Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ternary dual Z-scheme graphitic carbon nitride/ultrathin metal–organic framework nanosheet/Ag3PO4 photocatalysts for boosted photocatalytic performance under visible light†
Tomoharu Kusutakia,
Hideyuki Katsumata *a,
Ikki Tateishib,
Mai Furukawaa and
Satoshi Kanecoab
*a,
Ikki Tateishib,
Mai Furukawaa and
Satoshi Kanecoab
aDepartment of Chemistry for Materials, Graduate School of Engineering, Mie University, Tsu, Mie 514-8507, Japan. E-mail: hidek@chem.mie-u.ac.jp; Fax: +81-59231-9425; Tel: +81-59231-9425
bMie Global Environment Center for Education & Research, Mie University, Tsu, Mie 514-8507, Japan
First published on 2nd December 2019
Abstract
Ternary graphitic carbon nitride/ultrathin metal–organic framework nanosheet/Ag3PO4 (CNUA) composite photocatalysts were prepared under ultrasonic irradiation in tetrahydrofuran. The aim was to use them as photocatalysts for the degradation of organic pollutants in water. The crystal structure, surface morphology, optical properties, and chemical composition of the photocatalytic materials were investigated using X-ray diffraction, scanning electron microscopy, UV-vis diffuse reflectance spectroscopy, and X-ray photoelectron spectroscopy (XPS). The XPS analysis revealed the formation of Ag nanoparticles, which play an important role as an electronic mediator and photosensitizer in the composite during the synthesis. The photocatalytic activity of the composites in the degradation of 2-chlorophenol (2-CP) under visible light (>420 nm) was evaluated. Among the synthesized photocatalysts, the optimized CNUA with 10 wt% of g-C3N4 with respect to Ag3PO4 (CN10UA), exhibited the best photocatalytic performance in the degradation of 2-CP, which was almost decomposed completely upon ∼5 min of visible-light irradiation. Furthermore, the stability of the CN10UA photocatalyst could be maintained at a high level even after four cycling experiments, while that of pure Ag3PO4 declined significantly. The enhanced photocatalytic performance results from efficient charge separation through the dual Z-scheme mechanism involving Ag(0) bridges in the g-C3N4/Ag/Ag3PO4 and Ag3PO4/Ag/UMOFN pathways. The analysis of the photoluminescence of the catalysts also provided evidence for charge transport via the dual Z-scheme mechanism. In addition, radical scavenging tests confirmed that h+ and O2˙− are the main radical reactive species responsible for the photodegradation of 2-CP. The findings of this study enhance our understanding of the construction and mechanism of dual Z-scheme-type photocatalysts.
Introduction
Global issues, such as energy shortage and environmental pollution, are important problems that need to be solved for the sustainable development of modern society. In recent years, photodegradation of organic pollutants in photocatalytic reaction systems has attracted considerable interest as one of the most promising methods for environmental remediation.1 Recently, metal–organic frameworks (MOFs), in which metal cations and polydentate ligands are cross-linked, have attracted much interest as promising porous materials,2–4 because of their outstanding properties such as a large surface area, high porosity, excellent adsorption selectivity, and chemical stability. In addition, MOFs have also been widely utilized in photocatalysis, separation, gas storage, chemical sensors, and biomedicine; because their properties can be easily modulated by changing the size and chemical environment of the pore space.5–17 In particular, studies on the application of MOFs in photocatalysis have received much attention from the scientific community. According to Xamena et al., MOF-5 composed of Zn4O13 quantum dots and terephthalate linkers, shows excellent photocatalytic activity in the degradation of phenolic molecules under UV light.18 Further, Hardi et al. reported that MIL-125, constructed from titanium-oxo-hydroxo clusters and dicarboxylate linkers, shows high thermal stability and excellent photocatalytic activity owing to the formation of a Ti(III)–Ti(IV) mixed valence state in MIL-125 under UV-visible irradiation.19 In addition, ultrathin MOF nanosheets (UMOFNs), which are constructed from Ni2+, Co2+, and terephthalate linkers, exhibit high electrical conductivity, fast charge migration and high percentage of exposed active sites, and can serve as two-dimensional photocatalysts.20 Moreover, these properties of bimetal-UMOFNs are superior to those of single-metal-UMOFNs because of the interaction between Ni2+ and Co2+ via the bridging O2− and the optimization of the orbitals occupancy in the coordinatively unsaturated metals.21 However, the application of these MOFs as photocatalysts is hindered due to some drawbacks in terms of low effective separation of photogenerated charge carriers, poor photostability, and their absorption lying in the UV range and not in the visible one. Therefore, it is important to form a heterojunction between MOFs and visible-light-responsive photocatalysts.22In recent years, a considerable number of studies have been carried out in the field of photocatalysis on graphitic carbon nitride (g-C3N4), which is a metal-free photocatalyst. It is generally known that g-C3N4 has excellent properties, such as superior photoelectronic characteristics23,24 and high stability in water systems,25,26 as well as photocatalytic performance under visible-light irradiation.27,28 However, it has insufficient photocatalytic activity for practical application owing to the fast recombination rate of photogenerated electron–hole pairs. Therefore, development of composite photocatalysts based on g-C3N4is recommended for achieving efficient charge separation.29 For example, TiO2/g-C3N4,30 WO3/g-C3N4,31 ZnO/g-C3N4,32 SnO2/g-C3N4,33 graphene oxide (GO)/g-C3N4,34 CdS/g-C3N4,35 AgBr/g-C3N4,36 UMOFN/g-C3N4,37 ZIF-5/g-C3N4,38 and UiO-66/g-C3N4 (ref. 39) have been reported. These composite photocatalysts show remarkably enhanced photocatalytic performances as compared to that of pure g-C3N4. This is because the constructed heterojunction system facilitates efficient separation of photogenerated carriers, which could lead to higher photocatalytic performance.
