limonene, heptanoic acid/2-propenyl ester and acetic acid/1-phenylethyl ester (P < 0.01). The results indicated that the changes in the physicochemical characteristics of SSO during storage, including PV, AV and flavor, had significant relationships.
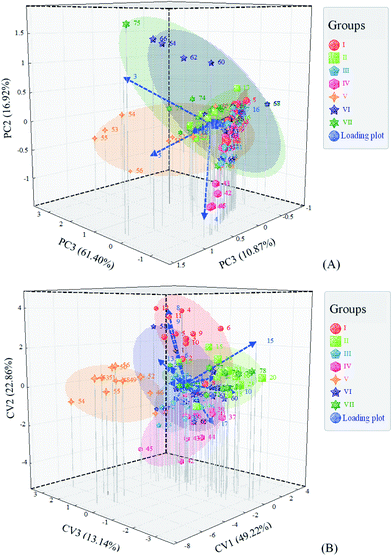
The differences of SSO products in the GC profile of VC were further investigated by multivariate statistical analysis combined with chemometrics methods. Principal component analysis (PCA) and multivariate analysis of variance (MANOVA) were performed to draw the score plot and loading plot, to explore the potential VCs responsible for the between-group variability (Fig. 4A and B). Three principal components (PCs) explained 89.19% of the total variance in the PCA score plot. Samples in group V, VI and VII were all scattered in a relatively large plane, indicating their great intragroup variations in VC composition. The main factors contributing to the variations were variables 3 (6.45 min), 4 (6.48 min) and 5 (6.60 min), which were all assigned to hexanal (6.45–6.60 min). The MANOVA score plot displayed a different distribution of samples compared to the PCA one. Three canonical variables (CVs) could explain 85.22% of the total variance. The variables 8 and 9, which were known as silicon compounds not belonging to SSO, were the main factors leading to the dispersion of samples in group I. The variables 10 (heptanal) and 15 (octanal) mainly contributed to the separation of light-exposure groups (II and VII). Group V was almost separated from other groups, and the main contributor was variable 13 (2-pentyl-furan). The results implied that hexanal, heptanal, octanal and 2-pentyl-furan were the main VCs related to the effects of light exposure and high temperature during storage.
4. Conclusion
SSO products stored under various conditions showed different changes in their physicochemical characteristics during the 11 month storage. Light exposure and high temperature (≥40 °C) both accelerated the oxidation of FA in SSO. Especially, the degradation of peroxides produced by primary oxidation was strengthened, which was associated with the decrease of PV, the increase of AV and the deterioration of flavor. The FAs of SSO might decrease due to oxidation-degradation and hydrolysis, but overall their percentages of composition did not change regardless of storage condition and time, as well as FTIR characteristics. However, the change of SSO in the VC composition was closely related to improper storage conditions, such as light exposure and high temperature (≥55 °C). 3-Methyl-2,5-furandione, acetic acid/1-phenylethyl ester, 2-pentyl-furan and limonene might be the main VCs contributing the desirable flavor of SSO, in which 3-methyl-2,5-furandione showed significant decreases in all the groups. By contrast, light exposure and high temperature enhanced the accumulation of aldehydes, especially hexanal and (E)-2-heptenal. As a major product from the decomposition of linoleate hydroperoxide, hexanal principally contributed to the undesirable flavor and showed an increasing percentage of VC composition in all the groups. Therefore, 3-methyl-2,5-furandione, hexanal and (E)-2-heptenal could be used as marker compounds for the quality control of SSO. SSO in a PET bottle should be stored at a relatively low temperature (<40 °C) in dark, and a long-time transportation at an extreme high-temperature (70 °C) should be also avoided.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFD0401103).References
- S. Şahin, E. Sayim and R. Samli, Korean J. Chem. Eng., 2017, 34, 2284–2292 CrossRef.
- D. Wang, W. Fan, Y. Guan, H. Huang, T. Yi and J. Ji, LWT–Food Sci. Technol., 2018, 98, 268–275 CrossRef CAS.
- A. Bendini, S. Barbieri, E. Valli, K. Buchecker, M. Canavari and T. G. Toschi, Eur. J. Lipid Sci. Technol., 2011, 113, 1375–1384 CrossRef CAS.
- R. Upadhyay, S. Sehwag and H. Niwas Mishra, Food Chem., 2017, 218, 496–504 CrossRef CAS PubMed.
