Open Access Article
Open Access ArticleA simple and convenient synthesis of 3-salicyloylquinoline-4-carboxylic esters from chromone and isatin†
Xuequan Wang *a,
Zhixin Yanga,
Weihang Miua,
Pingting Yea,
Mengjiao Baia,
Suyue Duana and
Xianfu Shen*b
*a,
Zhixin Yanga,
Weihang Miua,
Pingting Yea,
Mengjiao Baia,
Suyue Duana and
Xianfu Shen*b
aKey Laboratory of Natural Pharmaceutical and Chemical Biology of Yunnan Province, School of Science, Honghe University, Mengzi, Yunnan 661100, China. E-mail: huolixuanfeng@126.com
bCenter for Yunnan-Guizhou Plateau Chemical Functional Materials and Pollution Control, Qujing Normal University, 655011, P. R. China
First published on 12th November 2019
Abstract
A simple and convenient synthesis of 3-salicyloylquinoline-4-carboxylic esters has been developed through an AlCl3-catalyzed reaction of easily available Baylis–Hillman adducts from chromones and isatin-derivatives. This reaction involves esterification, cyclization and ring opening in a one-step process, and provides an efficient approach for easy access to a series of valuable salicyloylquinoline derivatives with high yields. Moreover, this protocol offers several advantages, such as availability of starting materials, economic availability, operational simplicity and mild reaction conditions.
Introduction
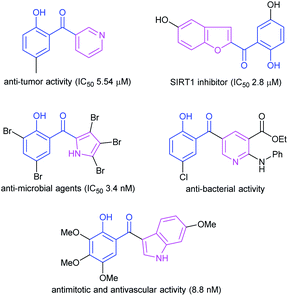
Quinoline motifs are important subunits of many drugs and natural products, and are ubiquitous in nature.1 These classes of compounds exhibit a wide range of remarkable biological and pharmacological activities such as anti-microbial,2 anti-cancer,3 anti-tubercular,4 anti-plasmodial,5 anti-malarial,6 anti-trypanosomal,7 anti-HIV,8 anti-inflammatory9 and anti-psoriasis10 activities.Groups such as salicyloyl, present in some active natural products, are associated with significant biological properties.11 Therefore, introduction of a salicyloyl group into a molecular structure has been a useful strategy in structural modifications of heterocyclic compounds, and has made brilliant achievements in the structure modification of pyridine,12 benzofuran,13 indole,14 coumarin15 and pyrrole.16 Most of these compounds display potent biological properties, including anti-tumor,17 anti-bacterial,18 photo-antioxidant,19 anti-mitotic and anti-vascular14 activities; they could also act as SIRT1 inhibitors13 (Fig. 1). Presently, several methods have been developed to assemble heterocyclic compounds with salicyloyl group.20 However, the reports about the construction of salicyloylquinoline are seldom, and the structural diversity is limited by the choice of starting materials. The approaches for salicyloylquinoline include TMSCl-mediated cyclization of 3-formylchromone with various anilines21 and aromatic nitro-group reduction of Baylis–Hillman alcohols from chromones and 2-nitrobenzaldehydes.22
In view of the significance and utility both quinoline and salicyloyl group, there is considerable interest in exploring novel synthetic approaches to various salicyloylquinoline from common, commercially available starting materials.
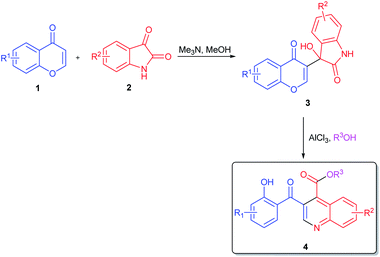
Chromones are used as building blocks for constructing various biological heterocycles. The application of chromone in the synthesis of salicyloyl-heterocycles has previously been reported.23 Interestingly, chromones can act as novel activated alkenes in the Baylis–Hillman coupling with isatin-derivatives, heteroaromatic-aldehydes and nitrobenzaldehydes. Meanwhile, the Baylis–Hillman adducts can be transformed into heterocyclic framework.24 Based on the above studies, and our ongoing interesting in constructing salicyloylquinoline, we report a simple and convenient protocol for the synthesis of 3-salicyloylquinoline-4-carboxylic esters from the Baylis–Hillman adducts of chromones and isatin-derivatives (Scheme 1).