Over the past few years, many researchers have shown interest in Ag3PO4as a photocatalyst because of its excellent photocatalytic activity under visible-light irradiation. In addition, the photocatalytic performance of Ag3PO4 can be further improved by modifying its surface properties.40–43 There is, however, a particular disadvantage in using Ag3PO4 as a photocatalyst; an excessive amount of Ag metal is deposited on the surface of Ag3PO4 due to its photo-corrosion during the photocatalytic reaction. The deposited metallic Ag interferes with charge transfer, light adsorption, and adsorption of organic pollutants. Therefore, the structure and photocatalytic activity of Ag3PO4 gradually deteriorate. In order to solve this problem, considerable attention has been paid to the development of Ag3PO4 composite photocatalysts such as g-C3N4/Ag3PO4,44 ZnO/Ag3PO4,45 MoSe2/Ag3PO4,46 TiO2/Ag3PO4,47 GO/Ag3PO4,48 HKUST-1/Ag3PO4,49 NH2-MIL-125/Ag3PO4,50 and AgBr/Ag3PO4.51 In comparison with pure Ag3PO4, these photocatalysts show superior photocatalytic performance owing to the formation of heterojunctions.
Furthermore, in recent years, various ternary semiconductors have been developed in order to further improve the photocatalytic performance of binary photocatalysts. The ternary photocatalysts with suitable matching energy band structures provide more efficient charge separation than binary photocatalysts. Wang et al. prepared a ternary GO/Ag3PO4/AgBr composite via an in situ anion-exchange method, and it showed higher photocatalytic degradation of rhodamine B (RhB) under visible-light irradiation in comparison with those of pure Ag3PO4 and Ag3PO4/AgBr.52 Yan et al. demonstrated that ternary Ag3PO4/GO/g-C3N4 photocatalysts synthesized by a chemical precipitation method has superior photocatalytic activity in RhB degradation than single or binary photocatalysts. In this ternary system, Ag3PO4, GO, and g-C3N4 serve as the photosensitizer, cocatalyst, and visible-light-driven photocatalyst, respectively, and together they enhance the photocatalytic activity under visible-light irradiation.53 Xu et al. synthesized a magnetic Ag3PO4/TiO2/Fe3O4 nanocomposite that presented excellent properties including cycling stability, long-term durability, and effective charge separation because of the heterojunction structure in the ternary system.54 As reported by Liu et al., the ternary g-C3N4/Ag3PO4/Ag2MoO4 photocatalysts exhibited efficient charge separation and superior water oxidation under visible-light irradiation owing to the generation of dual Z-scheme-type Ag bridges.55 According to our previous studies, tetrahedral UMOFN/Ag3PO4 core–shell photocatalysts have great potential for application in environmental purification technologies.56 The synergistic effects of the tetrahedral structure and heterojunction can be harnessed in the formation of useful ternary photocatalysts when combined with a third material. Therefore, the formation of a ternary heterojunction, such as in the g-C3N4/UMOFNs/Ag3PO4 system, could lead to further improvement in the photocatalytic activity. However, to the best of our knowledge, there are no reports on ternary g-C3N4/UMOFNs/Ag3PO4 composite photocatalysts.
In this study, we developed a new ternary g-C3N4/UMOFNs/Ag3PO4 composite photocatalyst to further enhance the photocatalytic activity. The ternary g-C3N4/UMOFNs/Ag3PO4 photocatalysts were easily fabricated in a tetrahydrofuran (THF) solution under ultrasound irradiation. The photocatalytic performance of the ternary g-C3N4/UMOFNs/Ag3PO4 photocatalysts was evaluated by performing the photodegradation of 2-chlorophenol (2-CP) under visible-light irradiation (>420 nm) in their presence. Furthermore, the crystal structure, optical properties, chemical composition, and surface morphology of the photocatalytic samples were characterized, and the degradation mechanism of 2-CP under the catalysis of ternary g-C3N4/UMOFNs/Ag3PO4 photocatalysts was carefully studied in detail.
Experimental
Materials and methods
UMOFNs were synthesized according to a previously reported procedure.20 N,N-Dimethylformamide (DMF, 32 mL), ethanol (2 mL), and distilled water (2 mL) were mixed by sonication for 10 min. Then, terephthalic acid (0.75 mmol) was added to the mixed solution. Next, CoCl2·6H2O (0.375 mmol) and NiCl2·6H2O (0.375 mmol) were simultaneously dissolved in the mixed solution. After the complete dissolution of the two salts, triethylamine (0.8 mL) was rapidly added to the mixture, and it was ultrasonicated for 8 h in a sealed glassware. Finally, the obtained product was centrifuged, washed four times with ethanol, and dried under vacuum at 30 °C to recover a pale pink powder of UMOFNs.
g-C3N4 was synthesized according to a previously reported procedure.44 In a typical synthesis, 10 g of urea was placed in an alumina crucible with a cover, and it was calcined in an electric furnace at 550 °C for 5 h at the heating rate of 20 °C min−1. After cooling naturally to room temperature, the obtained g-C3N4 was ground into a powder for further use.
The ternary g-C3N4/UMOFNs/Ag3PO4 (CNUA) composite photocatalysts were prepared via ultrasound irradiation in a THF solution. First, the prepared UMOFNs (20 mg) and an appropriate amount of g-C3N4 were uniformly dispersed in tetrahydrofuran (100 mL) through sonication for 30 min. Then, the prepared Ag3PO4 tetrahedron (400 mg) was added to the mixed solution, and the mixture was sonicated in the dark for 6 h. The so-obtained CNUA was centrifuged, washed four times with ethanol, and dried under vacuum at 30 °C. The weight ratio of UMOFNs to Ag3PO4 was optimized according to a previously published method.56 A series of CNUA samples were prepared by varying the amount of g-C3N4in the composite, and samples with 5, 10, 20, 30, 50, and 70 wt% of g-C3N4 were referred to as CN5UA, CN10UA, CN20UA, CN30UA, CN50UA, and CN70UA, respectively. For comparison, binary composites of UMOFN/Ag3PO4 (UA) without g-C3N4 and g-C3N4/Ag3PO4 (CNA) with 10 wt% g-C3N4 and no UMOFNs were also prepared according to the same procedure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) water/acetonitrile. A total organic carbon (TOC) analyser (TOC-VE, Shimadzu) was used to investigate the decrease in the TOC of the 2-CP solution during the photocatalytic reaction. For the radical scavenging test, various scavengers were added to the reaction system under the same experimental conditions as those used for the photocatalytic degradation experiment. A 30 mM ammonium oxalate (AO) solution, 30 mM t-butyl alcohol (TBA) solution, and 10 mM 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMPO) solution were used as scavengers for detecting the generation of h+, ·OH, and O2˙−, respectively.
