Open Access Article
Open Access ArticleAllylation of isatin-derived N-Boc-hydrazones followed by Pd-catalyzed carboamination reaction: an entry to 3-spiro-pyrazolidyl-oxindoles†
Stefano Gazzotti ,
Marco Manenti,
Leonardo Lo Presti
,
Marco Manenti,
Leonardo Lo Presti and
Alessandra Silvani
and
Alessandra Silvani *
*
Dipartimento di Chimica, Universitá di Milano, Via Golgi 19, Milano, 20133, Italy. E-mail: alessandra.silvani@unimi.it
First published on 20th November 2019
Abstract
The indium-mediated allylation of novel 3-(2-Boc-hydrazono)indolin-2-one derivatives, followed by a palladium-catalysed carboamination reaction, is described to afford unprecedented spirocyclic oxindoles in good yields. The method provides an efficient access to both cis and trans diastereoisomers of highly functionalized compounds, bearing an N-Boc, 5-substituted pyrazolidine ring at the C3-oxindole spiro junction. The versatility of the method is fully demonstrated starting from a series of substituted isatins and employing a variety of aryl halides in the key cyclization step.
Introduction
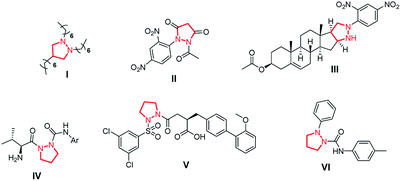
The pyrazolidine five-membered ring, characterized by the presence of two adjacent nitrogen atoms, can be considered as a cyclic hydrazine moiety. Among natural products containing a nitrogen–nitrogen bond,1 such a heterocycle is displayed by the highly unusual alkaloid garceine (I),2 isolated from Lotus garcinii in 2001, but not investigated for biological properties because of insufficient material (Fig. 1).Owing to their particular stability and, at the same time, peculiar electronic properties, pyrazolidines and their derivatives are potentially capable of providing improved physicochemical properties in their interaction with biological systems.3 Indeed, this heterocyclic ring is present as a partial structure in a number of synthetic compounds, displaying a wide range of bioactivities, such as, for example, antibacterial (II),4 anticancer (III)5 and anticonvulsant (IV).6
In addition, pyrazolidine-amino acid derivatives, acting as azaproline analogues, have been shown to have application as peptidomimetics, displaying inhibitory activities against enzymes (serine peptidase dipeptidyl IV inhibitor, V)7 and receptors (VLA-4 antagonist, VI).8
Despite a recurrent interest in pyrazolidine 3,5-diones9–11 and N,N-disubstituted derivatives,12 other kinds of pyrazolidine-based frameworks have received minor attention, and their application in the context of drug discovery has yet to be fully explored. Over the last decade the synthetic effort towards highly functionalized pyrazolidine derivatives has greatly increased, leading to the development of efficient protocols, such as cycloadditions of hydrazones and olefins,13 1,3-dipolar cycloadditions of azomethine imines,14 carboamination reactions,15 and amination reactions of allenes.16
As part of our interest in the synthesis of 3,3-disubstituted oxindole derivatives and related spirocompounds,17 we looked into the biological significance of the pyrazolidine ring, conceiving its combination with the relevant oxindole nucleus, by means of a spiro arrangement of the two ring systems. The conjugation of privileged heterocycles into spiro structures is a challenging application of the molecular hybridization concept,18 a viable and effective approach envisioning the rational design of new functional compounds through the structural fusion of two pharmacophoric subunits into one chemical entity. Since spiro compounds have an intrinsic three-dimensionality, they are able to access unexplored chemical space, often displaying improved biological interactions and being more likely to be successfully developed as drugs.19
At the best of our knowledge, only a recent work describes spirocyclic pyrazolidines, achieved by means of a gold-catalyzed three-component spirocyclization, starting from alkynols, hydrazines and carbonyl components, mainly aldehydes.20
No methods have been reported for the preparation of pyrazolidines derived from cyclic ketones and, of course, of oxindoles bearing an N-jointed pyrazolidine ring at the key C3 position.
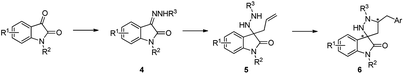
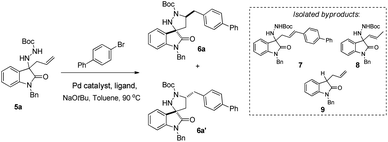
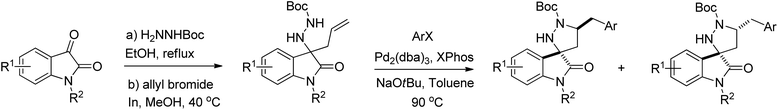
Relying on our previous experience with isatin-derived ketimines and 3-amino substituted oxindoles,17 we envisioned 3-allyl-3-hydrazinylindolin-2-ones 5, obtainable from 3-hydrazonoindolin-2-ones 4, as suitable substrates for a palladium-catalysed carboamination reaction, aimed to the synthesis of the unprecedented 1′,5′-disubstituted spiro[indoline-3,3′-pyrazolidin]-2-one scaffold 6 (Scheme 1).
Herein, we report the synthesis of a large family of highly functionalized 1′-Boc, 5′-arylmethyl spiro[indoline-3,3′-pyrazolidin]-2-ones, attainable as separable 3′-5′-cis and 3′-5′-trans diastereoisomers, thus demonstrating for the first time the suitability of the Pd-catalysed carboamination reaction of hydrazine derivatives for the synthesis of spiro compounds.
Results and discussion
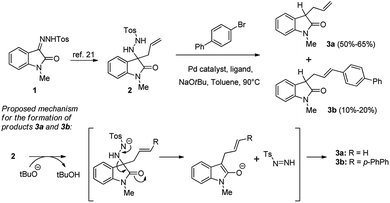
We started our investigation by taking into consideration the already known isatin-derived N-tosyl hydrazone 1 and the corresponding imino allylation product 2,21 besides 4-bromo-1,1′-biphenyl as aryl halide. We tested various reaction conditions for the Pd-catalysed carboamination step. However, treatment of compound 2 with different Pd catalysts (Pd(OAc)2, Pd2(dba)3) in presence of various ligands (dppe, dpe-phos, BINAP) afforded high amounts of compounds 3a and 3b, instead of the expected spiro compound (Scheme 2).Likely, N-tosyl hydrazine 2 is not stable in the basic reaction conditions employed and, due to the presence of NaOtBu, it easily decomposes, ejecting the tosyl anion and forming a diazonium anion. Loss of molecular nitrogen results in protonation of the substrate, affording the already known compound 3a.22 The formation of moderate amounts of compound 3b can be explained as the result of a standard Heck reaction between 3a and the 4-bromo-1,1′-biphenyl.
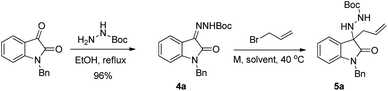
Thinking about more appropriate hydrazone N-substituents, we turned our attention to reaction of N-benzyl isatin with tert-butyl carbazate. After refluxing for two hours in ethanol, the unprecedented N-Boc hydrazone 4a was obtained in nearly quantitative yield (Table 1, reaction). For the subsequent allylation reaction of 4a, we initially explored the cheap tin powder, in combination with allyl bromide in a simple Barbier-type one-pot procedure, which avoids the use of the toxic allylic tri-butyltin reagent.23 The reaction proved to be unsuccessful in MeOH (Table 1, entry 1), while it afforded the desired N-Boc allyl hydrazine derivative 5a when the solvent was changed to THF (entry 2).
| Entry | M (powder) | Solvent | T (h) | Yieldb (%) |
|---|---|---|---|---|
| a Compound 4a was prepared starting from N-benzyl isatin (2 mmol), tert-butyl carbazate (2 mmol), in ethanol (0.3 M), at reflux for two hours. All allylation reactions were conducted with 4a (0.25 mmol), allyl bromide (0.5 mmol) and metal powder (0.5 mmol).b Isolated yields. | ||||
| 1 | Sn | MeOH | 48 | <5 |
| 2 | Sn | THF | 48 | 70 |
| 3 | In | THF/NH4Cl sat 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | <5 |
| 4 | In | MeOH/NH4Cl sat 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 | 40 |
| 5 | In | MeOH | 3 | 94 |
Aiming at improving the yield and, at the same time, at experimenting an environmentally benign chemical process, we then looked at the indium-promoted allylation in aqueous media.21 Unlike most Barbier-type allylation methods, indium-mediated reactions are insensitive to moisture, relying on facile and practical reaction conditions coupled with minimal side reactions. Treatment of a THF/NH4Cl (aq. saturated) solution of hydrazone 4a with allyl bromide in the presence of indium afforded compound 5a only in traces (entry 3). The yield improved when the reaction was conducted in a 3/1 MeOH/NH4Cl (aq. saturated) solution (entry 4) and it was ultimately satisfying when only MeOH was adopted as a solvent (entry 5). An excess of allyl bromide and indium powder proved to be necessary for an almost quantitative yield, probably due to progressive metal inactivation.
Once a multi-gram scale procedure for the preparation of the hydrazine substrate 5a was achieved, we moved our attention on the key Pd-catalysed carboamination reaction. Among all possible variables that can be considered for the screening of the reaction conditions, we chose to focus preliminary on Pd complexes and ligand additives. We investigated the effect of Pd(0)- and Pd(II)-based complexes and of several phosphines, at various degrees of basicity and steric encumbrance. After some preliminary screening on solvent and temperature, all reactions were carried out in toluene, at 90 °C, using NaOtBu as the base and 4-bromo-1,1′-biphenyl as reference aryl halide (Table 2).
| Entry | Pd cat | Lb | T (h) | Target spiro compounds | Isolated byproducts | |||
|---|---|---|---|---|---|---|---|---|
| Yieldc (6a + 6a′%) | drd (6a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a′) 6a′) |
7 (%) | 8 (%) | 9 (%) | ||||
| a Reactions were carried out on a 0.25 mmol scale, with 1.0 equiv. of 5a, 1.3 equiv. of 4-bromo-1,1′-biphenyl, 1.3 equiv. of NaOtBu, 4 mol% of Pd catalyst, 4 mol% of ligand, toluene (0.20 M).b Dpe-phos = bis[(2-diphenylphosphino)phenyl]ether; BINAP= (±)-2,2′-bis(diphenylphosphino)-1,1′-binaphthalene; TFP = tris(2-furyl)phosphine; TCP = triscyclohexylphospine; TTP = tris(o-tolyl)phosphine; X-phos = 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl.c Sum of the isolated yields for each diastereoisomer.d Determined by 1H NMR of the crude reaction product. | ||||||||
| 1 | Pd(OAc)2 | dpe-phos | 12 | 0 | — | 32 | 53 | |
| 2 | Pd(OAc)2 | TCP | 12 | 45 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
49 | ||
| 3 | Pd(OAc)2 | TTP | 3 | 87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||
| 4 | Pd(OAc)2 | TFP | 3 | 87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
13 | ||
| 5 | PdCl2(MeCN)2 | TFP | 24 | 79 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
14 | ||
| 6 | PdCl2(MeCN)2 | BINAP | 24 | 44 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 | 3 | |
| 7 | PdCl2(MeCN)2 | PPh3 | 24 | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
21 | ||
| 8 | PdCl2(PPh3)2 | — | 24 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29 | ||
| 9 | Pd(PPh3)4 | — | 24 | 69 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
19 | ||
| 10 | Pd2(dba)3 | TTP | 3 | 96 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | ||
| 11 | Pd2(dba)3 | X-phos | 3 | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||
We started our investigation from Pd(OAc)2 catalyst, coupled with various phosphine-based ligands. Reaction employing dpe-phos failed to give the desired spiro products, affording only compound 7 from the competing Heck arylation, and compound 8, from the Pd-promoted isomerization of the substrate 5a double bond (entry 1). Switching to triscyclohexylphospine (TCP), we were pleased to observe the formation of diastereoisomeric 6a and 6a′ target compounds (dr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), together with a considerable amount of byproduct 9,24 deriving from decomposition of the starting N-Boc-hydrazine (entry 2). A significant yield improvement and reduction of reaction times was achieved exploiting tris(o-tolyl)phosphine (TTP) or tris(2-furyl)phosphine (TFP), but without any increase of diastereoisomeric ratios (entries 3 and 4). Switching to PdCl2(MeCN)2, PdCl2(PPh3)2 or Pd(PPh3)4 complexes did not lead to any contribution, in terms of better yield or dr (entries 5–9). On the other hand, Pd2(dba)3 proved to be the catalyst of choice, both when paired with TTP or with X-phos, affording the desired 3-spiro-pyrazolidyl-oxindoles 6a and 6a′ in excellent yield, albeit in negligible dr (entries 10–11). The known propensity of the Pd2(dba)3 complex to reduce the rate of β-hydrogen elimination in “PdII-σ-alkyl” complexes,25 can likely explain the ability of this catalyst to drive the reaction towards the intramolecular key carboamination, rather than towards the competing standard intermolecular Heck reaction.