- A. I. Méndez and E. Falqué, Food Control, 2007, 18, 521–529 CrossRef.
- E. N. Guiotto, V. Y. Ixtaina, S. M. Nolasco and M. C. Tomás, J. Am. Oil Chem. Soc., 2014, 91, 767–776 CrossRef CAS.
- D. Wang, Y. Meng, X. Zhao, W. Fan, T. Yi and X. Wang, LWT–Food Sci. Technol., 2019, 111, 55–61 CrossRef CAS.
- R. Kowalski, Food Chem., 2009, 112, 820–830 CrossRef CAS.
- R. Aly and U. Ravid, Isr. J. Plant Sci., 2008, 56, 273–278 CAS.
- G. Ramírez, G. Hough and A. Contarini, J. Food Qual., 2001, 24, 195–204 CrossRef.
- R. Romanić, E. Dimić, V. Lazić and V. Vujasinović, Acta Aliment., 2009, 38, 319–327 CrossRef.
- R. Wang, China Oils Fats, 2016, 41, 1–3 Search PubMed.
- J. C. Fransoo and C. Y. Lee, Prod. Oper. Manag., 2013, 22, 253–268 CrossRef.
- S.-y. Zhao, R.-l. Tian, C.-g. Wang and W.-j. Wang, Equip. Environ. Eng., 2007, 4, 44–47 Search PubMed.
- N. Vlachos, Y. Skopelitis, M. Psaroudaki, V. Konstantinidou, A. Chatzilazarou and E. Tegou, Anal. Chim. Acta, 2006, 573–574, 459–465 CrossRef CAS PubMed.
- J. Vilela, L. Coelho and J. M. M. M. de Almeida, Cogent Food Agric., 2015, 1, 1020254 Search PubMed.
- Q. Zhang, C. Liu, Z. Sun, X. Hu, Q. Shen and J. Wu, Food Chem., 2012, 132, 1607–1613 CrossRef CAS PubMed.
- Á. Keszler, T. Kriska and A. Németh, J. Agric. Food Chem., 2000, 48, 5981–5985 CrossRef PubMed.
- National Health and Family Planning Commission of China, Standard, 2016, GB5009.227 Search PubMed.
- National Health and Family Planning Commission of China, Standard, 2016, GB5009.229 Search PubMed.
- J. Yao, C.-X. Zhang, Y.-R. Cai, H.-F. Wang, Y. Yi and L.-M. Wang, J. Food Saf. Food Qual., 2018, 9, 1072–1078 Search PubMed.
- F. Gutiérrez and J. L. Fernández, J. Agric. Food Chem., 2002, 50, 571–577 CrossRef PubMed.
- X. Li, H. Zhu, C. F. Shoemaker and S. C. Wang, J. Am. Oil Chem. Soc., 2014, 91, 1559–1570 CrossRef CAS.
- A. Kanavouras, Food Packag. Shelf Life, 2019, 21, 100336 CrossRef.
- M.-J. Kim, J. W. Park, J. Y. Kim, K. W. Park, S.-J. Lee, J.-K. Jang and J. H. Lee, Food Sci. Food Biotechnol., 2013, 22, 1–6 CAS.
- M. J. Akhtar, M. Jacquot, E. Arab-Tehrany, C. Gaïani, M. Linder and S. Desobry, Food Chem., 2010, 120, 395–401 CrossRef CAS.
- Y. Yi, J. Yao, W. Xu, L.-M. Wang and H.-X. Wang, RSC Adv., 2019, 9, 27347–27360 RSC.
- M. J. Lerma-García, G. Ramis-Ramos, J. M. Herrero-Martínez and E. F. Simó-Alfonso, Food Chem., 2010, 118, 78–83 CrossRef.
- K. D. Petersen, K. K. Kleeberg, G. Jahreis and J. Fritsche, Int. J. Food Sci. Nutr., 2012, 63, 160–169 CrossRef CAS PubMed.
- H. R. Halim, D. P. Hapsari, A. Junaedi, A. W. Ritonga, A. Natawijaya, R. Poerwanto, Sobir, W. D. Widodo and D. D. Matra, Data Brief., 2019, 23, 1–3 Search PubMed.
- F. K. Bangash, T. Ahmad, S. Atta and A. Zeb, J. Chin. Chem. Soc., 2004, 51, 991–995 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09215c |
| This journal is © The Royal Society of Chemistry 2019 |