50 (v/v) water/acetonitrile. A total organic carbon (TOC) analyser (TOC-VE, Shimadzu) was used to investigate the decrease in the TOC of the 2-CP solution during the photocatalytic reaction. For the radical scavenging test, various scavengers were added to the reaction system under the same experimental conditions as those used for the photocatalytic degradation experiment. A 30 mM ammonium oxalate (AO) solution, 30 mM t-butyl alcohol (TBA) solution, and 10 mM 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMPO) solution were used as scavengers for detecting the generation of h+, ·OH, and O2˙−, respectively.Results and discussion
Material characterization
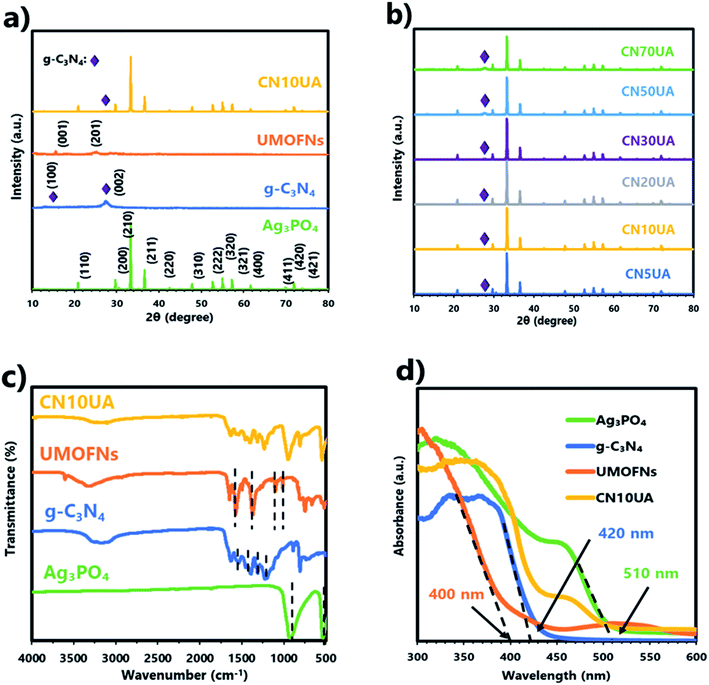
In order to identify the crystal structure and composition of the as-synthesized materials, the XRD patterns of g-C3N4, UMOFNs, Ag3PO4, and CN10UA were analysed (Fig. 1a). For g-C3N4, the two characteristic diffraction peaks observed at 12.7 and 27.5° correspond to the (1 0 0) and (0 0 2) planes of the material, respectively. The former peak is attributed to the interlayer stacking of aromatic systems, while the latter one is attributed to in-plane structural packing.44 For UMOFNs, the same XRD pattern as that reported previously was obtained,20 confirming that UMOFNs were successfully synthesized. In the XRD pattern of Ag3PO4, the positions of all characteristic diffraction peaks are in good agreement with those of a body-centred cubic structure of Ag3PO4 (JCPDS no. 06-0505). In addition, the narrow and sharp peaks reveal that the prepared Ag3PO4 particles are highly crystalline without any other impurities. The intensity ratio between the signals of (2 2 2) and (2 0 0) planes is 0.96, suggesting that tetrahedral Ag3PO4 composed of {1 1 1} facets was successfully synthesized.57 In the XRD pattern of CN10UA, a weak peak derived from g-C3N4 and the characteristic peaks corresponding to Ag3PO4 could be observed, while no peaks attributable to UMOFNs were observed because of their lower concentration and relatively weaker crystallinity in comparison with those of Ag3PO4. This result is consistent with a previous report on UMOFN/Ag3PO4 photocatalyst.56 Moreover, no other characteristic diffraction peaks are observed, indicating that the CN10UA composite photocatalyst is a highly pure material without any impurities. The diffraction peaks of CNUA samples with different concentrations of g-C3N4 (5, 10, 20, 30, 50, and 70 wt%) are presented in Fig. 1b. As the weight ratio of g-C3N4 is gradually increased, the peak intensity derived from g-C3N4 becomes stronger in the XRD pattern of CNUA. These results indicate the successful preparation of CNUA systems with ternary heterojunctions.The FTIR spectra of g-C3N4, UMOFNs, Ag3PO4, and CNUA with different concentrations of g-C3N4 (5, 10, 20, 30, 50, and 70 wt%) were also acquired to further determine the composition of the photocatalytic materials. As shown in Fig. 1c, the two characteristic peaks at 539 and 923 cm−1 in the FTIR spectrum of Ag3PO4 result from the bending vibration of P–O bond and asymmetric stretching vibration of PO43−, respectively.44 The FTIR spectrum of UMOFNs shows two strong peaks at 1370 and 1647 cm−1 corresponding to the asymmetric and symmetric stretching vibrations of the carboxyl group in terephthalic acid, respectively.56 The two peaks at 1016 and 1102 cm−1 are attributed to the C–N stretching vibration of DMF. The appearance of these peaks indicates the presence of DMF solvent in the pores of UMOFNs because they could not be removed during the preparation of UMOFNs.56 The peak at ∼600–800 cm−1 is attributed to the vibration of the metal–oxygen bond in UMOFNs.56 In the case of g-C3N4, the characteristic peaks located at 1227, 1312, 1454, 1536, and 1628 cm−1 could be attributed to the C–N stretching vibration.44 Further, the peak at 807 cm−1 and the broad peak at ∼3200 cm−1 are attributed to the breathing of triazine units and N–H stretching vibration, respectively.44 In the CN10AU composite photocatalyst, prominent peaks corresponding to g-C3N4 and Ag3PO4 appeared, while no peaks attributed to UMOFN are observed due to its lower concentration in comparison with that of Ag3PO4. Furthermore, these peaks are slightly shifted to higher wave numbers, suggesting the presence of weak interactions between g-C3N4, UMOFNs, and Ag3PO4 (Fig. S1†).20 These results are in good agreement with those of the XRD analysis.
The surface morphology of the photocatalytic materials was observed by SEM and TEM. Fig. 2a and e show that g-C3N4 has an irregularly stacked structure. Further, Fig. 2b and f show that Ag3PO4 particles have a tetrahedral morphology with 1 μm side length and exposed {1 1 1} facets, which have higher surface energy than other facets.57 Fig. 2c and g show that UMOFNs are ultra-thin nanosheets with slightly rolled edges. Finally, the SEM images of CN10UA in Fig. 2d and h reveal that Ag3PO4 tetrahedrons are incorporated into g-C3N4 without undergoing any morphological changes. However, UMOFNs cannot be identified due to their ultra-thin two-dimensional nanosheet structure. In addition, EDX mapping analysis was performed to further confirm the distribution of elements in CN10UA, (Fig. S2†). As shown in Fig. S2,† C, N, O, Co, Ni, Ag and P elements exist uniformly in CN10UA, indicating that the hetero-structure of the ternary photocatalyst is composed of UMOFNs, g-C3N4 and Ag3PO4 without other impurities.
