Open Access Article
Open Access ArticleNaYF4:Yb,Tm@TiO2 core@shell structures for optimal photocatalytic degradation of ciprofloxacin in the aquatic environment†
Yongmei Ma and
Siyue Li
and
Siyue Li *
*
Research Center for Ecohydrology, Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, Chongqing 400714, China. E-mail: syli2006@163.com; Fax: +86 23 65935000; Tel: +86 23 65935058
First published on 18th October 2019
Abstract
The removal of antibiotic residues in the aquatic environment is still a big challenge in environmental protection. Here, we developed NaYF4:Yb,Tm@TiO2 as a highly efficient photocatalyst for photocatalytic degradation of ciprofloxacin (CIP), a representative antibiotic in water under simulated solar irradiation. NaYF4:Yb,Tm@TiO2 can efficiently utilize a broad spectrum of solar energy to improve the efficiency of ciprofloxacin removal from an aquatic environment. The optimum operation conditions of photocatalyst dosage, pH value, and initial concentrations of CIP were determined by a series of contrast experiments. The dynamic process of CIP removal was monitored by UV-vis spectrophotometry, and can be well predicted by a pseudo first order model. The optimal conditions of photocatalyst dosage, initial concentration of CIP and pH value for CIP photocatalytic degradation were 1 g L−1, 10−5 M and 8, respectively. This study provides an efficient method for antibiotic removal and enables a promising strategy for other organic water pollutant treatments.
Introduction
The widespread use of antibiotics in humans, animals and aquaculture results in vast residuals in aquatic environments.1,2 The persistence of antibiotic residues may cause a series of problems in ecosystems and public health because of the poor biodegradability of antibiotics.3 Therefore, the removal of antibiotic residues in aquatic environments is of great concern in environmental protection. In this case, many methods have been developed to deal with antibiotic residues, such as advanced oxidation treatment,4 membrane treatments,5 adsorption technology6 and photoelectron-Fenton reactions.7 However, most of these methods are costly and time-consuming. Also, some technologies need professionals to operate them. Therefore, it is still a challenge to develop an environmentally friendly, simple and efficient approach to remove antibiotic residues from aquatic environments.Photocatalytic degradation technology has been considered as an effective and promising technology to remove antibiotic residues from water. Among the most photocatalysts, titanium dioxide (TiO2) has aroused the increasing concern due to the extraordinary chemical stability, high photocatalytic activity, biocompatible features, low cost, wide application and environmental friendliness.8–10 However, the photons absorption capacity of TiO2 is poor due to the broad band gap of ca. 3.2 eV, which requires ultraviolet (UV) light activation.11 UV light accounts for only 5% of sunlight,12 which limits the application of TiO2 in environment restoration. Effort therefore have been focused on extending the range of absorption wavelength of TiO2 to the visible or even NIR region for improving the photocatalytic efficiency.13–15 The TiO2 absorption wavelength rang can be extended by numerous reformative methods, such as metals and nonmetallic ions doping, cationic substitutions, anionic doping, and so on.16–18 However, most of these methods suffer from expensive requirement, complex treated process and overall catalytic capability decreasing.19,20 Therefore, it remains an urgent problem to improve the photons absorption and photocatalytic capacity of TiO2 at the same time.