Results and discussion
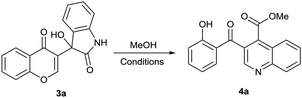
3-Salicyloylquinoline-4-carboxylic esters were synthesized as shown in Scheme 1. Chromones were coupled with isatin-derivatives through Baylis–Hillman reaction in the presence of methanolic trimethylamine (25% w/w) to generate 3-hydroxy-3-(4-oxo-4H-chromen-3-yl)indolin-2-ones 3.24 With the Baylis–Hillman adducts 3 on hand, our efforts focused on transforming from 3 to 4. To optimize the reaction conditions, we examined a variety of acid catalysts taking the reaction of 3a with methanol as a model. The results were listed in the Table 1.| a Reaction conditions: 3a (0.5 mmol) and methanol (3 mL) at reflux under air.b Isolated yield.c Not detected. | |||
|---|---|---|---|
| Entry | Catalyst (equiv.) | Time (h) | Yieldb (%) |
| 1 | TMSCl (0.5) | 1 | Trace |
| 2 | TfOH (0.5) | 1 | Trace |
| 3 | TFA (0.5) | 1 | n.d.c |
| 4 | HCl (0.5) | 1 | Trace |
| 5 | ZnCl2 (0.5) | 1 | n.d. |
| 6 | AlCl3 (0.5) | 1 | 54% |
| 7 | AlCl3 (0.7) | 1 | 71% |
| 8 | AlCl3 (1.0) | 1 | 85% |
As shown in Table 1, different types of acids, such as ZnCl2, TFA, TMSCl, TfOH and HCl were employed as catalysts, but only TMSCl, TfOH and HCl afforded trace amounts of the desired product 4a (Table 1, entry 1–5). To our delight, when AlCl3 (0.5 equiv.) was used, compound 4a was obtained in a yield of 54% (Table 1, entry 6). In order to improve the efficiency of the reaction, different amounts of AlCl3 were investigated (Table 1, entry 7 and 8), and the investigation indicated that the optimal amount of catalyst was AlCl3 with 1 equiv. in 85% yield (Table 1, entry 8).
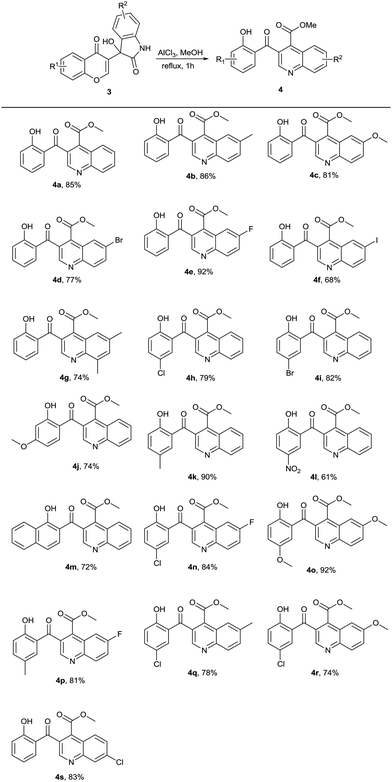
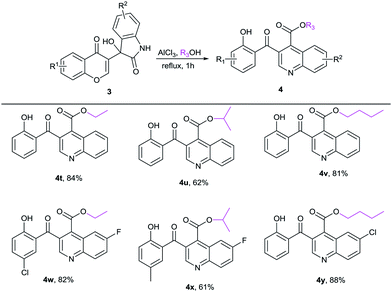
Under the above optimum reaction conditions, we tested the substrate scope for the synthesis of 3-salicyloylquinoline-4-carboxylic esters 4. Firstly, a range of 3-hydroxy-3-(4-oxo-4H-chromen-3-yl)indolin-2-ones 3 were investigated, and the representative results were listed in Table 2. Baylis–Hillman adducts 3 with various R1 or R2 group in MeOH proceeded smoothly to afford the desired products in good yields with 61–92% (4a–4s).