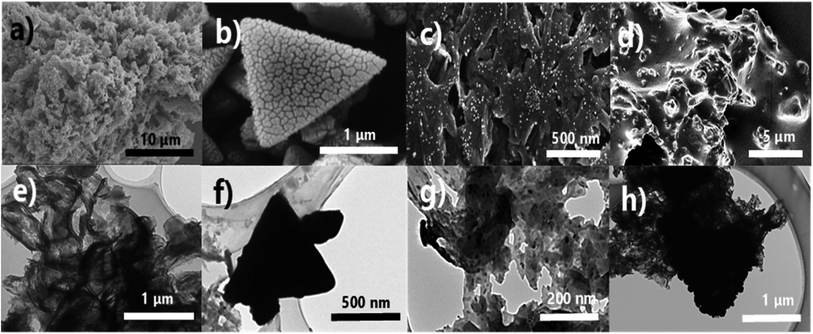 | ||
| Fig. 2 SEM (a–d) and TEM (e–h) images of (a and e) g-C3N4 (b and f) Ag3PO4, (c and g) UMOFNs, and (d and h) CN10UA. | ||
The porosity and specific surface area of photocatalytic materials were determined by the analysis of nitrogen adsorption–desorption isotherms. As shown in Fig. S3,† CN10UA demonstrates a type IV isotherm according to the IUPAC classification, confirming that it has both mesopores and micropores. In addition, the Brunauer–Emmett–Teller (BET) surface areas of materials used in this study are summarized in Table 1. The BET surface area of Ag3PO4 is 0.78 m2 g−1. With an increase in the g-C3N4 content, the BET surface area of CNUA increases from 3.66 m2 g−1 (CN5UA) to 9.65 m2 g−1 (CN10UA), 10.11 m2 g−1 (CN20UA), 10.13 m2 g−1 (CN30UA), 18.26 m2 g−1 (CN50UA), and 26.88 m2 g−1 (CN70UA). It is well known that a photocatalyst with a higher BET surface area facilitates effective adsorption of organic pollutants onto its surface and provides more photocatalytic active sites. Therefore, it is reasonable to assume that the composite catalyst would improve the efficiency of the photocatalytic reaction because the surface area of the material increased with the introduction of g-C3N4 into Ag3PO4.
| Samples | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| Ag3PO4 | 0.78 | 0.0015 | 7.82 |
| UMOFNs | 6.32 | 0.0196 | 12.63 |
| g-C3N4 | 89.24 | 0.6521 | 29.22 |
| UA | 1.60 | 0.0045 | 11.36 |
| CAN | 5.06 | 0.0235 | 18.65 |
| CN5UA | 3.66 | 0.0143 | 15.67 |
| CN10UA | 9.65 | 0.0788 | 32.65 |
| CN20UA | 10.11 | 0.0756 | 29.91 |
| CN30UA | 10.13 | 0.0861 | 33.45 |
| CN50UA | 18.26 | 0.1481 | 32.45 |
| CN70UA | 26.88 | 0.2122 | 31.55 |
The optical properties and band structures of samples were studied by UV-vis diffuse reflectance spectroscopy. As shown in Fig. 1d, the adsorption edge of g-C3N4, UMOFNs, and Ag3PO4 were determined to be 420, 380, and 510 nm, respectively. Based on the Kubelka–Munk formula, the band gap energies (Eg) of g-C3N4, UMOFNs, and Ag3PO4 were calculated to be 2.89, 3.01, and 2.43 eV, respectively (Fig. S4a†). In addition, valence band (VB) XPS analysis was performed to estimate the position of the valence band edge (EVB). For g-C3N4, UMOFNs, and Ag3PO4, the positions of the EVB were determined to be 1.43, 1.62, and 2.61 eV, respectively (Fig. S4b†). Therefore, their conduction band (ECB) positions were calculated to be −1.46, −1.39, and +0.18 eV, respectively, according to the empirical equation.
XPS analysis was performed to verify the chemical composition and environments of CN10UA (Fig. 3 and S5†). In Fig. S5a,† the XPS survey spectra indicates the existence of C, N, O, P, Co, Ni, and Ag in the ternary system, thus suggesting the coexistence of g-C3N4, UMOFNs, and Ag3PO4 in the ternary composite photocatalyst. Furthermore, other characteristic peaks are not observed, indicating that the ternary CN10UA composite is a high-purity photocatalyst without any impurities. As shown in Fig. 3a, the C 1s narrow-scan XPS spectrum has two strong peaks at 284.4 and 287.7 eV corresponding to sp2-hybridized carbons (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in aromatic hydrocarbons and sp2-hybridized nitrogen atoms (C
C) in aromatic hydrocarbons and sp2-hybridized nitrogen atoms (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in the triazine ring of g-C3N4 and/or carbonate species (C–O) in UMOFNs, respectively.44,56 In the N 1s narrow-scan XPS spectrum in Fig. 3b, four peaks located at 398.3, 400.2, 401.4, and 404.4 eV are attributed to sp2-hybridized N (C
N) in the triazine ring of g-C3N4 and/or carbonate species (C–O) in UMOFNs, respectively.44,56 In the N 1s narrow-scan XPS spectrum in Fig. 3b, four peaks located at 398.3, 400.2, 401.4, and 404.4 eV are attributed to sp2-hybridized N (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C), sp3-hybridized nitrogen (N–(C3)), amino functional groups (N–H and
N–C), sp3-hybridized nitrogen (N–(C3)), amino functional groups (N–H and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH) in g-C3N4, and the charge effects, respectively.44 The O 1s peaks are located at 530.8 and 532.5 eV in Fig. S5b.