2), together with a considerable amount of byproduct 9,24 deriving from decomposition of the starting N-Boc-hydrazine (entry 2). A significant yield improvement and reduction of reaction times was achieved exploiting tris(o-tolyl)phosphine (TTP) or tris(2-furyl)phosphine (TFP), but without any increase of diastereoisomeric ratios (entries 3 and 4). Switching to PdCl2(MeCN)2, PdCl2(PPh3)2 or Pd(PPh3)4 complexes did not lead to any contribution, in terms of better yield or dr (entries 5–9). On the other hand, Pd2(dba)3 proved to be the catalyst of choice, both when paired with TTP or with X-phos, affording the desired 3-spiro-pyrazolidyl-oxindoles 6a and 6a′ in excellent yield, albeit in negligible dr (entries 10–11). The known propensity of the Pd2(dba)3 complex to reduce the rate of β-hydrogen elimination in “PdII-σ-alkyl” complexes,25 can likely explain the ability of this catalyst to drive the reaction towards the intramolecular key carboamination, rather than towards the competing standard intermolecular Heck reaction.
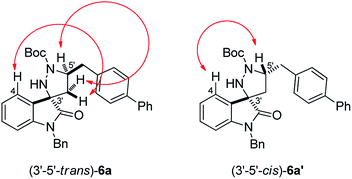
Diastereoisomeric 3-spiro-pyrazolidyl-oxindoles 6a and 6a′ proved to be easily separable by flash chromatography, allowing a full mono- and bidimensional NMR characterization, including determination of the relative stereochemistry. In particular, NOESY experiments allowed assigning the 3′-5′-trans configuration to diastereoisomer 6a and the 3′-5′-cis configuration to diastereoisomer 6a′. Diagnostic NOE interactions between oxindole H-4 and selected pyrazolidine protons were identified for both diastereoisomers (Fig. 2).
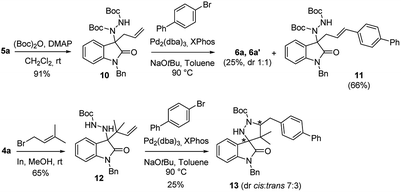
In order to evaluate possible improvements in dr, we made some focused modifications to the N-Boc hydrazine substrate. Aiming at exploring the effects of more steric constraints in the transition states, we selected N,N2-Boc hydrazine 10 and 2,2-dimethylbut-3-en-1-yl hydrazine 12, easily prepared from compounds 5a and 4a, respectively (Scheme 3).
From the Pd-catalysed reaction on substrate 10, the mono-Boc spiro derivatives 6a and 6a′ could be recovered in the usual dr, together with a substantial amount of the Heck product 11. Reaction of 12 afforded the desired 4-dimethyl ethyl spiropyrazolidine 13, in modest yield and only slightly higher dr.
With the best conditions in hand, we proceeded with the investigation of the reaction scope, varying R1 and R2 substituents in the starting isatin and ArX in the carboamination step (Table 3).
| Entry | 5 (R1, R2) | Yield (5%)b | ArX | T (h) | 6 | Yield (6%)c | dr (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′)d 6′)d |
|---|---|---|---|---|---|---|---|
| a Compounds 5 were prepared on a 0.3 mmol scale, starting from the proper R1,R2-substituted isatin (1.0 equiv.), tert-butyl carbazate (1 equiv.), in ethanol (0.3 M), at reflux for two hours, to give the corresponding N-Boc hydrazone intermediates 4. Allylation reactions were conducted on 4 (1 equiv.), allyl bromide (2 equiv.) and metal powder (2 equiv.), in methanol (0.1 M), at 40 °C for 3 hours. Carboamination reactions were carried out on a 0.2 mmol scale, with 1.0 equiv. of 5, 1.3 equiv. of aryl halide, 1.3 equiv. of NaOtBu, 4 mol% of Pd2(dba)3, 4 mol% of XPhos, toluene (0.2 M).b Isolated yields.c Sum of the isolated yields for each diastereoisomer.d Determined by 1H NMR of the crude reaction product.e No reaction. | |||||||
| 1 | 5a (H, Bn) | 94 | p-Br-biphenyl | 3 | 6a, 6a′ | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 5a (H, Bn) | 94 | p-Br-anisole | 6 | 6b, 6b′ | 98 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 5a (H, Bn) | 94 | p-Br-nitrobenzene | 12 | 6c, 6c′ | 53 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 5a (H, Bn) | 94 | p-Br-benzophenone | 4 | 6d, 6d′ | 92 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 5a (H, Bn) | 94 | p-Br-acetophenone | 3 | 6e, 6e′ | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 5a (H, Bn) | 94 | o-Br-toluene | 10 | 6f, 6f′ | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
| 7 | 5a (H, Bn) | 94 | 2-Br-1,3,5-trimethylbenzene | 5 | 6g, 6g′ | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
| 8 | 5a (H, Bn) | 94 | p-I-toluene | 6 | 6h, 6h′ | 63 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 5b (H, Me) | 82 | p-Br-biphenyl | 12 | 6i, 6i′ | 53 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 5c (H, 3,4-diCl-Ph(CH)2CH2) | 93 | p-Br-biphenyl | 12 | 6j, 6j′ | 69 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 5d (5-Me, Bn) | 98 | p-Br-biphenyl | 12 | 6k, 6k′ | 50 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 5e (5-Br, Bn) | 82 | p-Br-biphenyl | 12 | 6l, 6l′ | 34 | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 5f (6-Cl, Bn) | 25 | p-Br-biphenyl | 12 | 6m, 6m′ | 72 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 5g (7-CF3, Bn) | 75 | p-Br-biphenyl | 12 | 6n, 6n′ | 47 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 5h (5-NO2, Bn) | 71 | p-Br-biphenyl | 24 | 6o, 6o′ | nre | |
| 16 | 5i (5-OMe, Bn) | 15 | |||||
| 17 | 5j (4-Cl, Bn) | nre | |||||
In reactions with 5a, most of aryl halides are tolerated in the Pd-catalysed step (entries 1–8). The target spiro compounds are generally obtained in high yields, in the presence of either electron-withdrawing, -donating or bulky substituents on the aromatic Ar-X ring. On the other hand, the electronic characteristics and positions of the substituents at the isatin starting compounds affected more heavily the outcome of the synthetic process. To this regard, the allylation step appeared to be troublesome in two cases (entries 13, 16), affording the intermediate hydrazines derivatives 5f and 5i in modest yields, likely for electronic reasons. Starting from N-benzyl, 4-Cl-isatin (entry 17), the corresponding hydrazine derivative 5j could not be achieved, probably because of the steric hindrance at the oxindole C3-position, due to the presence of the C4-substituent. Finally, the presence of a 5-NO2 substituent on the substrate 5h (entry 15) proved to be incompatible with a successful Pd-catalysed reaction, probably due to the instability of the nitro group in basic conditions.
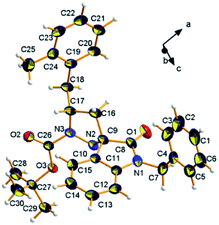
In order to further confirm the relative stereochemistry of the two diastereoisomeric series of products 6, as determined by NMR, a single crystal of the diastereoisomer 6f′ was subjected to single-crystal X-ray diffraction analysis. The experiment unambiguously assigns the 3′S*,5′R* (cis) relative configuration, highlighting at the same time the preferred solid-state conformation (Fig. 3. For a full discussion of the crystallographic results, see the ESI†).
Conclusion
We developed an efficient strategy for the synthesis of a novel class of oxindole-based spiro compounds, bearing the unusual pyrazolidine ring at the C3 stereogenic center. The reactivity of 3-hydrazonoindolin-2-ones under various allylation conditions was explored, developing a robust protocol for the multi-gram conversion of variously substituted isatins into the corresponding N-Boc allyl hydrazine derivatives. Through a screening of the carboamination reaction conditions, it was possible to react effectively such intermediates with a variety of aryl halides, affording the cyclized spiro compounds in satisfactory yields. Both cis and trans diastereoisomers, with respect to the pyrazolidine ring, were obtained. Further work is currently underway, aimed at establishing spirooxindole-fused pyrazolidines as possible lead compounds for drug discovery programs.Experimental section
General procedures
All commercial materials (Aldrich, Fluorochem) were used without further purification. All solvents were of reagent grade or HPLC grade. Reactions requiring anhydrous conditions were performed under nitrogen atmosphere. All reactions were monitored by thin layer chromatography (TLC) on precoated silica gel Merck 60 F254; spots were visualized with UV light (254 nm) or by treatment with KMnO4 solution in water or ninhydrin solution in ethanol. Products were purified by flash chromatography (FC) on silica gel 60 (230–400 mesh). Yields refer to isolated compounds estimated to be >95% pure as determined by 1H NMR. NMR spectra were recorded on Bruker 300 or Advance 400 spectrometers, using TMS as an internal standard. 13C NMR spectra have been recorded using the APT pulse sequence. Chemical shifts are reported in parts per million relative to the residual solvent, coupling constants (J) are given in Hz. Multiplicities in 1H NMR are reported as follows: s = singlet, d = doublet, t = triplet, m = multiplet, br s = broad singlet. High-resolution MS spectra (HRMS) were recorded with a Thermo Fisher LCQ Fleet ion trap mass spectrometer, equipped with an ESI source.Typical procedure (TP-A) for the synthesis of N-Boc hydrazone derivatives (4a–4j)
A mixture of R1,R2-substituted isatin (0.3 mmol) and tert-butyl carbazate (0.3 mmol) were suspended in ethanol (0.3 M) and the reaction was stirred at reflux for 2 hours. The reaction was cooled down to room temperature, and then the solid was filtered and washed with cold ethanol. The pure product was obtained without need of purification.Typical procedure (TP-B) for the synthesis of N-Boc hydrazine derivatives (5a–5j)
To a suspension of N-Boc hydrazone derivative (0.3 mmol) in methanol (0.1 M), indium (0.6 mmol) and allyl bromide (0.6 mmol) were added. After stirring at 40 °C for three hours, saturated aqueous NH4Cl solution was added and the mixture was extracted twice with ethyl acetate. The combined organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford the crude product, which was purified by FC as indicated below.Typical procedure (TP-C) for the synthesis of 3-spiro-Pyrazolidyl-Oxindoles derivatives (6a,6a′–6n,6n′)
The N-Boc hydrazine derivative (0.2 mmol), the aryl halide (0.26 mmol), sodium tert-butoxide (0.26 mmol), Pd2(dba)3 (4 mol%) and XPhos (4 mol%) were suspended in anhydrous toluene (0.2 M) and the reaction was stirred at 90 °C. After the completion of reaction (monitored by TLC), saturated aqueous NH4Cl solution was added and the reaction was extracted twice with ethyl acetate. The combined organic layer was then dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford the crude product. This was purified by FC (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, as described below), affording the desired product, as separated (3′-5′-trans) and (3′-5′-cis) diastereoisomers (except for 6l, 6l′).
ethyl acetate, as described below), affording the desired product, as separated (3′-5′-trans) and (3′-5′-cis) diastereoisomers (except for 6l, 6l′).