Moreover, the reactivity of alcohol was also tested, and the results were shown in Table 3. The structures of alcohols showed little impact on the results.
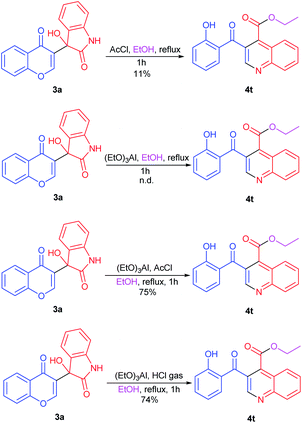
In order to probe the mechanism of this reaction, control experiments were further carried out (Scheme 2). The reactions were conducted under acetyl chloride and triethoxyaluminum respectively. The results showed that the 4t was obtained in 11% yield with 3 equiv. of HCl which generated from acetyl chloride with alcohol. And in the presence of triethoxyaluminum, the product 4t was not detected. However, when acetyl chloride (3 equiv.) and triethoxyaluminum (1 equiv.) were added simultaneously, the reaction proceeded smoothly and afforded 4t in 75% yield. Meanwhile, reaction of 3a with triethoxyaluminum in EtOH was tested in the presence of HCl gas. The reaction proceeded smoothly to afford the desired product in 74% yield. This result indicates that the formation of 4 was based on the cooperative effect of HCl and aluminum alkoxides.
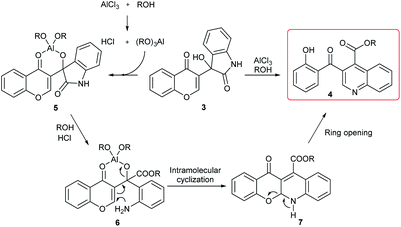
On the basis of the above observations and the previously literature reports,22,25 a plausible mechanism for the formation of 4 is presented in Scheme 3. The initial step involves the alcohol exchange and coordination of 3 with aluminum alkoxides to generate intermediate 5. Alcoholysis of intermediate 5 produces 6, which undergoes an intramolecular Michael addition and elimination to provide tetracyclic intermediate 7. Finally, ring opening by cleaving the hemiaminal in 7 gives the final product 4.
Conclusions
In conclusion, an AlCl3-catalyzed synthesis of 3-salicyloylquinoline-4-carboxylic esters from Baylis–Hillman adducts derived from chromones and isatin-derivatives has been developed. This synthetic approach provides a simple, economical, convenient and operationally process for producing biologically active salicyloylquinoline derivatives in good yields. Further research on the reaction mechanism and the application of these salicyloylquinoline derivatives are underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21602050), Applied Basic Research Project of Yunnan (2017FD154), and Yunnan Province Department of Education Fund (2016ZZX218).Notes and references
- (a) X. M. Chu, C. Wang, W. Liu, L. L. Liang, K. K. Gong, C. Y. Zhao and K. L. Sun, Eur. J. Med. Chem., 2019, 161, 101 CrossRef CAS; (b) O. Afzal, S. Kumar, M. R. Haider, M. R Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2015, 97, 871 CrossRef CAS PubMed.
- (a) G. G. Ladani and M. P. Patel, New J. Chem., 2015, 39, 9848 RSC; (b) P. Sridhar, M. Alagumuthu, S. Arumugam and S. R. Reddy, RSC Adv., 2016, 6, 64460 RSC; (c) J. Y. Zhang, S. Wang, Y. Y. Ba and Z. Xu, Eur. J. Med. Chem., 2019, 174, 1 CrossRef CAS.