† The former peak is assigned to the lattice oxygen in Ag3PO4 and/or metal–oxygen bonds (Ni–O and Co–O) in UMOFNs.44,56 Further, the latter peak is assigned to the hydroxyl groups or water molecules adsorbed on the surface of CN10UA.44 Fig. 3c presents the P 2p peak at 132.1 eV, which is derived from PO43− in Ag3PO4.44 Further, the two peaks at 781.6 and 784.9 eV in the Co 2p narrow-scan XPS spectrum (Fig. 3d), which could be attributed to Co 2p3/2 and its satellite peak, respectively, indicate the presence of Co2+ in UMOFNs.56 In Fig. 3e, the two peaks in the Ni 2p narrow-scan XPS spectrum are located at 856.8 and 862.3 eV, which correspond to Ni 2p3/2 and its satellite peak, respectively, suggesting the existence of Ni2+ in UMOFNs.56 As shown in Fig. 3f, there are two strong peaks and two weak peaks in the Ag narrow-scan XPS spectrum. The former peaks at 367.7 and 373.7 eV are attributed to Ag5/2 and Ag3/2 levels of Ag+ ions in Ag3PO4, respectively. Meanwhile, the latter peaks at 369.5 and 375.2 eV are attributed to the Ag0 nanoparticles; these peaks indicate the formation of Ag0 due to the migration of electrons from g-C3N4 to Ag+ ions during the synthesis of the composite photocatalysts.44,56 The trace amounts of Ag nanoparticles in CNUA serve both as an electron mediator and as a photosensitizer (owing to the surface plasm on resonance (SPR)) in the photocatalytic reaction.58
NH) in g-C3N4, and the charge effects, respectively.44 The O 1s peaks are located at 530.8 and 532.5 eV in Fig. S5b.† The former peak is assigned to the lattice oxygen in Ag3PO4 and/or metal–oxygen bonds (Ni–O and Co–O) in UMOFNs.44,56 Further, the latter peak is assigned to the hydroxyl groups or water molecules adsorbed on the surface of CN10UA.44 Fig. 3c presents the P 2p peak at 132.1 eV, which is derived from PO43− in Ag3PO4.44 Further, the two peaks at 781.6 and 784.9 eV in the Co 2p narrow-scan XPS spectrum (Fig. 3d), which could be attributed to Co 2p3/2 and its satellite peak, respectively, indicate the presence of Co2+ in UMOFNs.56 In Fig. 3e, the two peaks in the Ni 2p narrow-scan XPS spectrum are located at 856.8 and 862.3 eV, which correspond to Ni 2p3/2 and its satellite peak, respectively, suggesting the existence of Ni2+ in UMOFNs.56 As shown in Fig. 3f, there are two strong peaks and two weak peaks in the Ag narrow-scan XPS spectrum. The former peaks at 367.7 and 373.7 eV are attributed to Ag5/2 and Ag3/2 levels of Ag+ ions in Ag3PO4, respectively. Meanwhile, the latter peaks at 369.5 and 375.2 eV are attributed to the Ag0 nanoparticles; these peaks indicate the formation of Ag0 due to the migration of electrons from g-C3N4 to Ag+ ions during the synthesis of the composite photocatalysts.44,56 The trace amounts of Ag nanoparticles in CNUA serve both as an electron mediator and as a photosensitizer (owing to the surface plasm on resonance (SPR)) in the photocatalytic reaction.58
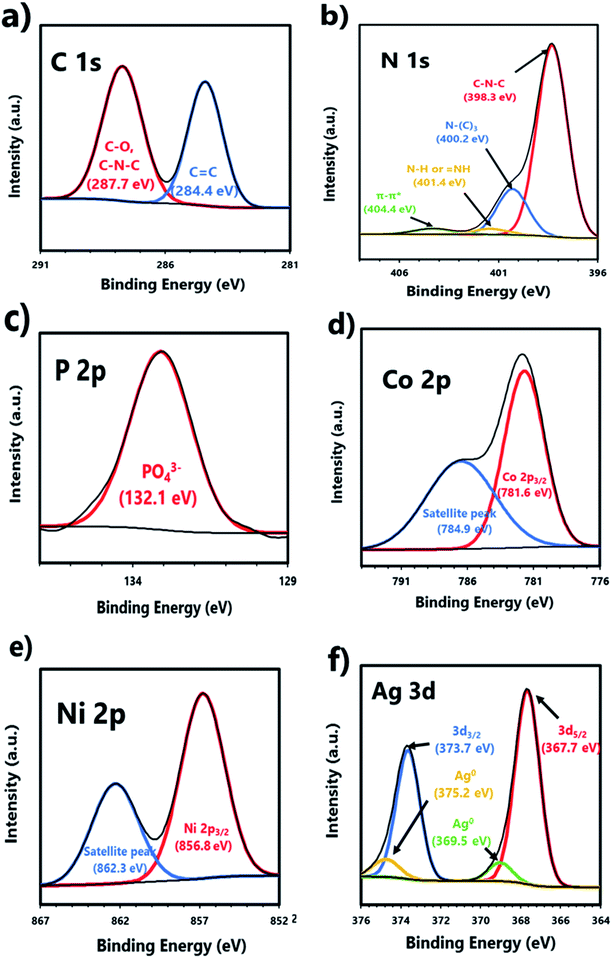 | ||
| Fig. 3 XPS spectra of CN10UA: (a) C 1s, (b) N 1s, (c) P 2p, (d) Co 2p, (e) Ni 2p, and (f) Ag 3d spectra. | ||
Photocatalytic activity of the ternary photocatalysts
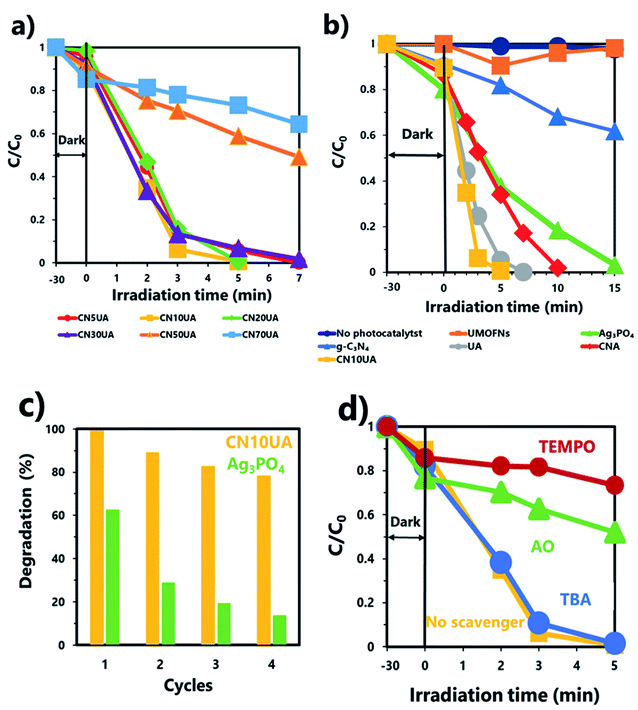
The photocatalytic performance of the as-synthesized samples was determined by evaluating the efficiency of photodegradation of 2-CP under their catalysis. Fig. 4a shows the photocatalytic activity of CNUA samples with different amounts of g-C3N4 (5, 10, 20, 30, 50, and 70%). Among the ternary photocatalysts, CN10UA exhibited the best photocatalytic activity. In addition, the apparent first-order rate constants (kapp) for a series of CNUA samples were calculated using the pseudo-first-order kinetics equation. The value of kapp follows the order of CN70UA (0.06 min−1) < CN50UA (0.10 min−1) < CN30UA (0.56 min−1) < CN5UA (0.57 min−1) < CN20UA (0.84 min−1) < CN10UA (0.93 min−1), as shown in Fig. S6a.† Apparently, CN10UA has the highest kapp value among the prepared CNUA samples. Thus, the optimal amount of g-C3N4 required to form the ternary system was determined to be 10 wt%. Fig. 4b shows that 2-CP did not undergo any degradation in the absence of photocatalysts, indicating that its self-photolysis reaction during the photocatalytic process is negligible and could be neglected. In the presence of pure Ag3PO4, the photocatalytic efficiency of 2-CP reached 97.1% within 15 min. When Ag3PO4 is combined with UMOFNs or g-C3N4, the photocatalytic activity of the binary photocatalyst, UA or CNA, is improved as compared to that of pure Ag3PO4. Remarkably, after the formation of the ternary system consisting of g-C3N4, UMOFNs, and Ag3PO4, the photocatalytic activity increased significantly. The ternary photocatalyst showed higher photocatalytic activity than those of the binary or single-component photocatalysts, and completely degraded 2-CP in only 5 min. The enhanced photocatalytic activity of ternary photocatalysts results from more efficient and fast charge separation through dual Z-scheme mechanism, as discussed in the following section. The TOC removal rate was also analysed to investigate the degree of mineralization of 2-CP over Ag3PO4 and CN10UA. As shown in Fig. S7,† TOC removal efficiencies of 22.1 and 98.7% in 120 min were