Analytical data of products (4a–4j, 5a–5i, 6a,6a′–6n,6n′, 3b, 7–13)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z
1 Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E mixture) δ 12.27 (m, br, 0.6H), 8.79 (m, br, 0.4H), 7.71 (d, br, J = 7.8 Hz, 0.6H), 7.56 (d, br, J = 7.8 Hz, 0.4H), 7.42 (t, J = 7.8 Hz, 0.4H), 7.35 (t, J = 7.8 Hz, 0.6H), 7.10 (t, J = 7.8 Hz, 1H), 6.90 (d, J = 7.8 Hz, 0.4H), 6.85 (d, J = 7.8 Hz, 0.6H), 3.25 (s, 1.8H), 3.24 (s, 1.2H), 1.57 (s, 3.6H), 1.55 (s, 5.4H). 13C NMR (100 MHz, CDCl3, 1.5
E mixture) δ 12.27 (m, br, 0.6H), 8.79 (m, br, 0.4H), 7.71 (d, br, J = 7.8 Hz, 0.6H), 7.56 (d, br, J = 7.8 Hz, 0.4H), 7.42 (t, J = 7.8 Hz, 0.4H), 7.35 (t, J = 7.8 Hz, 0.6H), 7.10 (t, J = 7.8 Hz, 1H), 6.90 (d, J = 7.8 Hz, 0.4H), 6.85 (d, J = 7.8 Hz, 0.6H), 3.25 (s, 1.8H), 3.24 (s, 1.2H), 1.57 (s, 3.6H), 1.55 (s, 5.4H). 13C NMR (100 MHz, CDCl3, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z
1 Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E mixture) δ 160.8 and 160.7 (1C), 155.6 and 155.2 (1C), 152.4, 145.6 and 135.1 (1C), 136.0 and 116.2 (1C), 133.0 and 131.3 (1C), 124.6 and 123.8 (1C), 123.3 and 121.8 (1C), 110.0 and 109.4 (1C), 84.2 and 83.8 (1C), 29.0 and 28.8 (3C), 26.9. HRMS (ESI) m/z: 276.1349 [M + H]+; calcd for C14H18N3O3+, 276.1343.
E mixture) δ 160.8 and 160.7 (1C), 155.6 and 155.2 (1C), 152.4, 145.6 and 135.1 (1C), 136.0 and 116.2 (1C), 133.0 and 131.3 (1C), 124.6 and 123.8 (1C), 123.3 and 121.8 (1C), 110.0 and 109.4 (1C), 84.2 and 83.8 (1C), 29.0 and 28.8 (3C), 26.9. HRMS (ESI) m/z: 276.1349 [M + H]+; calcd for C14H18N3O3+, 276.1343.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 tautomer mixture) δ 12.29 (m, br, 0.7H), 8.05 (s, br, 0.3H), 7.57 (s, br, 0.7H), 7.37–7.23 (m, 3.7H), 7.16–7.00 (m, 2H), 6.84 (d, br, J = 7.8 Hz, 0.3H), 6.65 (d, J = 7.8 Hz, 0.7H), 6.50 (d, J = 7.8 Hz, 0.3H), 4.91 (s, 1.4H), 4.70 (s, br, 0.6H), 2.40–1.89 (m, br, 0.3H), 2.29 (s, 3H), 1.55 (s, 6.3H), 1.51 (s, 2.7H). 13C NMR (100 MHz, CDCl3) δ 168.4 and 161.6 (1C), 168.3 and 154.9 (1C), 152.4 and 58.2 (1C), 139.9 and 137.9 (1C), 136.7 and 135.2 (1C), 133.1 and 131.9 (1C), 131.9–121.9 (7C), 120.0 and 118.2 (1C), 109.4 and 107.9 (1C), 82.6 and 78.6 (1C), 43.4 and 43.1 (1C), 28.6 and 28.2 (3C), 21.1 and 21.0 (1C). HRMS (ESI) m/z: 366.1804 [M + H]+; calcd for C21H24N3O3+, 366.1812.
3 tautomer mixture) δ 12.29 (m, br, 0.7H), 8.05 (s, br, 0.3H), 7.57 (s, br, 0.7H), 7.37–7.23 (m, 3.7H), 7.16–7.00 (m, 2H), 6.84 (d, br, J = 7.8 Hz, 0.3H), 6.65 (d, J = 7.8 Hz, 0.7H), 6.50 (d, J = 7.8 Hz, 0.3H), 4.91 (s, 1.4H), 4.70 (s, br, 0.6H), 2.40–1.89 (m, br, 0.3H), 2.29 (s, 3H), 1.55 (s, 6.3H), 1.51 (s, 2.7H). 13C NMR (100 MHz, CDCl3) δ 168.4 and 161.6 (1C), 168.3 and 154.9 (1C), 152.4 and 58.2 (1C), 139.9 and 137.9 (1C), 136.7 and 135.2 (1C), 133.1 and 131.9 (1C), 131.9–121.9 (7C), 120.0 and 118.2 (1C), 109.4 and 107.9 (1C), 82.6 and 78.6 (1C), 43.4 and 43.1 (1C), 28.6 and 28.2 (3C), 21.1 and 21.0 (1C). HRMS (ESI) m/z: 366.1804 [M + H]+; calcd for C21H24N3O3+, 366.1812.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate from 7
ethyl acetate from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Yellow foam (94% yield). 1H NMR (400 MHz, CDCl3) δ 7.46 (d, br, J = 7.2 Hz, 1H), 7.34–7.22 (m, 5H), 7.17 (t, br, J = 7.5 Hz, 1H), 7.04 (t, br, J = 7.5 Hz, 1H), 6.85 (d, J = 7.8 Hz, 1H), 6.08 (m, br, 1H), 5.52 (m, 1H), 5.09 (d, br, J = 17.0 Hz, 1H), 5.00 (d, br, J = 10.2 Hz, 1H), 4.94 (d, br, J = 16.0 Hz, 1H), 4.87 (d, br, J = 16.0 Hz, 1H), 4.81–4.47 (m, br, 1H), 2.73 (dd, br, J = 13.3 and 6.2 Hz, 1H), 2.64 (dd, br, J = 13.3 and 8.5 Hz, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.0, 143.2, 135.6, 130.7, 129.2, 128.7 (2C), 127.8, 127.6, 127.2 (2C), 125.2, 122.6, 120.1, 109.1, 80.5, 68.6, 43.8, 39.6, 28.1 (3C). HRMS (ESI) m/z: 416.1938 [M + Na]+; calcd for C23H27N3NaO3+, 416.1945.
7). Yellow foam (94% yield). 1H NMR (400 MHz, CDCl3) δ 7.46 (d, br, J = 7.2 Hz, 1H), 7.34–7.22 (m, 5H), 7.17 (t, br, J = 7.5 Hz, 1H), 7.04 (t, br, J = 7.5 Hz, 1H), 6.85 (d, J = 7.8 Hz, 1H), 6.08 (m, br, 1H), 5.52 (m, 1H), 5.09 (d, br, J = 17.0 Hz, 1H), 5.00 (d, br, J = 10.2 Hz, 1H), 4.94 (d, br, J = 16.0 Hz, 1H), 4.87 (d, br, J = 16.0 Hz, 1H), 4.81–4.47 (m, br, 1H), 2.73 (dd, br, J = 13.3 and 6.2 Hz, 1H), 2.64 (dd, br, J = 13.3 and 8.5 Hz, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.0, 143.2, 135.6, 130.7, 129.2, 128.7 (2C), 127.8, 127.6, 127.2 (2C), 125.2, 122.6, 120.1, 109.1, 80.5, 68.6, 43.8, 39.6, 28.1 (3C). HRMS (ESI) m/z: 416.1938 [M + Na]+; calcd for C23H27N3NaO3+, 416.1945.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (82% yield). 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 7.8 Hz, 1H), 7.29 (t, J = 7.8 Hz, 1H), 7.07 (t, J = 7.8 Hz, 1H), 6.80 (d, J = 7.8 Hz, 1H), 5.98 (m, br, 1H), 5.48 (m, 1H), 5.08–4.94 (m, 2H), 3.18 (s, 3H), 3.18–2.77 (m, br, 1H), 2.67 (dd, J = 15.6 and 6.8 Hz, 1H), 2.55 (dd, J = 15.6 and 8.8 Hz, 1H), 1.31 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.1, 143.9, 130.6, 129.2, 127.7, 125.2, 122.5, 119.8, 107.9, 80.3, 68.6, 39.5, 28.1 (3C), 26.1. HRMS (ESI) m/z: 340.1636 [M + Na]+; calcd for C17H23N3NaO3+, 340.1632.
3). Yellow foam (82% yield). 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 7.8 Hz, 1H), 7.29 (t, J = 7.8 Hz, 1H), 7.07 (t, J = 7.8 Hz, 1H), 6.80 (d, J = 7.8 Hz, 1H), 5.98 (m, br, 1H), 5.48 (m, 1H), 5.08–4.94 (m, 2H), 3.18 (s, 3H), 3.18–2.77 (m, br, 1H), 2.67 (dd, J = 15.6 and 6.8 Hz, 1H), 2.55 (dd, J = 15.6 and 8.8 Hz, 1H), 1.31 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.1, 143.9, 130.6, 129.2, 127.7, 125.2, 122.5, 119.8, 107.9, 80.3, 68.6, 39.5, 28.1 (3C), 26.1. HRMS (ESI) m/z: 340.1636 [M + Na]+; calcd for C17H23N3NaO3+, 340.1632.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 1
ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Dark yellow foam (93% yield). 1H NMR (300 MHz, CDCl3) δ 7.47 (d, br, J = 7.8 Hz, 1H), 7.42–7.19 (m, 3H), 7.17–7.03 (m, 2H), 6.79 (d, br, J = 7.8 Hz, 1H), 6.41 (d, br, J = 15.6 Hz, 1H), 6.16 (dt, br, J = 15.6 and 4.9 Hz, 1H), 5.98 (m, br, 1H), 5.50 (m, br, 1H), 5.07 (d, J = 17.6 Hz, 1H), 5.01 (d, J = 10.8 Hz, 1H), 5.46 (d, br, J = 4.9 Hz, 2H), 2.81–2.50 (m, br, 1H), 2.71 (dd, J = 13.7 and 6.8 Hz, 1H), 2.60 (dd, J = 13.7 and 8.8 Hz, 1H), 1.30 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.0, 156.2, 143.0, 136.4, 132.7, 131.5, 130.7, 130.4, 129.9, 129.3, 128.2, 127.8, 125.6, 125.4, 124.9, 122.7, 120.1, 108.7, 80.5, 68.6, 41.5, 39.7, 28.1 (3C). HRMS (ESI) m/z: 510.1028 [M + Na]+; calcd for C25H27Cl2N3NaO3+, 510.1322.
1). Dark yellow foam (93% yield). 1H NMR (300 MHz, CDCl3) δ 7.47 (d, br, J = 7.8 Hz, 1H), 7.42–7.19 (m, 3H), 7.17–7.03 (m, 2H), 6.79 (d, br, J = 7.8 Hz, 1H), 6.41 (d, br, J = 15.6 Hz, 1H), 6.16 (dt, br, J = 15.6 and 4.9 Hz, 1H), 5.98 (m, br, 1H), 5.50 (m, br, 1H), 5.07 (d, J = 17.6 Hz, 1H), 5.01 (d, J = 10.8 Hz, 1H), 5.46 (d, br, J = 4.9 Hz, 2H), 2.81–2.50 (m, br, 1H), 2.71 (dd, J = 13.7 and 6.8 Hz, 1H), 2.60 (dd, J = 13.7 and 8.8 Hz, 1H), 1.30 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.0, 156.2, 143.0, 136.4, 132.7, 131.5, 130.7, 130.4, 129.9, 129.3, 128.2, 127.8, 125.6, 125.4, 124.9, 122.7, 120.1, 108.7, 80.5, 68.6, 41.5, 39.7, 28.1 (3C). HRMS (ESI) m/z: 510.1028 [M + Na]+; calcd for C25H27Cl2N3NaO3+, 510.1322.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (98% yield). 1H NMR (300 MHz, CDCl3) δ 7.33–7.20 (m, 6H), 6.95 (d, br, J = 7.8 Hz, 1H), 6.53 (d, br, J = 7.8 Hz, 1H), 5.98 (m, br, 1H), 5.50 (m, 1H), 5.08 (d, br, J = 17.6 Hz, 1H), 4.99 (d, br, J = 10.7 Hz, 1H), 4.92 (d, br, J = 15.6 Hz, 1H), 4.81 (d, br, J = 15.6 Hz, 1H), 3.72 (m, br, 1H), 2.71 (dd, J = 12.7 and 5.8 Hz, 1H), 2.62 (dd, J = 12.7 and 8.8 Hz, 1H), 2.29 (s, 3H), 1.33 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.3, 156.0, 140.8, 135.7, 132.0, 130.8, 129.4, 128.7 (2C), 127.8, 127.5, 127.2 (2C), 125.9, 120.0, 108.9, 80.5, 68.6, 43.8, 39.6, 28.1 (3C), 21.1. HRMS (ESI) m/z: 430.2096 [M + Na]+; calcd for C24H29N3NaO3+, 430.2101.