Different from the traditional methods to extend the absorption of TiO2, a promising strategy is transferring visible and near infrared lights into UV lights, which can activate TiO2 particles better and improve photocatalytic efficiency. Upconversion (UC) materials have attracted much attention due to the special property of transferring NIR light into visible and UV light.21,22 It is an effective method to introduce UC materials to photocatalysts, which can improve the photocatalytic efficiency and utilization efficiency of sunlight. The hybrid structure of UC and UV-active photocatalysts had been synthesized, and used for environmental conservation.23–25 In our earlier work, we have prepared composite photocatalyst (NaYF4:Yb,Tm@TiO2/Ag) which has the good photocatalytic function for organic pollutants degradation under Xe lamp irradiation.26 However, that report did not research the optimization conditions of the composite photocatalyst to remove organic pollutants, such as the concentration of organic pollutants, photocatalyst dosage and pH value. It is essential to optimize these parameters, which would affect the organic pollutants removal in practical application. May For example, (i) the concentration of organic pollutants in water can influence their contact with photocatalysts; (ii) the light penetration can be influenced by photocatalyst dosage;27 (iii) the pH value of solution can also influence the contact between organic pollutants and photocatalyst.28,29
In this study, ciprofloxacin (CIP), a common fluoroquinolone antibiotic, was employed to explore the optimum operation conditions of NaYF4:Yb,Tm@TiO2 for antibiotic residues removal and its photocatalytic degradation mechanism. The optimized operation conditions of photocatalyst dosage, pH value, and initial concentrations of CIP were determined by a series of contrast experiments. The dynamic process of CIP removal by NaYF4:Yb,Tm@TiO2 under Xe lamp was monitored by UV-vis spectrophotometer to evaluate its photocatalytic efficiency. These results indicated that this photocatalytic platform can efficiently remove various organic pollutants in aquatic environments. The original contribution of this study was that we obtained the optimum operation conditions to effectively remove the CIP by NaYF4:Yb,Tm@TiO2 under Xe lamp and the possible mechanism of this reaction.
Experimental section
Chemicals and materials
Titanium n-butoxide (Ti(OBu)4), sodium citrate, TmCl3, NaF, YbCl3·6H2O, and YCl3·6H2O were purchased from Shanghai Reagent Co., all the chemicals were analytical reagents grade and directly used without further treatment. The solutions were prepared by distilled water and no pH regulation. All experiments were conducted under room temperature (25 °C) in water systems.Synthesis of NaYF4:Yb,Tm@TiO2
The NaYF4:Yb, Tm@TiO2 core@shell structures were prepared according to our earlier work.26Photocatalytic experiments
CIP was used to evaluate the photocatalytic activities of the NaYF4:Yb,Tm@TiO2 under the irradiation of a Xe lamp (set at 200 W). In a typical process, the NaYF4:Yb,Tm@TiO2 particles were dispersed into CIP solution in a 100 mL quartz tube under different conditions, such as pH value, NaYF4:Yb,Tm@TiO2 dosage and the concentrations of CIP. 1 M HCl or NaOH solution was used to adjust the initial pH of CIP solution during the photocatalytic degradation process. To obtain the real initial of CIP solution before the photocatalytic degradation, the solution was stirred for 2 h in the dark to reach an adsorption–desorption equilibrium between NaYF4:Yb,Tm@TiO2 and CIP solution. Subsequently, the quartz tube was exposed to irradiation from a Xe lamp, 3 mL of CIP aqueous solution was intermittently collected at given time intervals for centrifugation. The filtrate was measured by UV-vis spectroscopy.Apparatus
The scanning electron microscopy (SEM) images were recorded by a Sirion 200 field-emission scanning electron microscope. X-ray scattering patterns were taken by analyzing the powder samples on a Philips X-Pert Pro X-ray diffractometer (XRD) with Cu Kα radiation. A JEOL 2010 high resolution transmission electron microscope with X-ray energy dispersive spectroscopy capabilities was used to record transmission electron microscopy (TEM) images, and operated at an acceleration voltage of 200 kV. The UV-vis absorbance spectra of the CIP solution were measured by Lambda 35 UV-vis spectrometer (PerkinElmer, Waltham, MA, USA).Results and discussion
Hexagonal prisms of NaYF4:Yb,Tm with average side length about 400 nm were successfully synthesized (Fig. 1A–C). A straightforward method was developed to decorate TiO2 on the surface of NaYF4:Yb,Tm. Fig. 1D–F showed that all elements of Yb, Tm and Ti were distributed throughout the entire particle. More importantly, after coating TiO2, the core@shell structure of NaYF4:Yb,Tm@TiO2 was clearly observed from SEM and TEM images (Fig. 1B and C).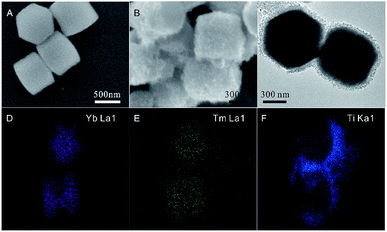 | ||
| Fig. 1 (A) SEM image of NaYF4:Yb,Tm. (B) SEM and (C) TEM images of NaYF4:Yb,Tm@TiO2. (D–F) Elemental mapping of NaYF4:Yb,Tm@TiO2 particles, including Yb La1, Tm La1 and Ti Ka1. | ||
The XRD pattern of NaYF4:Yb,Tm (Fig. 2) was consistent with the standard values of hexagonal phase NaYF4 crystals (JCPDS files no. 28-1192). The new peaks at 2θ = 25.3°, 38°, 48.1°, 55.4°, 62.9°, 68.9°, 75.2°, 83.2° in the curve of NaYF4:Yb,Tm@TiO2 were consistent with the standard values of anatase-phase TiO2 (JCPDS files no. 21-1272). This result further evidenced the successful decorating of TiO2 on the surface of NaYF4:Yb,Tm.