achieved using Ag3PO4 and CN10UA as photocatalysts, respectively, suggesting that the construction of the ternary system also led to enhanced photocatalytic mineralization activity. This result is in good agreement with the results of the photocatalytic degradation.The reusability of the photocatalyst is a very important factor for its practical application, and recycling experiments were carried out using pure Ag3PO4 and CN10UA to evaluate their reusability. As shown in Fig. 4c, pure Ag3PO4 showed poor photocatalytic stability during the 2-CP photodegradation. However, the photocatalytic efficiency of CN10UA remained as high as 78.1% even after four reaction cycles, indicating that the construction of the ternary system also led to improved stability of the catalyst under visible light. In addition, the recycled photocatalyst was characterized by XRD, SEM and DRS to investigate the stability and possible reaction mechanism. The XRD pattern of the used CN10UA photocatalyst in Fig. S8a† shows the appearance of a relatively weak diffraction peak at 38.1° corresponding to the (1 1 1) plane of Ag0 (JCPDS no. 65-8425). This result suggests that Ag+ ions in the Ag3PO4 are reduced to produce additional Ag0 nanoparticles on CNUA during photocatalysis. That is, Ag+ in CNUA is reduced to Ag0 not only during the synthetic process but also during the photocatalytic degradation of 2-CP. On the other hand, a stronger peak at 38.1° compared to that of used CN10UA and two weak peaks at 44.3 and 64.3° can be observed in the XRD patterns of the used Ag3PO4, corresponding to (1 1 1), (2 0 0), and (2 2 0) planes of Ag0, respectively. These differences indicate that CN10UA has higher photo-corrosion resistance as compared to pure Ag3PO4 due to the consumption of electrons from the conduction band (CB) of Ag3PO4. The SEM images of the used photocatalyst in Fig. S9† show that the surface morphology of CN10UA remains almost the same as that of a fresh one after four reaction cycles. The DRS of the used photocatalyst in Fig. S8b† shows that the intensity of the absorption band in the visible range is significantly increased after visible-light irradiation due to the formation of additional Ag0 species during the photocatalytic reaction. In general, it is known that an excessive amount of Ag nanoparticles interferes with light adsorption, contact of the catalyst with organic pollutants, and charge migration on Ag3PO4, thereby decreasing the photocatalytic performance.56 Therefore, the slight deterioration of the photocatalytic activity during the cycling experiments can be attributed to the deposition of additional Ag0 nanoparticles on the photocatalyst during the photocatalytic process.
Possible photocatalytic mechanism
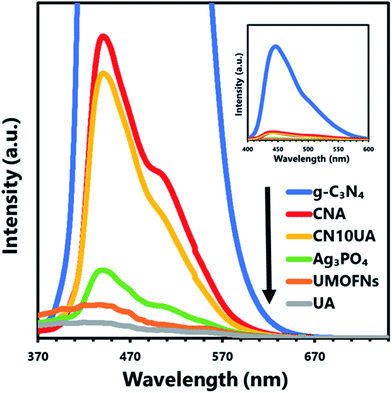
To investigate the main reactive species involved in the photocatalytic degradation of 2-CP over CN10UA, radical scavenging tests were conducted under the same experimental conditions as the photocatalytic degradation experiment. AO, TBA, and TEMPO were added to the reaction system for detecting the generation of h+, ·OH, and O2˙−, respectively. Fig. 4d shows that the addition of TBA had no effect on the degradation reaction, while the addition of AO and TEMPO led to the suppression of the photocatalytic degradation. In addition, the values of kapp were calculated in Fig. S6b,† and their order was as follows: TEMPO (0.03 min−1) < AO (0.09 min−1) < TBA (0.75 min−1) < No scavenger (0.93 min−1). These results indicate that h+ and O2˙− play an important role as the main reactive species in the photocatalytic degradation of 2-CP. In particular, O2˙− played a more significant role in the degradation of 2-CP.Furthermore, PL analysis was performed to further investigate the charge transfer pathway in the ternary composite. In Fig. 5, the PL spectrum of the binary CNA photocatalyst shows higher intensity than that of pure Ag3PO4, indicating the fast recombination rate of photogenerated electrons in the CB of Ag3PO4 and holes in the VB of g-C3N4 through the Ag bridge; that is, g-C3N4/Ag/Ag3PO4 pathways were constructed in CNA. A similar phenomenon was observed in a previous work.44 The PL intensity of ternary CN10UA photocatalyst is lower than that of binary CNA, because of the efficient transport of photogenerated electron–hole pairs via Ag electron separation centres in Ag3PO4/Ag/UMOFNs pathways.49,56 Taking these results into account, we infer that the photogenerated electrons on the ternary CNUA photocatalysts are transferred through dual Z-scheme pathways during the photocatalytic reaction, leading to the efficient charge separation of photoinduced electron–holes on the ternary CNUA composite photocatalysts.