- (a) W. B. Kuang, R. Z. Huang, Y. L. Fang, G. B. Liang, C. H. Yang, X. L. Ma and Y. Zhang, RSC Adv., 2018, 8, 24376 RSC; (b) T. Su, J. C. Zhu, R. Q. Sun, H. H. Zhang, Q. H. Huang, X. D. Zhang, R. L. Du, L. Q. Qiu and R. H. Cao, Eur. J. Med. Chem., 2019, 178, 154 CrossRef CAS; (c) W. T Gao, Z. Y. Li, Q. Q. Xu and Y. Li, RSC Adv., 2018, 8, 38844 RSC.
- (a) M. Akula, P. Yogeeswari, D. Sriram, M. Jhac and A. Bhattacharya, RSC Adv., 2016, 6, 46073 RSC; (b) B. Tanwar, A. Kumar, P. Yogeeswari, D. Sriram and A. K. Chakraborti, Bioorg. Med. Chem. Lett., 2016, 26, 5960 CrossRef CAS.
- Y. Q. Hu, C. Gao, S. Zhang, L. Xu, Z. Xu, L. S. Feng, X. Wu and F. Zhao, Eur. J. Med. Chem., 2017, 139, 22 CrossRef CAS PubMed.
- (a) S. Vandekerckhove and M. D'hooghe, Bioorg. Med. Chem., 2015, 23, 5098 CrossRef CAS; (b) K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245 CrossRef CAS PubMed.
- (a) H. Zhang, J. Collins, R. Nyamwihura, S. Ware, M. Kaiser and I. V. Ogungbe, Bioorg. Med. Chem. Lett., 2018, 28, 1647 CrossRef CAS; (b) S. N. Chanquiaa, F. Larreguia, V. Puenteb, C. Labriolac, E. Lombardob and G. G. Liñaresa, Bioorg. Chem., 2019, 83, 526 CrossRef.
- (a) K. C. Sekgota, S. Majumder, M. Isaacs, D. Mnkandhla, H. C. Hoppe, S. D. Khanye, F. H. Kriel, J. Coates and P. T. Kaye, Bioorg. Chem., 2017, 75, 310 CrossRef CAS; (b) P. Shah, D. Naik, N. Jariwala, D. Bhadane, S. Kumar, S. Kulkarni, K. K. Bhutani and I. P. Singh, Bioorg. Chem., 2018, 80, 591 CrossRef CAS.
- J. Liu, C. J. Li, L. Ni, J. Z. Yang, L. Li, C. X. Zang, X. Q. Bao, D. Zhang and D. M. Zhang, RSC Adv., 2015, 5, 80553 RSC.
- W. J. Zhang, P. H. Lia, M. C. Zhao, Y. H. Gua, C. Z. Dong, H. X. Chen and Z. Y. Du, Bioorg. Chem., 2019, 88, 102899 CrossRef CAS.
- (a) Y. H. Jo, B. Shin, Q. Liu, K. Y. Lee, D. C. Oh, B. Y. Hwang and M. K. Lee, J. Nat. Prod., 2014, 77, 2361 CrossRef CAS; (b) K. Yamanaka, K. S. Ryan, T. A. M. Gulder, C. C. Hughes and B. S. Moore, J. Am. Chem. Soc., 2012, 134, 12434 CrossRef CAS; (c) X. N. Shi, Y. He, X. Y. Zhang and X. S. Fan, Org. Chem. Front., 2017, 4, 1967 RSC.
- (a) S. M. B. Maezono, T. N. Poudel, L. K. Xia and Y. R. Lee, RSC Adv., 2016, 6, 82321 RSC; (b) E. Raj Baral, K. Sharma, M. S. Akhtar and Y. R. Lee, Org. Biomol. Chem., 2016, 14, 10285 RSC.
- J. H. Wu, Y. Li, K. X. Chen, H. L. Jiang, M. H. Xu and D. L. Liu, Eur. J. Med. Chem., 2013, 60, 441 CrossRef CAS.