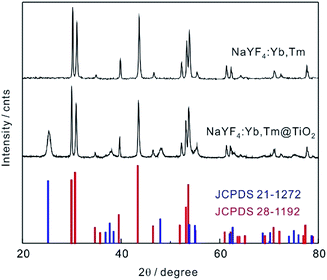 | ||
| Fig. 2 XRD patterns of NaYF4:Yb,Tm, NaYF4:Yb,Tm@TiO2 and standard XRD patterns of NaYF4 (JCPDS 28-1192) and anatase-phase TiO2 (21-1272). | ||
In our previous work, we have proved that NaYF4:Yb,Tm can convert NIR light into UV light to activate TiO2 and induce the photocatalytic activity of NaYF4:Yb,Tm@TiO2 under NIR irradiation. This compound photocatalyst can efficiently utilize different bands of the solar spectrum, which enables a promising application in photocatalytic degradation of organic pollutant in water. For example, the capacity of TiO2 for photocatalytic degradation antibiotic can be improved after introducing NaYF4:Yb,Tm under Xe lamp irradiation (Fig. S1†). In order to maximize the photocatalytic efficiency of the photocatalyst under Xe lamp irradiation, CIP was used to study the optimization conditions of the composite photocatalyst for organic pollutants removal, including pH value, NaYF4:Yb,Tm@TiO2 dosage and the concentrations of CIP.
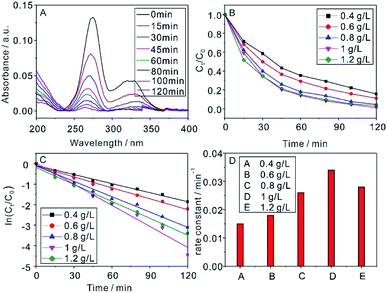
3 mL of CIP solution was taken out and centrifuged after irradiation a designated time by Xe lamp, and the supernatant was measured by UV-vis spectroscopy. The influence of NaYF4:Yb,Tm@TiO2 dosage on the efficiency of CIP removal has been shown in Fig. 3. Fig. 3A displayed the absorbance spectra of CIP (1 × 10−5 M) photocatalytic degraded by 1 g L−1 NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation as a function of the irradiation time. As expected, the absorption intensity of CIP at 274 nm decreased with irradiation time increase, indicating the photocatalytic degradation of CIP under current condition. The photocatalytic degradation activity of NaYF4:Yb,Tm@TiO2 was evaluated by comparing the filtrate concentration at each designated irradiation time to that at time zero. The photocatalytic degradation degree of CIP can be revealed by the plots of the time-dependent curves of the Ct/C0 ratio (Fig. 3B). C0 represent the initial concentration of CIP and Ct was the measured concentration of CIP at designated irradiation time t. Ct was obtained from comparing 274 nm absorbance intensity of CIP with that of standard CIP solution. After 2 h irradiation, about 75%, 89%, 96%, 99% and 97% of CIP were photocatalytic degraded under the NaYF4:Yb,Tm@TiO2 dosage of 0.4, 0.6, 0.8, 1 and 1.2 g L−1, respectively. In order to further evaluate the influence of NaYF4:Yb,Tm@TiO2 dosage on the efficiency of CIP removal, Fig. 3C showed the kinetics of the photocatalytic degradation reactions, which can be described as pseudo first order by eqn (1).26
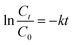 | (1) |