Based on the results of PL, DRS, XPS, and radical trapping studies on the ternary photocatalyst, a possible reaction mechanism of 2-CP photodegradation via the dual Z-scheme pathway over CN10UA under-visible light irradiation is proposed, as illustrated in Fig. 6. First, the trace amounts of Ag nanoparticles formed during the photocatalyst preparation are deposited on the composite due to the migration of electrons from g-C3N4 to Ag3PO4, as evidenced from XPS analysis. These Ag nanoparticles play an important role as dual Z-scheme channels in the g-C3N4/Ag/Ag3PO4 and Ag3PO4/Ag/UMOFNs pathways. Moreover, the Ag nanoparticles can be activated through the SPR effect under visible-light irradiation. In the g-C3N4/Ag/Ag3PO4 system, g-C3N4 and Ag3PO4 can be activated by visible light to generate electron–hole pairs. The photogenerated electrons in the CB of Ag3PO4 are easily transferred to the Ag metal, and then they recombine with the photogenerated holes from the VB of g-C3N4 via the Ag bridge. As a result, the electrons left on the g-C3N4 reduce O2 to O2˙−, which oxidizes 2-CP. Meanwhile, the holes on Ag3PO4 directly degrade 2-CP owing to the great ability of PO43− to attract the 2-CP electrons and the high positive potential of the VB that mainly consists of Ag 4d and O 2p orbitals.57 In the other system of Ag3PO4/Ag/UMOFNs, Ag nanoparticles absorb the visible light as a photosensitizer, and aids the formation of electron–hole pairs by the SPR effect. The SPR-induced electrons migrate to the CB of UMOFNs to reduce O2 to O2˙−. In contrast, the SPR-induced holes recombine with the photogenerated electrons from Ag3PO4 due to the lower Fermi level of Ag nanoparticles (Ef = +0.80 V vs. NHE).43 Therefore, the dual Z-scheme could facilitate more positive and negative potentials of VB and CB, respectively, which are favourable for the oxidation of 2-CP and the reduction of O2, respectively. Moreover, the electrons in the CB of Ag3PO4 flow into the Ag nanoparticles through the two Z-scheme pathways, instead of remaining in the CB of Ag3PO4, which could prevent the self-reduction of Ag3PO4 and the production of H2O2 from the reduction of O2 also occurred on the CB of Ag3PO4 through a two-electron process (E° (O2/H2O2) = +0.68 V vs. NHE).59,60 Consequently, the dual Z-scheme-type ternary CN10UA photocatalyst facilitates fast charge separation, leading to its high stability and enhanced photocatalytic activity in 2-CP photodegradation.
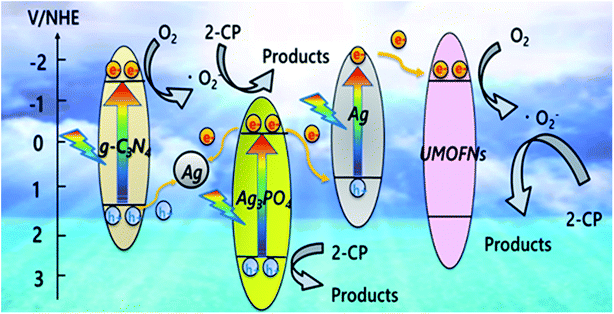 | ||
| Fig. 6 Possible reaction mechanism of the photodegradation of 2-CP over CN10UA under visible-light irradiation. | ||
Conclusions
We successfully synthesized ternary CNUA photocatalysts via ultrasound irradiation in a THF solution. The photocatalytic activity of the as-prepared materials was evaluated by employing these in the photodegradation of 2-CP under visible light irradiation. The ternary CN10UA photocatalyst shows better photocatalytic activity in comparison with binary or single-component photocatalysts; it facilitated almost complete degradation of 2-CP in only 5 min. Moreover, CN10UA also demonstrated high stability and effective charge separation. The observed enhancement in the photocatalytic activity of CN10UA results from fast charge transport through dual Z-scheme channels, via the g-C3N4/Ag/Ag3PO4 and Ag3PO4/Ag/UMOFNs pathways. In this system, the Ag bridges served both as an electron mediator and a photosensitizer via the SPR effect, and they remarkably promoted the rate of charge transport. In addition, PL analysis confirmed the transition of the photogenerated electrons on the ternary CNUA photocatalysts through dual Z-scheme pathways during the photocatalytic reaction. The findings of this study are expected to contribute to a better understanding of the construction and mechanism of dual Z-scheme type photocatalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research (B) No. 18H02013 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.Notes and references
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366–2388 RSC.
- Y. B. Huang, J. Liang, X. S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC.
- L. Zeng, X. Guo, C. He and C. Duan, ACS Catal., 2016, 6, 7935–7947 CrossRef CAS.
- S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574–1589 CrossRef CAS PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed. Engl., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- Z. Hu, J. B. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127–1129 CrossRef CAS PubMed.
- Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC.
- F. X. L. Xamena, A. Corma and H. Garcia, J. Phys. Chem. C, 2007, 111, 80–85 CrossRef.
- M. D. Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Ferey, J. Am. Chem. Soc., 2009, 131, 10857–10859 CrossRef PubMed.
- Y. Liang, R. Shang, J. Lu, L. Liu, J. Hu and W. Cui, ACS Appl. Mater. Interfaces, 2018, 10, 8758–8769 CrossRef CAS PubMed.
- S. Zhao, Y. Wang, J. Dong, C. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. Khattak, N. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao andZ and Y. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- J. He, Z. Yan, J. Wang, J. Xie, L. Jiang, Y. Shi, F. Yuan, F. Yu and Y. Sun, Chem. Commun., 2013, 49, 6761–6763 RSC.