3). Yellow foam (98% yield). 1H NMR (300 MHz, CDCl3) δ 7.33–7.20 (m, 6H), 6.95 (d, br, J = 7.8 Hz, 1H), 6.53 (d, br, J = 7.8 Hz, 1H), 5.98 (m, br, 1H), 5.50 (m, 1H), 5.08 (d, br, J = 17.6 Hz, 1H), 4.99 (d, br, J = 10.7 Hz, 1H), 4.92 (d, br, J = 15.6 Hz, 1H), 4.81 (d, br, J = 15.6 Hz, 1H), 3.72 (m, br, 1H), 2.71 (dd, J = 12.7 and 5.8 Hz, 1H), 2.62 (dd, J = 12.7 and 8.8 Hz, 1H), 2.29 (s, 3H), 1.33 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.3, 156.0, 140.8, 135.7, 132.0, 130.8, 129.4, 128.7 (2C), 127.8, 127.5, 127.2 (2C), 125.9, 120.0, 108.9, 80.5, 68.6, 43.8, 39.6, 28.1 (3C), 21.1. HRMS (ESI) m/z: 430.2096 [M + Na]+; calcd for C24H29N3NaO3+, 430.2101.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (82% yield). 1H NMR (300 MHz, CDCl3) δ 7.57 (d, J = 1.8 Hz, 1H), 7.35–7.17 (m, 6H), 6.51 (d, J = 7.8 Hz, 1H), 5.91 (m, br, 1H), 5.50 (m, 1H), 5.10 (d, br, J = 16.6 Hz, 1H), 5.03 (d, br, J = 9.8 Hz, 1H), 4.91(d, br, J = 15.0 Hz, 1H), 4.83 (d, br, J = 15.0 Hz, 1H), 3.04–2.71 (m, br, 1H), 2.69 (dd, J = 13.7 and 6.8 Hz, 1H), 2.61 (dd, J = 13.7 and 8.8 Hz, 1H), 1.34 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.8, 156.2, 142.3, 135.1, 131.9, 130.2, 130.1, 128.8 (2C), 128.5, 127.7, 127.1 (2C), 120.5, 115.3, 110.6, 80.6, 69,1, 43.9, 39.6, 28.1 (3C). HRMS (ESI) m/z: 494.1055 [M + Na]+; calcd for C23H26BrN3NaO3+, 494.1050.
3). Yellow foam (82% yield). 1H NMR (300 MHz, CDCl3) δ 7.57 (d, J = 1.8 Hz, 1H), 7.35–7.17 (m, 6H), 6.51 (d, J = 7.8 Hz, 1H), 5.91 (m, br, 1H), 5.50 (m, 1H), 5.10 (d, br, J = 16.6 Hz, 1H), 5.03 (d, br, J = 9.8 Hz, 1H), 4.91(d, br, J = 15.0 Hz, 1H), 4.83 (d, br, J = 15.0 Hz, 1H), 3.04–2.71 (m, br, 1H), 2.69 (dd, J = 13.7 and 6.8 Hz, 1H), 2.61 (dd, J = 13.7 and 8.8 Hz, 1H), 1.34 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.8, 156.2, 142.3, 135.1, 131.9, 130.2, 130.1, 128.8 (2C), 128.5, 127.7, 127.1 (2C), 120.5, 115.3, 110.6, 80.6, 69,1, 43.9, 39.6, 28.1 (3C). HRMS (ESI) m/z: 494.1055 [M + Na]+; calcd for C23H26BrN3NaO3+, 494.1050.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate from 8
ethyl acetate from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). Dark yellow foam (25% yield). 1H NMR (300 MHz, CDCl3) δ 7.42–7.20 (m, 6H), 7.01 (d, br, J = 7.8 Hz, 1H), 6.33 (m, 1H), 5.92 (m, br, 1H), 5.50 (m, 1H), 5.07 (d, br, J = 16.6 Hz, 1H), 5.01 (d, br, J = 9.8 Hz, 1H), 4.89 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 3.35–2.84 (m, br, 1H), 2.69 (dd, J = 13.7 and 6.8 Hz, 1H), 2.60 (dd, J = 13.7 and 8.8 Hz, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.1, 144.4, 135.1, 134.9, 130.3, 128.9 (2C), 127.8, 127.1 (2C), 126.3, 126.2, 122.5, 120.5, 109.7, 80.6, 68.5, 43.9, 39.6, 28.1 (3C). HRMS (ESI) m/z: 450.1560 [M + Na]+; calcd for C23H26ClN3NaO3+, 450.1555.
6). Dark yellow foam (25% yield). 1H NMR (300 MHz, CDCl3) δ 7.42–7.20 (m, 6H), 7.01 (d, br, J = 7.8 Hz, 1H), 6.33 (m, 1H), 5.92 (m, br, 1H), 5.50 (m, 1H), 5.07 (d, br, J = 16.6 Hz, 1H), 5.01 (d, br, J = 9.8 Hz, 1H), 4.89 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 3.35–2.84 (m, br, 1H), 2.69 (dd, J = 13.7 and 6.8 Hz, 1H), 2.60 (dd, J = 13.7 and 8.8 Hz, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.3, 156.1, 144.4, 135.1, 134.9, 130.3, 128.9 (2C), 127.8, 127.1 (2C), 126.3, 126.2, 122.5, 120.5, 109.7, 80.6, 68.5, 43.9, 39.6, 28.1 (3C). HRMS (ESI) m/z: 450.1560 [M + Na]+; calcd for C23H26ClN3NaO3+, 450.1555.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (75% yield). 1H NMR (300 MHz, CDCl3) δ 7.74 (d, br, J = 6.9 Hz, 1H), 7.56 (d, br, J = 7.9 Hz, 1H), 7.33–7.07 (m, 6H), 5.91 (m, br, 1H), 5.48 (m, 1H), 5.20 (d, J = 16.6 Hz, 1H), 5.13 (d, J = 16.6 Hz, 1H), 5.06 (d, br, J = 17.6 Hz, 1H), 5.05 (d, br, J = 10.8 Hz, 1H), 3.34 (m, br, 1H), 2.74–2.56 (m, 2H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 178.8, 156.0, 141.5, 136.3, 130.9, 129.8, 129.1, 128.3 (2C), 127.5 (q, J = 5.9 Hz), 126.9, 125.8 (2C), 123.3 (q, J = 271.3 Hz), 122.2, 120.8, 112.8 (q, J = 33.9 Hz), 80.7, 67.0, 45.7, 40.0, 28.1 (3C). HRMS (ESI) m/z: 484.1824 [M + Na]+; calcd for C24H26F3N3NaO3+, 484.1818.
3). Yellow foam (75% yield). 1H NMR (300 MHz, CDCl3) δ 7.74 (d, br, J = 6.9 Hz, 1H), 7.56 (d, br, J = 7.9 Hz, 1H), 7.33–7.07 (m, 6H), 5.91 (m, br, 1H), 5.48 (m, 1H), 5.20 (d, J = 16.6 Hz, 1H), 5.13 (d, J = 16.6 Hz, 1H), 5.06 (d, br, J = 17.6 Hz, 1H), 5.05 (d, br, J = 10.8 Hz, 1H), 3.34 (m, br, 1H), 2.74–2.56 (m, 2H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 178.8, 156.0, 141.5, 136.3, 130.9, 129.8, 129.1, 128.3 (2C), 127.5 (q, J = 5.9 Hz), 126.9, 125.8 (2C), 123.3 (q, J = 271.3 Hz), 122.2, 120.8, 112.8 (q, J = 33.9 Hz), 80.7, 67.0, 45.7, 40.0, 28.1 (3C). HRMS (ESI) m/z: 484.1824 [M + Na]+; calcd for C24H26F3N3NaO3+, 484.1818.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (71% yield). 1H NMR (300 MHz, CDCl3) δ 8.32 (m, br, 1H), 8.13 (d, br, J = 8.7 Hz, 1H), 7.36–7.17 (m, 5H), 6.71 (d, J = 8.7 Hz, 1H), 6.00 (m, 1H), 5.49 (m, 1H), 5.15–5.00 (m, 2H), 4.97 (d, J = 15.6 Hz, 1H), 4.91 (d, J = 15.6 Hz, 1H), 3.54–2.87 (m, br, 1H), 2.75 (dd, J = 13.0 and 5.8 Hz, 1H), 2.56 (dd, J = 13.0 and 7.8 Hz, 1H), 1.31 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.4, 156.4, 148.9, 143.4, 134.5, 129.6, 129.1, 129.0 (2C), 127.2 (2C), 126.2, 121.2, 121.0, 120.0, 108.8, 81.3, 68.7, 44.2, 39.6, 28.0 (3C). HRMS (ESI) m/z: 461.1791 [M + Na]+; calcd for C23H26N4NaO5+, 461.1795.
3). Yellow foam (71% yield). 1H NMR (300 MHz, CDCl3) δ 8.32 (m, br, 1H), 8.13 (d, br, J = 8.7 Hz, 1H), 7.36–7.17 (m, 5H), 6.71 (d, J = 8.7 Hz, 1H), 6.00 (m, 1H), 5.49 (m, 1H), 5.15–5.00 (m, 2H), 4.97 (d, J = 15.6 Hz, 1H), 4.91 (d, J = 15.6 Hz, 1H), 3.54–2.87 (m, br, 1H), 2.75 (dd, J = 13.0 and 5.8 Hz, 1H), 2.56 (dd, J = 13.0 and 7.8 Hz, 1H), 1.31 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.4, 156.4, 148.9, 143.4, 134.5, 129.6, 129.1, 129.0 (2C), 127.2 (2C), 126.2, 121.2, 121.0, 120.0, 108.8, 81.3, 68.7, 44.2, 39.6, 28.0 (3C). HRMS (ESI) m/z: 461.1791 [M + Na]+; calcd for C23H26N4NaO5+, 461.1795.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (15% yield). 1H NMR (300 MHz, CDCl3) δ 7.34–7.20 (m, 5H), 7.09 (d, br, J = 2.9 Hz, 1H), 6.69 (dd, J = 8.7 and 2.9 Hz, 1H), 6.52 (d, J = 8.7 Hz, 1H), 6.00 (m, br, 1H), 5.51 (m, 1H), 5.08 (d, br, J = 17.6 Hz, 1H), 5.00 (d, br, J = 11.7 Hz, 1H), 4.91 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 3.75 (s, 3H), 2.71 (dd, J = 12.7 and 5.8 Hz, 1H), 2.62 (dd, J = 12.7 and 7.8 Hz, 1H), 2.52 (m, br, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.8, 156.7 (2C), 137.1, 136.3, 131.3, 129.7, 129.4 (2C), 128.2, 127.9 (2C), 120.8, 114.8 (br), 112.8 (br), 110.2, 81.1, 69.6, 56.5, 44.5, 40.3, 28.8 (3C). HRMS (ESI) m/z: 446.2056 [M + Na]+; calcd for C24H29N3NaO4+, 446.2050.