The rate constants (k, min−1) were evaluated from plots of ln(Ct/C0) vs. irradiation time t. The calculated rate constants of 0.4, 0.6, 0.8, 1 and 1.2 g L−1 of NaYF4:Yb,Tm@TiO2 dosage were 0.015, 0.018, 0.026, 0.034 and 0.028, respectively (Fig. 3D and Table S1†). As the increasing of photocatalyst dosage, the best reaction rate constant can be obtained when NaYF4:Yb,Tm@TiO2 dosage was 1 g L−1. After that, the reaction rate constant was decreased with increasing NaYF4:Yb,Tm@TiO2 dosage up to 1.2 g L−1. The turbidity induced by more photocatalyst dosage lead to light scattering, which can reduce the capacity of light penetration into the solution and counteract the effect of photocatalyst surface area. Both of these caused the efficiency of photocatalyst decrease for CIP removal.30 The R2 of pseudo first order kinetic model for CIP removal with NaYF4:Yb,Tm@TiO2 dosage of 0.4, 0.6, 0.8, 1 and 1.2 g L−1 were 0.989, 0.991, 0.991, 0.978 and 0.991, respectively (Table S1†). The high R2 values demonstrated the reasonable photocatalytic degradation kinetic experimental data handling based on the pseudo first order kinetic model.31
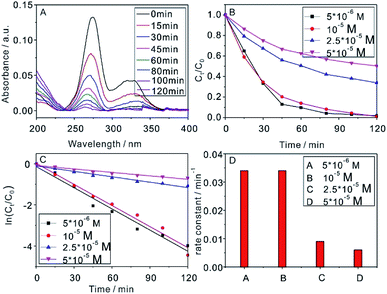
To further investigate the photocatalytic degradation capacity of the photocatalyst, different CIP concentrations were photocatalyzed with 1 g L−1 NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation (Fig. 4). Fig. 4A showed the absorbance spectra of CIP (1 × 10−5 M) photocatalyzed by 1 g L−1 NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation as a function of the reaction time. The time-dependent curves of Ct/C0 ratio was shown in Fig. 4B. After 2 h irradiation, the concentrations of 10−5 and 5 × 10−6 M CIP had been almost photocatalytic degraded. Meanwhile, only 66% and 50% CIP solution were photocatalytic degraded when the concentrations were 2.5 × 10−5 and 5 × 10−5 M, respectively. The kinetics of the photocatalytic degradation reactions also can be described as pseudo first order according to eqn (1) (Fig. 4C). The evaluated rate constants with the CIP concentrations of 5 × 10−5, 2.5 × 10−5, 10−5 and 5 × 10−6 M were 0.006, 0.009, 0.034 and 0.034, respectively (Fig. 4D and Table S2†). These results showed that the rate constant didn't increase when the concentration of CIP exceed 10−5 M under current conditions. Generally speaking, the reaction rate constant will increase with the pollutants concentration decrease. However, the capacity of the photocatalyst may not show efficiently when less pollutants reached to photocatalyst surface due to the low concentration. Thus, the choosing concentration of CIP in next experiments was 10−5 M. The R2 of pseudo first order kinetic model for CIP removal with the concentrations of 5 × 10−5, 2.5 × 10−5, 10−5 and 5 × 10−6 M were 0.969, 0.978, 0.963 and 0.937, respectively (Table S2†). The high R2 values also demonstrated the reasonable photocatalytic degradation kinetic experimental data handling based on the pseudo first order kinetic model.