- H. Y. Lee, C. Y. Chang, M. J. Lai, H. Y. Chuang, C. C. Kuo, C. Y. Chang, J. Y. Chang and J. P. Liou, Bioorg. Med. Chem. Lett., 2015, 23, 4230 CrossRef CAS.
- D. Belkhir-Talbi, M. Makhloufi-Chebli, S. Terrachet-Bouaziz, D. Hikem-Oukacha, N. Ghemmit, L. Ismaili, A. M. S. Silva and M. Hamdi, J. Mol. Struct., 2019, 1179, 495 CrossRef CAS.
- X. Y. Qi, H. Y. Xiang, Y. H. Yang and C. H. Yang, RSC Adv., 2015, 5, 98549 RSC.
- A. Sood, V. Sharma, A. Chaudhry, R. Kumar, S. Arora, Rajnikant, V. Gupta and M. P. S. Ishar, Bioorg. Med. Chem. Lett., 2014, 24, 4724 CrossRef CAS.
- (a) D. Schillaci, S. Petruso and V. Sciortino, Int. J. Antimicrob. Agents, 2005, 25, 338 CrossRef CAS; (b) A. V. Gadakh, C. Pandit, S. S. Rindhe and B. K. Karale, Bioorg. Med. Chem. Lett., 2010, 20, 5572 CrossRef CAS; (c) M. V. Raimondi, R. Listro, M. G. Cusimano, M. L. Franca, T. Faddetta, G. Gallo, D. Schillaci, S. Collina, A. Leonchiks and G. Barone, Bioorg. Med. Chem., 2019, 27, 721 CrossRef CAS.
- Y. Dobashi, J. I. Kondou and Y. Ohkatsu, Polym. Degrad. Stab., 2005, 89, 140 CrossRef CAS.
- (a) M. L. N. Rao and B. S. Ramakrishna, Eur. J. Org. Chem., 2017, 2017, 5080 CrossRef CAS; (b) A. S. Plaskon, S. V. Ryabukhin, D. M. Volochnyuk, A. N. Shivanyuk and A. A. Tolmachev, Tetrahedron, 2008, 64, 5933 CrossRef CAS; (c) V. O. Iaroshenko, S. Mkrtchyan, G. Ghazaryan, A. Hakobyan, A. Maalik, L. Supe, A. Villinger, A. Tolmachev, D. Ostrovskyi, V. Y. Sosnovskikh, T. V. Ghochikyan and P. Langer, Synthesis, 2011, 469 CrossRef CAS.
- (a) A. S. Plaskon, S. V. Ryabukhin, D. M. Volochnyuk, K. S. Gavrilenko, A. N. Shivanyuk and A. A. Tolmachev, J. Org. Chem., 2008, 73, 6010 CrossRef CAS; (b) S. V. Ryabukhin, A. S. Plaskon, D. M. Volochnyuk and A. A. Tolmachev, Synthesis, 2007, 1861 CAS.
- D. Basavaiah, R. J. Reddy and J. S. Rao, Tetrahedron Lett., 2006, 47, 73 CrossRef CAS.
- (a) K. K. Dong and Q. Huang, Tetrahedron Lett., 2019, 60, 1871 CrossRef CAS; (b) T. Z. Dai, Q. Y. Li, X. F. Zhang and C. H. Yang, J. Org. Chem., 2019, 84, 5913 CrossRef CAS.
- (a) S. Z. Luo, X. L. Mi, H. Xu, P. G. Wang and J. P. Cheng, J. Org. Chem., 2004, 69, 8413 CrossRef CAS PubMed; (b) D. Basavaiah and A. J. Rao, Tetrahedron Lett., 2003, 44, 4365 CrossRef CAS.
- P. Zhou, B. Hu, S. Y. Zhao, Q. H. Zhang, Y. Q. Wang, X. Li and F. C. Yu, Tetrahedron Lett., 2018, 59, 3116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures. 1H NMR and 13C NMR spectra for synthesized compounds or other electronic format. See DOI: 10.1039/c9ra08124k |
| This journal is © The Royal Society of Chemistry 2019 |