3). Yellow foam (15% yield). 1H NMR (300 MHz, CDCl3) δ 7.34–7.20 (m, 5H), 7.09 (d, br, J = 2.9 Hz, 1H), 6.69 (dd, J = 8.7 and 2.9 Hz, 1H), 6.52 (d, J = 8.7 Hz, 1H), 6.00 (m, br, 1H), 5.51 (m, 1H), 5.08 (d, br, J = 17.6 Hz, 1H), 5.00 (d, br, J = 11.7 Hz, 1H), 4.91 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 3.75 (s, 3H), 2.71 (dd, J = 12.7 and 5.8 Hz, 1H), 2.62 (dd, J = 12.7 and 7.8 Hz, 1H), 2.52 (m, br, 1H), 1.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.8, 156.7 (2C), 137.1, 136.3, 131.3, 129.7, 129.4 (2C), 128.2, 127.9 (2C), 120.8, 114.8 (br), 112.8 (br), 110.2, 81.1, 69.6, 56.5, 44.5, 40.3, 28.8 (3C). HRMS (ESI) m/z: 446.2056 [M + Na]+; calcd for C24H29N3NaO4+, 446.2050.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (98% yield, separated diastereoisomers, 1
3). Yellow foam (98% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6a. 1H NMR (400 MHz, CDCl3) δ 7.65–7.57 (m, 4H), 7.48 (t, br, J = 7.7 Hz, 2H), 7.44–7.22 (m, 9H), 7.16 (t, br, J = 7.8 Hz, 1H), 6.93 (t, br, J = 7.8 Hz, 1H), 6.85 (m, br, 1H), 6.65 (d, br, J = 7.8 Hz, 1H), 5.07–4.71 (m, br, 3H), 3.23 (dd, J = 13.2 and 3.8 Hz, 1H), 3.15 (dd, J = 13.2 and 7.0 Hz, 1H), 2.60 (dd, J = 12.8 and 8.2 Hz, 1H), 2.25 (dd, J = 12.8 and 7.9 Hz, 1H), 1.56 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.2, 156.7 (br), 144.4, 141.5, 140.4, 137.5, 136.3, 131.1 (2C), 130.6, 129.5 (2C), 129.4 (2C), 128.3, 128.0 (3C), 127.9 (2C), 127.7 (2C), 126.2, 123.4, 123.2, 110.0, 81.4, 67.3, 61.0, 44.0 (2C), 41.1, 29.1 (3C). HRMS (ESI) m/z: 568.2566 [M + Na]+; calcd for C35H35N3NaO5+, 568.2571. Diastereoisomer 6a′. 1H NMR (400 MHz, CDCl3) δ 7.62–7.53 (m, 4H), 7.45 (t, br, J = 7.7 Hz, 2H), 7.41–7.24 (m, 9H), 7.17 (t, br, J = 7.8 Hz, 1H), 7.05 (d, br, J = 7.8 Hz, 1H), 7.00 (t, br, J = 7.8 Hz, 1H), 6.74 (d, br, J = 7.8 Hz, 1H), 5.01 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 4.75 (m, br, 1H), 3.46 (dd, J = 13.2 and 4.4 Hz, 1H), 2.96 (dd, J = 13.2 and 9.0 Hz, 1H), 2.44 (dd, J = 12.3 and 8.5 Hz, 1H), 2.35 (dd, J = 12.3 and 7.3 Hz, 1H), 1.54 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 156.4 (br), 142.5, 141.6, 140.3, 137.7, 135.8, 132.3, 130.5 (2C), 129.6 (2C), 129.4 (2C), 128.5, 128.0 (4C), 127.9 (2C), 127.7 (2C), 124.0, 123.4, 110.4, 81.8, 68.7, 61.8, 45.4, 44.9, 41.6, 29.1 (3C). HRMS (ESI) m/z: 568.2576 [M + Na]+; calcd for C35H35N3NaO5+, 568.2571.
1 dr). Diastereoisomer 6a. 1H NMR (400 MHz, CDCl3) δ 7.65–7.57 (m, 4H), 7.48 (t, br, J = 7.7 Hz, 2H), 7.44–7.22 (m, 9H), 7.16 (t, br, J = 7.8 Hz, 1H), 6.93 (t, br, J = 7.8 Hz, 1H), 6.85 (m, br, 1H), 6.65 (d, br, J = 7.8 Hz, 1H), 5.07–4.71 (m, br, 3H), 3.23 (dd, J = 13.2 and 3.8 Hz, 1H), 3.15 (dd, J = 13.2 and 7.0 Hz, 1H), 2.60 (dd, J = 12.8 and 8.2 Hz, 1H), 2.25 (dd, J = 12.8 and 7.9 Hz, 1H), 1.56 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.2, 156.7 (br), 144.4, 141.5, 140.4, 137.5, 136.3, 131.1 (2C), 130.6, 129.5 (2C), 129.4 (2C), 128.3, 128.0 (3C), 127.9 (2C), 127.7 (2C), 126.2, 123.4, 123.2, 110.0, 81.4, 67.3, 61.0, 44.0 (2C), 41.1, 29.1 (3C). HRMS (ESI) m/z: 568.2566 [M + Na]+; calcd for C35H35N3NaO5+, 568.2571. Diastereoisomer 6a′. 1H NMR (400 MHz, CDCl3) δ 7.62–7.53 (m, 4H), 7.45 (t, br, J = 7.7 Hz, 2H), 7.41–7.24 (m, 9H), 7.17 (t, br, J = 7.8 Hz, 1H), 7.05 (d, br, J = 7.8 Hz, 1H), 7.00 (t, br, J = 7.8 Hz, 1H), 6.74 (d, br, J = 7.8 Hz, 1H), 5.01 (d, J = 15.6 Hz, 1H), 4.82 (d, J = 15.6 Hz, 1H), 4.75 (m, br, 1H), 3.46 (dd, J = 13.2 and 4.4 Hz, 1H), 2.96 (dd, J = 13.2 and 9.0 Hz, 1H), 2.44 (dd, J = 12.3 and 8.5 Hz, 1H), 2.35 (dd, J = 12.3 and 7.3 Hz, 1H), 1.54 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 156.4 (br), 142.5, 141.6, 140.3, 137.7, 135.8, 132.3, 130.5 (2C), 129.6 (2C), 129.4 (2C), 128.5, 128.0 (4C), 127.9 (2C), 127.7 (2C), 124.0, 123.4, 110.4, 81.8, 68.7, 61.8, 45.4, 44.9, 41.6, 29.1 (3C). HRMS (ESI) m/z: 568.2576 [M + Na]+; calcd for C35H35N3NaO5+, 568.2571.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (98% yield, separated diastereoisomers, 1
3). Yellow foam (98% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6b. 1H NMR (300 MHz, CDCl3) δ 7.33–7.18 (m, 7H), 7.14 (d, br, J = 7.8 Hz, 1H), 6.92 (t, br, J = 7.8 Hz, 1H), 6.89 (d, br, J = 8.8 Hz, 2H), 6.74 (d, br, J = 7.8 Hz, 1H), 6.62 (d, J = 7.8 Hz, 1H), 5.00–4.97 (m, br, 3H), 4.11 (m, br, 1H), 3.81 (s, 3H), 3.11–2.97 (m, 2H), 2.51 (dd, J = 12.7 and 7.8 Hz, 1H), 2.13 (dd, J = 12.7 and 7.8 Hz, 1H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.2, 159.2, 157.4 (br), 144.3, 136.3 (2C), 131.6 (2C), 130.7, 130.3, 129.5 (3C), 128.3, 127.9 (2C), 123.5, 114.7 (2C), 110.0, 81.7, 68.3, 61.1, 56.0, 44.1 (2C), 40.4, 29.1 (3C). HRMS (ESI) m/z: 522.2368 [M + Na]+; calcd for C30H33N3NaO4+, 522.2363. Diastereoisomer 6b′. 1H NMR (300 MHz, CDCl3) δ 7.41–7.09 (m, 9H), 7.03–6.92 (m, 2H), 6.84 (d, br, J = 7.7 Hz, 1H), 6.70 (d, br, J = 7.8 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.77 (d, J = 15.6 Hz, 1H), 4.71–4.57 (m, br, 2H), 3.77 (s, 3H), 3.30 (dd, J = 13.7 and 4.9 Hz, 1H), 2.84 (dd, J = 13.7 and 9.8 Hz, 1H), 2.35 (dd, J = 12.7 and 8.8 Hz, 1H), 2.24 (dd, J = 12.7 and 7.8 Hz, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 159.1, 152.9 (br), 142.5, 135.8, 132.3, 131.0 (2C), 130.5, 129.5 (3C), 128.4, 127.9 (2C), 124.0, 123.4, 114.7 (2C), 110.3, 81.6, 68.6, 61.8, 55.9, 45.1, 44.9, 40.8, 29.1 (3C). HRMS (ESI) m/z: 522.2367 [M + Na]+; calcd for C30H33N3NaO4+, 522.2363.
1 dr). Diastereoisomer 6b. 1H NMR (300 MHz, CDCl3) δ 7.33–7.18 (m, 7H), 7.14 (d, br, J = 7.8 Hz, 1H), 6.92 (t, br, J = 7.8 Hz, 1H), 6.89 (d, br, J = 8.8 Hz, 2H), 6.74 (d, br, J = 7.8 Hz, 1H), 6.62 (d, J = 7.8 Hz, 1H), 5.00–4.97 (m, br, 3H), 4.11 (m, br, 1H), 3.81 (s, 3H), 3.11–2.97 (m, 2H), 2.51 (dd, J = 12.7 and 7.8 Hz, 1H), 2.13 (dd, J = 12.7 and 7.8 Hz, 1H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.2, 159.2, 157.4 (br), 144.3, 136.3 (2C), 131.6 (2C), 130.7, 130.3, 129.5 (3C), 128.3, 127.9 (2C), 123.5, 114.7 (2C), 110.0, 81.7, 68.3, 61.1, 56.0, 44.1 (2C), 40.4, 29.1 (3C). HRMS (ESI) m/z: 522.2368 [M + Na]+; calcd for C30H33N3NaO4+, 522.2363. Diastereoisomer 6b′. 1H NMR (300 MHz, CDCl3) δ 7.41–7.09 (m, 9H), 7.03–6.92 (m, 2H), 6.84 (d, br, J = 7.7 Hz, 1H), 6.70 (d, br, J = 7.8 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.77 (d, J = 15.6 Hz, 1H), 4.71–4.57 (m, br, 2H), 3.77 (s, 3H), 3.30 (dd, J = 13.7 and 4.9 Hz, 1H), 2.84 (dd, J = 13.7 and 9.8 Hz, 1H), 2.35 (dd, J = 12.7 and 8.8 Hz, 1H), 2.24 (dd, J = 12.7 and 7.8 Hz, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 159.1, 152.9 (br), 142.5, 135.8, 132.3, 131.0 (2C), 130.5, 129.5 (3C), 128.4, 127.9 (2C), 124.0, 123.4, 114.7 (2C), 110.3, 81.6, 68.6, 61.8, 55.9, 45.1, 44.9, 40.8, 29.1 (3C). HRMS (ESI) m/z: 522.2367 [M + Na]+; calcd for C30H33N3NaO4+, 522.2363.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (53% yield, separated diastereoisomers, 1.5
3). Yellow foam (53% yield, separated diastereoisomers, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).Diastereoisomer 6c. 1H NMR (300 MHz, CDCl3) δ 8.18 (d, br, J = 8.8 Hz, 2H), 7.47 (d, br, J = 8.8 Hz, 2H), 7.33–7.09 (m, 7H), 7.06–6.94 (m, 2H), 6.66 (d, br, J = 7.7 Hz, 1H), 4.93 (m, br, 1H), 4.84 (d, br, J = 15.5 Hz, 1H), 4.76 (d, br, J = 15.5 Hz, 1H), 3.35 (dd, J = 13.2 and 5.8 Hz, 1H), 3.09 (dd, J = 13.2 and 7.8 Hz, 1H), 2.54 (dd, J = 12.7 and 7.8 Hz, 1H), 2.09 (dd, J = 13.2 and 8.8 Hz, 1H), 1.47 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 176.3, 156.9 (br), 146.9, 145.9, 143.8, 135.6, 130.5 (2C), 130.2, 130.0, 128.8 (2C), 127.7, 127.2 (2C), 123.7 (2C), 122.8, 122.4, 109.6, 81.1, 66.4, 60.2, 43.4, 42.3, 41.2, 28.3 (3C). HRMS (ESI) m/z: 537.2113 [M + Na]+; calcd for C29H30N4NaO5+, 537.2108. Diastereoisomer 6c′. 1H NMR (400 MHz, CDCl3) δ 8.20 (d, br, J = 8.8 Hz, 2H), 7.48 (d, br, J = 8.8 Hz, 2H), 7.38–7.15 (m, 7H), 7.04–6.97 (m, 2H), 6.76 (d, br, J = 7.7 Hz, 1H), 5.01 (d, J = 15.5 Hz, 1H), 4.80 (d, J = 15.5 Hz, 1H), 4.74 (m, br, 1H), 3.47 (dd, J = 13.2 and 5.0 Hz, 1H), 3.06 (dd, J = 13.2 and 8.5 Hz, 1H), 2.41–2.30 (m, 2H), 1.50 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.7, 153.1 (br), 147.6, 146.3, 142.5, 135.7, 132.1, 130.9 (2C), 129.8, 129.6 (2C), 128.6, 128.0 (2C), 124.5 (2C), 124.1, 123.4, 110.5, 82.1, 68.8, 61.3, 45.2, 45.0, 41.6, 29.0 (3C). HRMS (ESI) m/z: 537.2102 [M + Na]+; calcd for C29H30N4NaO5+, 537.2108.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (92% yield, separated diastereoisomers, 1
3). Yellow foam (92% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6d. 1H NMR (300 MHz, CDCl3) δ 7.80 (m, 2H), 7.62–7.18 (m, 12H), 7.07 (td, J = 7.8 and 1.9 Hz, 1H), 6.95 (t, br, J = 7.8 Hz, 1H), 6.88 (m, br, 1H), 6.65 (d, J = 7.8 Hz, 1H), 4.99 (m, br, 1H), 4.84 (d, br, J = 15.6 Hz, 1H), 4.76 (d, br, J = 15.6 Hz, 1H), 4.49–4.15 (m, br, 1H), 3.30 (dd, J = 13.7 and 4.9 Hz, 1H), 3.11 (dd, J = 13.7 and 7.8 Hz, 1H), 2.56 (dd, J = 13.7 and 8.8 Hz, 1H), 2.15 (dd, J = 13.7 and 8.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.0, 177.1, 157.4 (br), 144.4, 144.2, 143.6, 138.4, 136.8, 136.3, 133.1, 131.1 (2C), 130.7, 130.6 (2C), 130.4 (2C), 129.5 (2C), 129.0 (2C), 128.3, 127.9 (2C), 123.4, 123.1, 110.1, 81.6, 67.2, 60.9, 44.1 (2C), 41.8, 29.1 (3C). HRMS (ESI) m/z: 596.2526 [M + Na]+; calcd for C36H35N3NaO4+, 596.2520. Diastereoisomer 6d′. 1H NMR (300 MHz, CDCl3) δ 7.82–7.20 (m, 15H), 7.15 (td, J = 7.8 and 1.9 Hz, 1H), 6.97 (t, br, J = 7.8 Hz, 1H), 6.73 (d, J = 7.7 Hz, 1H), 4.99 (d, J = 15.6 Hz, 1H), 4.78 (d, J = 15.6 Hz, 1H), 4.78–4.66 (m, br, 2H), 3.46 (dd, J = 12.7 and 4.9 Hz, 1H), 2.99 (dd, J = 12.7 and 8.5 Hz, 1H), 2.36 (dd, J = 12.7 and 7.8 Hz, 1H), 2.30 (dd, J = 12.7 and 7.8 Hz, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.0, 174.8, 156.7 (br), 143.5, 142.5, 138.4, 136.8, 135.8, 133.0, 132.2, 131.2 (2C), 130.6 (2C), 130.0 (2C), 129.7, 129.6 (2C), 128.9 (2C), 128.5, 127.9 (2C), 124.0, 123.4, 110.4, 81.9, 68.7, 61.5, 45.3, 45.0, 41.9, 29.1 (3C). HRMS (ESI) m/z: 596.2527 [M + Na]+; calcd for C36H35N3NaO4+, 596.2520.