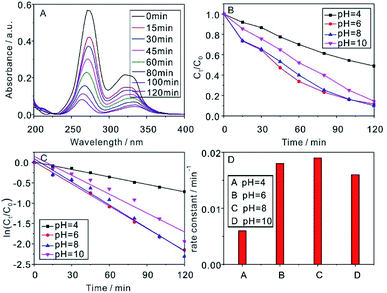
The solubility and lipophilicity of CIP are pH-dependent, because it has carboxylic acid group (pKa1 = 6.1) and an amine group in the piperazine moiety (pKa2 = 8.7).32 Therefore, the initial pH of the aqueous solution is a significant parameter in photocatalytic degradation process, which influences the activity and stability of photocatalyst and further influence the efficiency of CIP removal.33 Fig. 5 showed the actual influence of pH on the efficiency of CIP removal by NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation. Fig. 5A displayed the absorbance spectra of CIP (1 × 10−5 M) photocatalyzed by 1 g L−1 NaYF4:Yb,Tm@TiO2 at pH = 8 under Xe lamp irradiation as a function of the reaction time. Fig. 5B showed the time-dependent curves of Ct/C0 ratio and indicate the photocatalytic activity of NaYF4:Yb,Tm@TiO2. After 2 h irradiation, about 51%, 88%, 90% and 85% of CIP solution was photocatalytic degraded at pH = 4, 6, 8 and 10, respectively. According to eqn (1), the reaction rate constants were derived from the linearly fitted slope (Fig. 5C). The calculated values of rate constants at pH = 4, 6, 8 and 10 were 0.006, 0.018, 0.019 and 0.016, respectively (Fig. 5D and Table S3†). The results indicated that the biggest value of reaction rate constant was obtained at pH = 8. Under acidic conditions, the CIP molecule has positive charge because of protonization of the pyrazine ring. Under alkaline conditions, the CIP molecule exists as a monovalent anion (CIP–COO−) because of deprotonation of a carboxyl group. CIP and photocatalyst surface have the same charge both acidic and alkaline conditions, and the electrostatic repulsion force will hinder CIP molecule to the surface of photocatalyst.34,35 Therefore, in this study, when pH value is less than 8, rate constants increased with pH increase; however, when pH value is more than 8, rate constants decreased with pH increase. Under neutral conditions, the CIP molecule exists as the zwitterion, showing the lowest solubility and the highest hydrophobicity. And the hydrophobic interactions can promote more and more CIP molecule to reach the surface of photocatalyst.32 The photocatalytic degradation capacities of CIP peaked at pH = 8 in the research data. Actually, the neutral conditions were a better choice for this reaction. The R2 of pseudo first order kinetic model for CIP removal at pH = 4, 6, 8 and 10 were 0.997, 0.995, 0.981 and 0.946, respectively (Table S3†). The high R2 values also demonstrated the reasonable photocatalytic degradation kinetic experimental data handling based on the pseudo first order kinetic model.
In view of the fact that NaYF4:Yb,Tm can convert NIR light to UV light and further activate TiO2 particles, the mechanism of CIP photocatalytic degradation by NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation was elucidated and shown in Scheme 1. Under the irradiation of Xe lamp, TiO2 absorbed UV light converted by NaYF4:Yb,Tm, and excited electrons from valence band (CB) to conduction band (VB), creating electron–hole pairs.36 The electron–hole pairs will migrate to the surface of photocatalyst and take part in photocatalytic reactions. The excited electrons can react with the oxygen to form superoxide ˙O2− radical anions, and then react with H+ to form hydroxyl radicals (˙OH) at last. At the same time, holes can react with H2O to form ˙OH.37,38 The organic pollutant molecules will react with these oxidizing agents and then photocatalytic degraded by the oxidizing agents. Because of the strong oxidizing power of ˙OH, most of the organics will be oxidized finally to CO2 and H2O.