- Z. Zhu, X. Tang, T. Wang, W. Fan, Z. Liu, C. Li, P. Huo and Y. Yana, Appl. Catal., B, 2019, 241, 319–328 CrossRef CAS.
- O. Y. Poudievsky, A. S. Kondratyuk, O. A. Kozarenko, V. V. Cherepanov, V. G. Koshechko and V. D. Pokhodenko, Int. J. Hydrogen Energy, 2019, 33, 17922–17929 CrossRef.
- G. Zhang, Z. A. Lan, L. Lin, S. Lin and X. Wang, Chem. Sci., 2016, 7, 3062–3066 RSC.
- G. Zhang, Z. A. Lan and X. Wang, Chem. Sci., 2017, 8, 5261–5274 RSC.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- Y. Wang, X. Wang, A. Xinchen and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- X. Yang, L. Tian, X. Zhao, H. Tang, Q. Liu and G. Li, Appl. Catal., B, 2019, 244, 240–249 CrossRef CAS.
- C. Li, Z. Lou, Y. Yang, Y. Wang, Y. Lu, Z. Ye and L. Zhu, Langmuir, 2019, 353, 779–786 CrossRef PubMed.
- A. I. Navarro-Aguilar, S. Obregón, D. Sanchez-Martinez and D. B. Hernández-Urestia, J. Photochem. Photobiol., A, 2019, 384, 112010 CrossRef CAS.
- G. Di, Z. Zhu, H. Zhang, J. Zhu, Y. Qiu, D. Yin and S. Küppers, J. Colloid Interface Sci., 2019, 538, 256–266 CrossRef CAS PubMed.
- J. Singh, P. Kumari and S. Basu, J. Photochem. Photobiol., A, 2019, 371, 136–143 CrossRef CAS.
- K. Prakasha, J. V. Kumar, P. Latha, P. S. Kumar and S. Karuthapandian, J. Photochem. Photobiol., A, 2019, 370, 94–104 CrossRef.
- F. Al Marzouqi, Y. Kim and R. Selvaraj, New J. Chem., 2019, 43, 9784–9792 RSC.
- P. Yu, X. Zhou, Y. Yan, Z. Li and T. Zheng, Colloids Surf., B, 2019, 179, 170–179 CrossRef CAS PubMed.
- Y. Liang, R. Shang, J. Lu, W. An, L. Hu, L. Liu and W. Cui, Int. J. Hydrogen Energy, 2019, 44, 2797–2810 CrossRef CAS.
- Y. Gong, X. Zhao, H. Zhang, B. Yanga, K. Xiao, T. Guo, J. Zhang, H. Shao, Y. Wang and G. Yu, Appl. Catal., B, 2018, 233, 35–45 CrossRef CAS.
- X. Zhang, Y. Yang, W. Huang, Y. Yang, Y. Wang, C. He, N. Liu, M. Wu and L. Tang, Mater. Res. Bull., 2018, 99, 349–358 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- Y. Bi, H. Hu, Z. Jiao, H. Yu, G. Lu and J. Ye, Phys. Chem. Chem. Phys., 2012, 14, 14486–14488 RSC.
- H. Wang, L. He, L. Wang, P. Hu, L. Guo, X. Han and J. Li, CrystEngComm, 2012, 14, 8342–8344 RSC.
- B. Wang, L. Wang, Z. Hao and Y. Luo, Catal. Commun., 2015, 58, 117–121 CrossRef CAS.
- H. Katsumata, T. Sakai, T. Suzuki and S. Kaneco, Ind. Eng. Chem. Res., 2014, 53, 8018–8025 CrossRef CAS.
- J. Li, Z. Liu and Z. Zhu, J. Alloys Compd., 2015, 636, 229–233 CrossRef CAS.
- D. Li, H. Wang, H. Tang, X. Yang and Q. Liu, ACS Sustainable Chem. Eng., 2019, 7, 8466–8474 CrossRef CAS.
- S. B. Rawal, S. D. Sung and W. Lee, Catal. Commun., 2012, 17, 131–135 CrossRef CAS.
- G. Chen, M. Sun, Q. Wei, Y. Zhang, B. Zhu and B. Du, J. Hazard. Mater., 2013, 245, 86–93 CrossRef PubMed.
- F. A. Sofi, K. Majid and O. Mehraj, J. Alloys Compd., 2018, 737, 798–808 CrossRef CAS.
- R. M. Abdelhameed, D. M. Tobaldi and M. Karmaoui, J. Photochem. Photobiol., A, 2018, 351, 50–58 CrossRef CAS.
- H. Katsumata, T. Hayashi, M. Taniguchi, T. Suzuki and S. Kaneco, Mater. Sci. Semicond. Process., 2014, 25, 68–75 CrossRef CAS.
- H. Wang, L. Zou, Y. Shan and X. Wang, Mater. Res. Bull., 2018, 97, 189–194 CrossRef CAS.
- J. Yan, Z. Song, X. Wang, Y. Xu, W. Pu, H. Xu, S. Yuan and H. Li, Appl. Surf. Sci., 2019, 466, 70–77 CrossRef CAS.
- J. W. Xu, Z. D. Gao, K. Han, Y. Liu and Y. Y. Song, ACS Appl. Mater. Interfaces, 2014, 617, 15122–15131 CrossRef PubMed.
- W. Liu, J. Shen, X. Yang, Q. Liu and H. Tang, Appl. Surf. Sci., 2018, 456, 369–378 CrossRef CAS.
- T. Kusutaki, H. Katsumata, I. Tateishi, M. Furukawa and S. Kanco, ACS Omega, 2019, 4, 15975–15984 CrossRef CAS PubMed.
- D. J. Martin, N. Umezawa, X. Chen, J. Ye and J. Tang, Energy Environ. Sci., 2013, 6, 380–3386 RSC.
- J. Wan, E. Liu, J. Fan, X. Hu, L. Sun, C. Tang, Y. Yin, H. Li and Y. Hu, Ceram. Int., 2015, 41, 6933–6940 CrossRef CAS.
- H. Katsumata, M. Taniguchi, S. Kaneco and T. Suzuki, Catal. Commun., 2013, 34, 30–34 CrossRef CAS.
- Y. Xu, F. Ge, M. Xie, S. Huang, J. Qian, H. Wang, M. He, H. Xu and H. Li, Catal. Sci. Technol., 2019, 9, 2563–2570 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S9. See DOI: 10.1039/c9ra08292a |
| This journal is © The Royal Society of Chemistry 2019 |