1 dr). Diastereoisomer 6d. 1H NMR (300 MHz, CDCl3) δ 7.80 (m, 2H), 7.62–7.18 (m, 12H), 7.07 (td, J = 7.8 and 1.9 Hz, 1H), 6.95 (t, br, J = 7.8 Hz, 1H), 6.88 (m, br, 1H), 6.65 (d, J = 7.8 Hz, 1H), 4.99 (m, br, 1H), 4.84 (d, br, J = 15.6 Hz, 1H), 4.76 (d, br, J = 15.6 Hz, 1H), 4.49–4.15 (m, br, 1H), 3.30 (dd, J = 13.7 and 4.9 Hz, 1H), 3.11 (dd, J = 13.7 and 7.8 Hz, 1H), 2.56 (dd, J = 13.7 and 8.8 Hz, 1H), 2.15 (dd, J = 13.7 and 8.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.0, 177.1, 157.4 (br), 144.4, 144.2, 143.6, 138.4, 136.8, 136.3, 133.1, 131.1 (2C), 130.7, 130.6 (2C), 130.4 (2C), 129.5 (2C), 129.0 (2C), 128.3, 127.9 (2C), 123.4, 123.1, 110.1, 81.6, 67.2, 60.9, 44.1 (2C), 41.8, 29.1 (3C). HRMS (ESI) m/z: 596.2526 [M + Na]+; calcd for C36H35N3NaO4+, 596.2520. Diastereoisomer 6d′. 1H NMR (300 MHz, CDCl3) δ 7.82–7.20 (m, 15H), 7.15 (td, J = 7.8 and 1.9 Hz, 1H), 6.97 (t, br, J = 7.8 Hz, 1H), 6.73 (d, J = 7.7 Hz, 1H), 4.99 (d, J = 15.6 Hz, 1H), 4.78 (d, J = 15.6 Hz, 1H), 4.78–4.66 (m, br, 2H), 3.46 (dd, J = 12.7 and 4.9 Hz, 1H), 2.99 (dd, J = 12.7 and 8.5 Hz, 1H), 2.36 (dd, J = 12.7 and 7.8 Hz, 1H), 2.30 (dd, J = 12.7 and 7.8 Hz, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.0, 174.8, 156.7 (br), 143.5, 142.5, 138.4, 136.8, 135.8, 133.0, 132.2, 131.2 (2C), 130.6 (2C), 130.0 (2C), 129.7, 129.6 (2C), 128.9 (2C), 128.5, 127.9 (2C), 124.0, 123.4, 110.4, 81.9, 68.7, 61.5, 45.3, 45.0, 41.9, 29.1 (3C). HRMS (ESI) m/z: 596.2527 [M + Na]+; calcd for C36H35N3NaO4+, 596.2520.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (98% yield, separated diastereoisomers, 1
3). Yellow foam (98% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6e. 1H NMR (300 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 2H), 7.39 (d, J = 8.8 Hz, 2H), 7.33–7.21 (m, 5H), 7.15 (t, J = 7.8 Hz, 1H), 6.94 (t, J = 7.8 Hz, 1H), 6.85 (d, br, J = 7.8 Hz, 1H), 6.64 (d, J = 7.8 Hz, 1H), 4.95 (m, br, 1H), 4.83 (d, br, J = 15.6 Hz, 1H), 4.75 (d, br, J = 15.6 Hz, 1H), 4.27 (m, br, 1H), 3.26 (dd, J = 13.7 and 4.9 Hz, 1H), 3.07 (dd, J = 13.7 and 7.8 Hz, 1H), 2.60 (s, 3H), 2.52 (dd, J = 12.7 and 7.8 Hz, 1H), 2.10 (dd, J = 12.7 and 8.8 Hz, 1H), 1.50 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.9, 176.3, 156.8 (br), 143.6 (2C), 135.7, 135.6, 130.1 (3C), 128.8 (2C), 128.6 (2C), 127.6, 127.2 (2C), 125.2, 122.8, 122.5, 109.4, 80.9, 66.4, 60.2, 43.4, 41.9 (br), 41.0, 28.4 (3C), 26.7. HRMS (ESI) m/z: 534.2355 [M + Na]+; calcd for C31H33N3NaO4+, 534.2363. Diastereoisomer 6e′. 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 2H), 7.44–7.20 (m, 7H), 7.14 (t, J = 7.8 Hz, 1H), 7.01–6.92 (m, 2H), 6.71 (d, J = 7.8 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.76 (d, J = 15.6 Hz, 1H), 4.70 (m, br, 1H), 4.65 (s, br, 1H), 3.42 (dd, J = 13.7 and 4.9 Hz, 1H), 2.95 (dd, J = 13.7 and 8.8 Hz, 1H), 2.57 (s, 3H), 2.32 (dd, J = 12.7 and 8.8 Hz, 1H), 2.26 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.8, 174.1, 156.2 (br), 143.5, 141.8, 135.7, 135.1, 131.4, 129.6 (2C), 129.0, 128.9, 128.7 (2C), 127.9 (2C), 127.3 (2C), 123.4, 122.7, 109.8, 81.2, 68.0, 60.7, 44.5, 44.2, 41.0, 28.3 (3C), 26.6. HRMS (ESI) m/z: 5342.358 [M + Na]+; calcd for C31H33N3NaO4+, 534.2363.
1 dr). Diastereoisomer 6e. 1H NMR (300 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 2H), 7.39 (d, J = 8.8 Hz, 2H), 7.33–7.21 (m, 5H), 7.15 (t, J = 7.8 Hz, 1H), 6.94 (t, J = 7.8 Hz, 1H), 6.85 (d, br, J = 7.8 Hz, 1H), 6.64 (d, J = 7.8 Hz, 1H), 4.95 (m, br, 1H), 4.83 (d, br, J = 15.6 Hz, 1H), 4.75 (d, br, J = 15.6 Hz, 1H), 4.27 (m, br, 1H), 3.26 (dd, J = 13.7 and 4.9 Hz, 1H), 3.07 (dd, J = 13.7 and 7.8 Hz, 1H), 2.60 (s, 3H), 2.52 (dd, J = 12.7 and 7.8 Hz, 1H), 2.10 (dd, J = 12.7 and 8.8 Hz, 1H), 1.50 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.9, 176.3, 156.8 (br), 143.6 (2C), 135.7, 135.6, 130.1 (3C), 128.8 (2C), 128.6 (2C), 127.6, 127.2 (2C), 125.2, 122.8, 122.5, 109.4, 80.9, 66.4, 60.2, 43.4, 41.9 (br), 41.0, 28.4 (3C), 26.7. HRMS (ESI) m/z: 534.2355 [M + Na]+; calcd for C31H33N3NaO4+, 534.2363. Diastereoisomer 6e′. 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 2H), 7.44–7.20 (m, 7H), 7.14 (t, J = 7.8 Hz, 1H), 7.01–6.92 (m, 2H), 6.71 (d, J = 7.8 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.76 (d, J = 15.6 Hz, 1H), 4.70 (m, br, 1H), 4.65 (s, br, 1H), 3.42 (dd, J = 13.7 and 4.9 Hz, 1H), 2.95 (dd, J = 13.7 and 8.8 Hz, 1H), 2.57 (s, 3H), 2.32 (dd, J = 12.7 and 8.8 Hz, 1H), 2.26 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 197.8, 174.1, 156.2 (br), 143.5, 141.8, 135.7, 135.1, 131.4, 129.6 (2C), 129.0, 128.9, 128.7 (2C), 127.9 (2C), 127.3 (2C), 123.4, 122.7, 109.8, 81.2, 68.0, 60.7, 44.5, 44.2, 41.0, 28.3 (3C), 26.6. HRMS (ESI) m/z: 5342.358 [M + Na]+; calcd for C31H33N3NaO4+, 534.2363.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (98% yield, separated diastereoisomers, 1
3). Yellow foam (98% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 dr). Diastereoisomer 6f. 1H NMR (300 MHz, CDCl3) δ 7.35–7.10 (m, 10H), 7.01–6.89 (m, 2H), 6.63 (d, J = 7.8 Hz, 1H), 4.95 (m, 1H), 4.88 (d, br, J = 15.6 Hz, 1H), 4.73 (d, br, J = 15.6 Hz, 1H), 4.41 (m, br, 1H), 3.28 (dd, J = 13.7 and 4.9 Hz, 1H), 2.98 (dd, J = 13.7 and 7.8 Hz, 1H), 2.50 (dd, J = 12.7 and 7.8 Hz, 1H), 2.42 (s, 3H), 2.13 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.6, 156.7 (br), 143.7, 137.0, 136.3, 135.7, 130.5 (2C), 129.9, 128.8 (2C), 127.6, 127.2 (2C), 126.8, 126.0, 125.7, 122.7, 122.6, 109.4, 80.7, 66.5, 59.3, 43.3, 42.0, 38.5, 28.4 (3C), 19.9. HRMS (ESI) m/z: 506.2411 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414. Diastereoisomer 6f′. 1H NMR (300 MHz, CDCl3) δ 7.35–7.08 (m, 10H), 7.03–6.92 (m, 2H), 6.70 (d, J = 7.8 Hz, 1H), 4.98 (d, J = 15.6 Hz, 1H), 4.84–4.67 (m, 3H), 3.41 (dd, J = 12.7 and 4.9 Hz, 1H), 2.87 (dd, J = 12.7 and 8.8 Hz, 1H), 2.45 (s, 3H), 2.37 (dd, J = 12.7 and 7.8 Hz, 1H), 2.25 (dd, J = 12.7 and 7.8 Hz, 1H), 1.47 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.3, 156.1 (br), 141.8, 136.6, 136.2, 135.1, 131.7, 130.4 (2C), 128.9 (3C), 127.8, 127.3 (2C), 126.8, 126.1, 123.3, 122.7, 109.7, 81.1, 68.2, 59.6, 44.7, 44.2, 38.8, 28.4 (3C), 19.8. HRMS (ESI) m/z: 506.2419 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414.