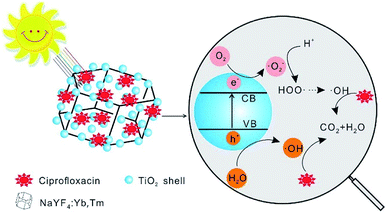 | ||
| Scheme 1 Illustrated mechanism of photocatalytic degradation of CIP on NaYF4:Yb,Tm@TiO2 under Xe lamp irradiation. | ||
Conclusions
In summary, NaYF4:Yb,Tm@TiO2 has been used as a highly efficient photocatalyst for photocatalytic degradation of ciprofloxacin in wastewater under Xe lamp irradiation. The kinetics of photocatalytic degradation reaction was monitored by UV spectroscopy, and simulated by pseudo first order model with suitable degrees of fitting. The influences of photocatalyst dosage, concentration of MG and pH values to the efficiency of CIP removal were investigated by analyzing the kinetics of photocatalytic reaction. 1 g L−1, 10−5 M and 8 were the optimal value of photocatalyst dosage, initial concentrations of CIP and pH, respectively to obtained the maximum efficiency of CIP degradation. This photocatalyst can efficiently utilize different range of solar energy, which improves its application in the field of water treatment, and enables a promising strategy to investigate the photocatalytic degradation of antibiotic.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC 21806164) and West Light Foundation of the Chinese Academy of Sciences (granted to Dr Yongmei Ma).Notes and references
- X. J. Wen, C. G. Niu, L. Zhang, C. Liang and G. M. Zeng, Appl. Catal., B, 2018, 221, 701–714 CrossRef CAS.
- P. C. Pinheiro, S. Fateixa, H. I. S. Nogueira and T. Trindade, Nanomaterials, 2019, 9, 31–49 CrossRef.
- T. S. Anirudhan, F. Shainy and J. Christa, J. Hazard. Mater., 2017, 324, 117–130 CrossRef CAS.
- C. L. Jiang, Y. F. Ji, Y. Y. Shi, J. F. Chen and T. M. Cai, Water Res., 2016, 106, 507–517 CrossRef CAS.
- V. Homem and L. Santos, J. Environ. Manage., 2011, 92, 2304–2347 CrossRef CAS.
- L. Liu, Q. F. Deng, T. Y. Ma, X. Z. Lin, X. X. Hou, Y. P. Liu and Z. Y. Yuan, J. Mater. Chem., 2011, 21, 16001–16009 RSC.
- E. Guinea, J. A. Garrido, R. M. Rodriguez, P. L. Cabot, C. Arias, F. Centellas and E. Brillas, Electrochim. Acta, 2010, 55, 2101–2115 CrossRef CAS.
- S. D. Zhao, J. R. Chen, Y. F. Liu, Y. Jiang, C. G. Jiang, Z. L. Yin, Y. G. Xiao and S. S. Cao, Chem. Eng. J., 2019, 367, 249–259 CrossRef CAS.
- S. H. Qin, W. Y. Cai, X. H. Tang and L. B. Yang, Analyst, 2014, 139, 5509–5515 RSC.
- Y. Jin, D. L. Jiang, D. Li and M. Chen, Catal.: Sci. Technol., 2017, 7, 2308–2317 RSC.
- J. Wang, G. Zhang, Z. H. Zhang, X. D. Zhang, G. Zhao, F. Y. Wen, Z. J. Pan, Y. Li, P. Zhang and P. L. Kang, Water Res., 2006, 40, 2143–2150 CrossRef CAS.
- Y. N. Tang, W. H. Di, X. S. Zhai, R. Y. Yang and W. P. Qin, ACS Catal., 2013, 3, 405–412 CrossRef CAS.
- Y. Shiraishi, H. Sakamoto, K. Fujiwara, S. Ichikawa and T. Hirai, ACS Catal., 2014, 4, 2418–2425 CrossRef CAS.
- D. Kim, B. C. Yeo, D. Shin, H. Choi, S. Kim, N. Park and S. S. Han, Phys. Rev. B, 2017, 95, 045209 CrossRef.
- L. Yang, Y. An, B. Dai, X. H. Guo, Z. Y. Liu and B. H. Peng, Korean J. Chem. Eng., 2017, 34, 476–483 CrossRef CAS.
- Z. G. Xiong and X. S. Zhao, J. Am. Chem. Soc., 2012, 134, 5754–5757 CrossRef CAS.
- A. Yu, Q. J. Wang, J. J. Wang and C. T. Chang, Catal. Commun., 2017, 90, 75–78 CrossRef CAS.
- K. L. Chow, Y. B. Man, N. F. Y. Tam, Y. Liang and M. H. Wong, J. Hazard. Mater., 2017, 322, 263–269 CrossRef CAS.
- L. Xu, E. M. P. Steinmiller and S. E. Skrabalak, J. Phys. Chem. C, 2012, 116, 871–877 CrossRef CAS.
- H. Y. Chuang and D. H. Chen, Nanotechnology, 2009, 20, 105704 CrossRef.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Y. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- A. Kumar, K. L. Reddy, S. Kumar, A. Kumar, V. Sharma and V. Krishnan, Acs Appl. Mater. Inter., 2018, 10, 15565–15581 CrossRef CAS PubMed.
- H. N. Huang, Z. Y. Wang, B. B. Huang, P. Wang, X. Y. Zhang, X. Y. Qin, Y. Dai, G. J. Zhou and M. H. Whangbo, Adv. Opt. Mater., 2018, 6, 1701331 CrossRef.
- W. Wang, M. Y. Ding, C. H. Lu, Y. R. Ni and Z. Z. Xu, Appl. Catal., B, 2014, 144, 379–385 CrossRef CAS.
- Y. M. Ma, H. L. Liu, Z. Z. Han, L. B. Yang and J. H. Liu, J. Mater. Chem. A, 2015, 3, 14642–14650 RSC.
- X. H. Huang, M. Leal and Q. L. Li, Water Res., 2008, 42, 1142–1150 CrossRef CAS.
- X. D. Wang and R. A. Caruso, J. Mater. Chem., 2011, 21, 20–28 RSC.
- A. Mills and S. LeHunte, J. Photochem. Photobiol., A, 1997, 108, 1–35 CrossRef CAS.
- J. K. Yang and S. M. Lee, Chemosphere, 2006, 63, 1677–1684 CrossRef CAS.
- B. S. Zhu, Y. Jia, Z. Jin, B. Sun, T. Luo, X. Y. Yu, L. T. Kong, X. J. Huang and J. H. Liu, Chem. Eng. J., 2015, 271, 240–251 CrossRef CAS.
- H. B. Li, D. Zhang, X. Z. Han and B. S. Xing, Chemosphere, 2014, 95, 150–155 CrossRef CAS.
- S. B. Wu, K. S. Zhang, X. L. Wang, Y. Jia, B. Sun, T. Luo, F. L. Meng, Z. Jin, D. Y. Lin, W. Shen, L. T. Kong and J. H. Liu, Chem. Eng. J., 2015, 262, 1292–1302 CrossRef CAS.
- H. B. Li, W. H. Wu, X. X. Hao, S. Wang, M. Y. You, X. Z. Han, Q. Zhao and B. S. Xing, Environ. Pollut., 2018, 243, 206–217 CrossRef CAS.
- H. Fu, X. B. Li, J. Wang, P. F. Lin, C. Chen, X. J. Zhang and I. H. Suffet, J. Environ. Sci., 2017, 56, 145–152 CrossRef.
- X. H. Li, G. Y. Chen, L. B. Yang, Z. Jin and J. H. Liu, Adv. Funct. Mater., 2010, 20, 2815–2824 CrossRef CAS.
- S. Furukawa, T. Shishido, K. Teramura and T. Tanaka, ACS Catal, 2012, 2, 175–179 CrossRef CAS.
- D. S. Muggli, J. T. McCue and J. L. Falconer, J. Catal., 1998, 173, 470–483 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: figure and tables. See DOI: 10.1039/c9ra08145c |
| This journal is © The Royal Society of Chemistry 2019 |