1.6 dr). Diastereoisomer 6f. 1H NMR (300 MHz, CDCl3) δ 7.35–7.10 (m, 10H), 7.01–6.89 (m, 2H), 6.63 (d, J = 7.8 Hz, 1H), 4.95 (m, 1H), 4.88 (d, br, J = 15.6 Hz, 1H), 4.73 (d, br, J = 15.6 Hz, 1H), 4.41 (m, br, 1H), 3.28 (dd, J = 13.7 and 4.9 Hz, 1H), 2.98 (dd, J = 13.7 and 7.8 Hz, 1H), 2.50 (dd, J = 12.7 and 7.8 Hz, 1H), 2.42 (s, 3H), 2.13 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.6, 156.7 (br), 143.7, 137.0, 136.3, 135.7, 130.5 (2C), 129.9, 128.8 (2C), 127.6, 127.2 (2C), 126.8, 126.0, 125.7, 122.7, 122.6, 109.4, 80.7, 66.5, 59.3, 43.3, 42.0, 38.5, 28.4 (3C), 19.9. HRMS (ESI) m/z: 506.2411 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414. Diastereoisomer 6f′. 1H NMR (300 MHz, CDCl3) δ 7.35–7.08 (m, 10H), 7.03–6.92 (m, 2H), 6.70 (d, J = 7.8 Hz, 1H), 4.98 (d, J = 15.6 Hz, 1H), 4.84–4.67 (m, 3H), 3.41 (dd, J = 12.7 and 4.9 Hz, 1H), 2.87 (dd, J = 12.7 and 8.8 Hz, 1H), 2.45 (s, 3H), 2.37 (dd, J = 12.7 and 7.8 Hz, 1H), 2.25 (dd, J = 12.7 and 7.8 Hz, 1H), 1.47 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.3, 156.1 (br), 141.8, 136.6, 136.2, 135.1, 131.7, 130.4 (2C), 128.9 (3C), 127.8, 127.3 (2C), 126.8, 126.1, 123.3, 122.7, 109.7, 81.1, 68.2, 59.6, 44.7, 44.2, 38.8, 28.4 (3C), 19.8. HRMS (ESI) m/z: 506.2419 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (70% yield, separated diastereoisomers, 1
3). Yellow foam (70% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6g. 1H NMR (300 MHz, CDCl3) δ 7.32–7.20 (m, 6H), 7.09–6.98 (m, 2H), 6.84 (s, 2H), 6.65 (d, J = 7.8 Hz, 1H), 5.01–4.86 (m, 3H), 4.69 (d, J = 15.6 Hz, 1H), 3.28 (dd, J = 13.7 and 7.8 Hz, 1H), 3.04 (dd, J = 13.7 and 6.8 Hz, 1H), 3.28 (dd, J = 12.7 and 7.8 Hz, 1H), 2.40 (s, 6H), 2.38–2.29 (m, 1H), 2.24 (s, 3H), 1.37 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.4, 157.0, 144.4, 137.5, 136.4, 136.2, 133.0, 130.6, 129.8 (3C), 129.6 (2C), 129.1, 128.3, 127.9, 126.6, 123.5, 123.4, 110.1, 81.2, 67.2, 59.4, 44.4, 44.1, 35.7, 28.8 (3C), 21.5, 21.4 (2C). HRMS (ESI) m/z: 534.2721 [M + Na]+; calcd for C32H37N3NaO3+, 534.2727. Diastereoisomer 6g′. 1H NMR (300 MHz, CDCl3) δ 7.35–7.21 (m, 5H), 7.14 (td, J = 7.8 and 2.9 Hz, 1H), 7.02–6.92 (m, 2H), 6.83 (s, 2H), 6.70 (d, J = 7.8 Hz, 1H), 4.96 (d, J = 15.6 Hz, 1H), 4.93–4.76 (m, 3H), 3.29 (dd, J = 13.7 and 6.8 Hz, 1H), 2.96 (dd, J = 13.7 and 6.8 Hz, 1H), 2.41 (s, 6H), 2.38–2.29 (m, 2H), 2.23 (s, 3H), 1.36 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 175.1, 156.5 (br), 142.5, 137.5 (2C), 136.3, 135.8, 132.8, 132.6, 129.8 (3C), 129.6 (3C), 128.5, 128.0, 124.0, 123.4, 110.4, 81.5, 69.0, 59.7, 45.3, 44.9, 35.4, 28.8 (3C), 21.5, 21.4 (2C). HRMS (ESI) m/z: 534.2719 [M + Na]+; calcd for C32H37N3NaO3+, 534.2727.
1 dr). Diastereoisomer 6g. 1H NMR (300 MHz, CDCl3) δ 7.32–7.20 (m, 6H), 7.09–6.98 (m, 2H), 6.84 (s, 2H), 6.65 (d, J = 7.8 Hz, 1H), 5.01–4.86 (m, 3H), 4.69 (d, J = 15.6 Hz, 1H), 3.28 (dd, J = 13.7 and 7.8 Hz, 1H), 3.04 (dd, J = 13.7 and 6.8 Hz, 1H), 3.28 (dd, J = 12.7 and 7.8 Hz, 1H), 2.40 (s, 6H), 2.38–2.29 (m, 1H), 2.24 (s, 3H), 1.37 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.4, 157.0, 144.4, 137.5, 136.4, 136.2, 133.0, 130.6, 129.8 (3C), 129.6 (2C), 129.1, 128.3, 127.9, 126.6, 123.5, 123.4, 110.1, 81.2, 67.2, 59.4, 44.4, 44.1, 35.7, 28.8 (3C), 21.5, 21.4 (2C). HRMS (ESI) m/z: 534.2721 [M + Na]+; calcd for C32H37N3NaO3+, 534.2727. Diastereoisomer 6g′. 1H NMR (300 MHz, CDCl3) δ 7.35–7.21 (m, 5H), 7.14 (td, J = 7.8 and 2.9 Hz, 1H), 7.02–6.92 (m, 2H), 6.83 (s, 2H), 6.70 (d, J = 7.8 Hz, 1H), 4.96 (d, J = 15.6 Hz, 1H), 4.93–4.76 (m, 3H), 3.29 (dd, J = 13.7 and 6.8 Hz, 1H), 2.96 (dd, J = 13.7 and 6.8 Hz, 1H), 2.41 (s, 6H), 2.38–2.29 (m, 2H), 2.23 (s, 3H), 1.36 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 175.1, 156.5 (br), 142.5, 137.5 (2C), 136.3, 135.8, 132.8, 132.6, 129.8 (3C), 129.6 (3C), 128.5, 128.0, 124.0, 123.4, 110.4, 81.5, 69.0, 59.7, 45.3, 44.9, 35.4, 28.8 (3C), 21.5, 21.4 (2C). HRMS (ESI) m/z: 534.2719 [M + Na]+; calcd for C32H37N3NaO3+, 534.2727.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (63% yield, separated diastereoisomers, 1
3). Yellow foam (63% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6h. 1H NMR (300 MHz, CDCl3) δ 7.32–7.10 (m, 10H), 6.92 (t, J = 7.8 Hz, 1H), 6.77 (m, br, 1H), 6.61 (d, J = 7.8 Hz, 1H), 4.98–4.67 (m, br, 4H), 3.12 (dd, J = 13.6 and 3.9 Hz, 1H), 3.03 (dd, J = 13.6 and 6.8 Hz, 1H), 2.51 (dd, J = 12.7 and 7.8 Hz, 1H), 2.34 (s, 3H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.1, 157.3, 144.3, 136.9, 136.3, 135.2, 130.6, 130.5 (2C), 130.0 (2C), 129.5 (2C), 128.3, 127.9 (2C), 122.3 (2C), 121.3, 110.0, 81.5, 67.3, 61.0, 44.5, 44.0, 41.0, 29.1 (3C), 21.7. HRMS (ESI) m/z: 506.2410 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414. Diastereoisomer 6h′. 1H NMR (400 MHz, CDCl3) δ 7.36–7.10 (m, 10H), 7.05–6.95 (m, 2H), 6.73 (d, J = 7.8 Hz, 1H), 5.00 (d, J = 15.6 Hz, 1H), 4.81 (d, J = 15.6 Hz, 1H), 4.73–4.62 (m, br, 2H), 3.37 (dd, J = 12.7 and 3.9 Hz, 1H), 2.88 (dd, J = 12.7 and 8.8 Hz, 1H), 2.39 (dd, J = 12.7 and 8.8 Hz, 1H), 2.34 (s, 3H), 2.28 (dd, J = 12.7 and 7.8 Hz, 1H), 1.55 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 156.5, 142.5, 136.9, 135.8, 135.3, 132.3, 130.0 (4C), 129.5 (3C), 128.5, 127.9 (2C), 124.0, 123.4, 110.3, 81.7, 68.6, 61.8, 45.2, 44.9, 41.3, 29.1 (3C), 21.7. HRMS (ESI) m/z: 506.2409 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414.
1 dr). Diastereoisomer 6h. 1H NMR (300 MHz, CDCl3) δ 7.32–7.10 (m, 10H), 6.92 (t, J = 7.8 Hz, 1H), 6.77 (m, br, 1H), 6.61 (d, J = 7.8 Hz, 1H), 4.98–4.67 (m, br, 4H), 3.12 (dd, J = 13.6 and 3.9 Hz, 1H), 3.03 (dd, J = 13.6 and 6.8 Hz, 1H), 2.51 (dd, J = 12.7 and 7.8 Hz, 1H), 2.34 (s, 3H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.1, 157.3, 144.3, 136.9, 136.3, 135.2, 130.6, 130.5 (2C), 130.0 (2C), 129.5 (2C), 128.3, 127.9 (2C), 122.3 (2C), 121.3, 110.0, 81.5, 67.3, 61.0, 44.5, 44.0, 41.0, 29.1 (3C), 21.7. HRMS (ESI) m/z: 506.2410 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414. Diastereoisomer 6h′. 1H NMR (400 MHz, CDCl3) δ 7.36–7.10 (m, 10H), 7.05–6.95 (m, 2H), 6.73 (d, J = 7.8 Hz, 1H), 5.00 (d, J = 15.6 Hz, 1H), 4.81 (d, J = 15.6 Hz, 1H), 4.73–4.62 (m, br, 2H), 3.37 (dd, J = 12.7 and 3.9 Hz, 1H), 2.88 (dd, J = 12.7 and 8.8 Hz, 1H), 2.39 (dd, J = 12.7 and 8.8 Hz, 1H), 2.34 (s, 3H), 2.28 (dd, J = 12.7 and 7.8 Hz, 1H), 1.55 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.8, 156.5, 142.5, 136.9, 135.8, 135.3, 132.3, 130.0 (4C), 129.5 (3C), 128.5, 127.9 (2C), 124.0, 123.4, 110.3, 81.7, 68.6, 61.8, 45.2, 44.9, 41.3, 29.1 (3C), 21.7. HRMS (ESI) m/z: 506.2409 [M + Na]+; calcd for C30H33N3NaO3+, 506.2414.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (53% yield, separated diastereoisomers, 1.5
3). Yellow foam (53% yield, separated diastereoisomers, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6i. 1H NMR (300 MHz, CDCl3) δ 7.62–7.52 (m, 4H), 7.49–7.22 (m, 7H), 6.96 (t, br, J = 7.8 Hz, 1H), 6.88 (m, br, 1H), 6.74 (d, br, J = 7.8 Hz, 1H), 4.88 (m, br, 1H), 3.24–3.12 (m, 2H), 3.10 (s, 3H), 2.49 (dd, J = 12.7 and 6.8 Hz, 1H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.4, 156.7 (br), 144.6, 140.9, 139.7, 136.9, 130.3 (2C), 130.0, 128.8 (2C), 127.3 (3C), 127.0 (2C), 125.6, 122.7, 122.4, 108.3, 80.8, 66.4, 60.2, 41.9, 40.5, 28.5 (3C), 26.0. HRMS (ESI) m/z: 492.2262 [M + Na]+; calcd for C29H31N3NaO3+, 492.2258. Diastereoisomer 6i′. 1H NMR (300 MHz, CDCl3) δ 7.58–7.47 (m, 4H), 7.46–7.28 (m, 5H), 7.09 (t, br, J = 7.8 Hz, 1H), 6.99 (d, br, J = 6.8 Hz, 2H), 6.83 (d, br, J = 7.8 Hz, 2H), 4.70 (m, br, 1H), 3.40 (dd, J = 12.7 and 4.9 Hz, 1H), 3.17 (s, 3H), 2.89 (dd, J = 12.7 and 9.8 Hz, 1H), 2.33 (dd, J = 12.7 and 8.8 Hz, 1H), 2.24 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.9, 153.8 (br), 145.8, 140.9, 139.6, 137.0, 131.5, 130.7, 129.8 (2C), 128.8 (2C), 127.3 (2C), 127.2, 127.0 (2C), 123.3, 123.0, 108.7, 81.0, 68.1, 61.1, 44.4, 40.9, 28.4 (3C), 26.1. HRMS (ESI) m/z: 492.2253 [M + Na]+; calcd for C29H31N3NaO3+, 492.2258.
1 dr). Diastereoisomer 6i. 1H NMR (300 MHz, CDCl3) δ 7.62–7.52 (m, 4H), 7.49–7.22 (m, 7H), 6.96 (t, br, J = 7.8 Hz, 1H), 6.88 (m, br, 1H), 6.74 (d, br, J = 7.8 Hz, 1H), 4.88 (m, br, 1H), 3.24–3.12 (m, 2H), 3.10 (s, 3H), 2.49 (dd, J = 12.7 and 6.8 Hz, 1H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.4, 156.7 (br), 144.6, 140.9, 139.7, 136.9, 130.3 (2C), 130.0, 128.8 (2C), 127.3 (3C), 127.0 (2C), 125.6, 122.7, 122.4, 108.3, 80.8, 66.4, 60.2, 41.9, 40.5, 28.5 (3C), 26.0. HRMS (ESI) m/z: 492.2262 [M + Na]+; calcd for C29H31N3NaO3+, 492.2258. Diastereoisomer 6i′. 1H NMR (300 MHz, CDCl3) δ 7.58–7.47 (m, 4H), 7.46–7.28 (m, 5H), 7.09 (t, br, J = 7.8 Hz, 1H), 6.99 (d, br, J = 6.8 Hz, 2H), 6.83 (d, br, J = 7.8 Hz, 2H), 4.70 (m, br, 1H), 3.40 (dd, J = 12.7 and 4.9 Hz, 1H), 3.17 (s, 3H), 2.89 (dd, J = 12.7 and 9.8 Hz, 1H), 2.33 (dd, J = 12.7 and 8.8 Hz, 1H), 2.24 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.9, 153.8 (br), 145.8, 140.9, 139.6, 137.0, 131.5, 130.7, 129.8 (2C), 128.8 (2C), 127.3 (2C), 127.2, 127.0 (2C), 123.3, 123.0, 108.7, 81.0, 68.1, 61.1, 44.4, 40.9, 28.4 (3C), 26.1. HRMS (ESI) m/z: 492.2253 [M + Na]+; calcd for C29H31N3NaO3+, 492.2258.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (69% yield, separated diastereoisomers, 1
3). Yellow foam (69% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6j. 1H NMR (300 MHz, CDCl3) δ 7.62–7.54 (m, 4H), 7.48–7.28 (m, 8H), 7.23 (t, br, J = 7.8 Hz, 1H), 7.12 (dd, J = 8.7 and 1.9 Hz, 1H), 6.95 (t, br, J = 7.8 Hz, 1H), 6.84–6.83 (m, 2H), 6.44 (d, br, J = 15.6 Hz, 1H), 6.14 (dt, J = 15.6 and 4.9 Hz, 1H), 4.93 (m, br, 1H), 4.45 (dd, br, J = 16.6 and 4.9 Hz, 1H), 4.31 (dd, br, J = 16.6 and 4.9 Hz, 1H), 3.19 (dd, J = 13.7 and 3.9 Hz, 1H), 3.10 (dd, J = 13.7 and 6.8 Hz, 1H), 2.54 (dd, J = 12.7 and 7.8 Hz, 1H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.2, 154.4 (br), 143.6, 140.9, 139.8, 136.9, 136.3, 132.7, 131.6, 130.5 (2C), 130.4 (2C), 130.0, 128.9 (2C), 128.2, 127.3 (4C), 127.0, 125.7, 125.6, 125.0, 122.8, 122.6, 109.0, 80.8, 66.4, 60.3, 41.4 (2C), 40.5, 28.5 (3C). HRMS (ESI) m/z: 662.1956 [M + Na]+; calcd for C37H35Cl2N3NaO3+, 662.1948. Diastereoisomer 6j′. 1H NMR (300 MHz, CDCl3) δ 7.61–7.20 (m, 13H), 7.15 (d, br, J = 8.7 Hz, 1H), 7.06–6.97 (m, 2H), 6.85 (d, J = 7.8 Hz, 1H), 6.48 (d, br, J = 15.6 Hz, 1H), 6.15 (dt, J = 15.6 and 5.9 Hz, 1H), 4.71 (m, br, 1H), 4.57–4.37 (m, 2H), 3.42 (dd, J = 12.7 and 3.9 Hz, 1H), 2.90 (dd, J = 12.7 and 8.8 Hz, 1H), 2.43–2.24 (m, 2H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.8, 151.0 (br), 141.7, 140.9, 139.6, 136.9, 136.1, 132.8, 131.7, 131.6, 131.0, 130.5, 129.8 (2C), 129.1, 128.8 (2C), 128.3, 127.3 (2C), 127.2, 127.0 (2C), 125.6, 124.5, 123.5, 122.9, 109.3, 81.1, 68.0, 61.2, 44.6, 42.2, 40.9, 28.4 (3C). HRMS (ESI) m/z: 662.1954 [M + Na]+; calcd for C37H35Cl2N3NaO3+, 662.1948.
1 dr). Diastereoisomer 6j. 1H NMR (300 MHz, CDCl3) δ 7.62–7.54 (m, 4H), 7.48–7.28 (m, 8H), 7.23 (t, br, J = 7.8 Hz, 1H), 7.12 (dd, J = 8.7 and 1.9 Hz, 1H), 6.95 (t, br, J = 7.8 Hz, 1H), 6.84–6.83 (m, 2H), 6.44 (d, br, J = 15.6 Hz, 1H), 6.14 (dt, J = 15.6 and 4.9 Hz, 1H), 4.93 (m, br, 1H), 4.45 (dd, br, J = 16.6 and 4.9 Hz, 1H), 4.31 (dd, br, J = 16.6 and 4.9 Hz, 1H), 3.19 (dd, J = 13.7 and 3.9 Hz, 1H), 3.10 (dd, J = 13.7 and 6.8 Hz, 1H), 2.54 (dd, J = 12.7 and 7.8 Hz, 1H), 2.17 (dd, J = 12.7 and 7.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 176.2, 154.4 (br), 143.6, 140.9, 139.8, 136.9, 136.3, 132.7, 131.6, 130.5 (2C), 130.4 (2C), 130.0, 128.9 (2C), 128.2, 127.3 (4C), 127.0, 125.7, 125.6, 125.0, 122.8, 122.6, 109.0, 80.8, 66.4, 60.3, 41.4 (2C), 40.5, 28.5 (3C). HRMS (ESI) m/z: 662.1956 [M + Na]+; calcd for C37H35Cl2N3NaO3+, 662.1948. Diastereoisomer 6j′. 1H NMR (300 MHz, CDCl3) δ 7.61–7.20 (m, 13H), 7.15 (d, br, J = 8.7 Hz, 1H), 7.06–6.97 (m, 2H), 6.85 (d, J = 7.8 Hz, 1H), 6.48 (d, br, J = 15.6 Hz, 1H), 6.15 (dt, J = 15.6 and 5.9 Hz, 1H), 4.71 (m, br, 1H), 4.57–4.37 (m, 2H), 3.42 (dd, J = 12.7 and 3.9 Hz, 1H), 2.90 (dd, J = 12.7 and 8.8 Hz, 1H), 2.43–2.24 (m, 2H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.8, 151.0 (br), 141.7, 140.9, 139.6, 136.9, 136.1, 132.8, 131.7, 131.6, 131.0, 130.5, 129.8 (2C), 129.1, 128.8 (2C), 128.3, 127.3 (2C), 127.2, 127.0 (2C), 125.6, 124.5, 123.5, 122.9, 109.3, 81.1, 68.0, 61.2, 44.6, 42.2, 40.9, 28.4 (3C). HRMS (ESI) m/z: 662.1954 [M + Na]+; calcd for C37H35Cl2N3NaO3+, 662.1948.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (50% yield, separated diastereoisomers, 1
3). Yellow foam (50% yield, separated diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Diastereoisomer 6k. 1H NMR (300 MHz, CDCl3) δ 7.58 (d, br, J = 7.8 Hz, 4H), 7.49–7.20 (m, 12H), 6.90 (d, br, J = 7.8 Hz, 1H), 6.49 (d, J = 7.8 Hz, 1H), 5.02 (m, br, 1H), 4.99–4.71 (m, br, 2H), 3.19 (dd, J = 12.7 and 3.9 Hz, 1H), 3.11 (dd, J = 12.7 and 6.8 Hz, 1H), 2.56 (dd, J = 12.7 and 7.8 Hz, 1H), 2.19 (dd, J = 12.7 and 7.8 Hz, 1H), 2.14 (s, 3H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.1, 158.5, 141.2, 140.8, 139.8, 137.0, 135.8, 132.3, 130.5 (2C), 130.1, 128.8 (2C), 128.7 (2C), 127.5, 127.3 (5C), 127.2, 127.0, 125.7, 123.3, 109.1, 80.7, 66.7, 60.3, 43.4 (2C), 40.6, 28.4 (3C), 20.9. HRMS (ESI) m/z: 582.2723 [M + Na]+; calcd for C36H37N3NaO3+, 582.2727. Diastereoisomer 6k′. 1H NMR (400 MHz, CDCl3) δ 7.65–7.54 (m, 4H), 7.45 (t, br, J = 7.8 Hz, 2H), 7.42–7.23 (m, 9H), 6.97 (d, br, J = 7.8 Hz, 1H), 6.85 (s, br, 1H), 6.62 (d, J = 7.8 Hz, 1H), 4,98 (d, J = 15.8 Hz, 1H), 4,80 (d, J = 15.8 Hz, 1H), 4.75 (m, br, 1H), 3.44 (dd, J = 12.7 and 4.9 Hz, 1H), 2.96 (dd, J = 12.7 and 8.8 Hz, 1H), 2.43 (dd, J = 12.7 and 8.5 Hz, 1H), 2.35 (dd, J = 12.7 and 7.6 Hz, 1H), 2.26 (s, 3H), 1.55 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 175.4, 158.0 (br), 142.4, 139.6, 139.4, 137.0, 135.3, 132.9, 131.7, 129.9 (2C), 129.1, 128.8 (2C), 128.7, 127.8, 127.3 (4C), 127.3, 127.2, 127.0 (2C), 123.6, 109.4, 81.0, 68.7, 61.2, 44.8, 44.2, 41.0, 28.4 (3C), 21.0. HRMS (ESI) m/z: 582.2720 [M + Na]+; calcd for C36H37N3NaO3+, 582.2727.
1 dr). Diastereoisomer 6k. 1H NMR (300 MHz, CDCl3) δ 7.58 (d, br, J = 7.8 Hz, 4H), 7.49–7.20 (m, 12H), 6.90 (d, br, J = 7.8 Hz, 1H), 6.49 (d, J = 7.8 Hz, 1H), 5.02 (m, br, 1H), 4.99–4.71 (m, br, 2H), 3.19 (dd, J = 12.7 and 3.9 Hz, 1H), 3.11 (dd, J = 12.7 and 6.8 Hz, 1H), 2.56 (dd, J = 12.7 and 7.8 Hz, 1H), 2.19 (dd, J = 12.7 and 7.8 Hz, 1H), 2.14 (s, 3H), 1.53 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.1, 158.5, 141.2, 140.8, 139.8, 137.0, 135.8, 132.3, 130.5 (2C), 130.1, 128.8 (2C), 128.7 (2C), 127.5, 127.3 (5C), 127.2, 127.0, 125.7, 123.3, 109.1, 80.7, 66.7, 60.3, 43.4 (2C), 40.6, 28.4 (3C), 20.9. HRMS (ESI) m/z: 582.2723 [M + Na]+; calcd for C36H37N3NaO3+, 582.2727. Diastereoisomer 6k′. 1H NMR (400 MHz, CDCl3) δ 7.65–7.54 (m, 4H), 7.45 (t, br, J = 7.8 Hz, 2H), 7.42–7.23 (m, 9H), 6.97 (d, br, J = 7.8 Hz, 1H), 6.85 (s, br, 1H), 6.62 (d, J = 7.8 Hz, 1H), 4,98 (d, J = 15.8 Hz, 1H), 4,80 (d, J = 15.8 Hz, 1H), 4.75 (m, br, 1H), 3.44 (dd, J = 12.7 and 4.9 Hz, 1H), 2.96 (dd, J = 12.7 and 8.8 Hz, 1H), 2.43 (dd, J = 12.7 and 8.5 Hz, 1H), 2.35 (dd, J = 12.7 and 7.6 Hz, 1H), 2.26 (s, 3H), 1.55 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 175.4, 158.0 (br), 142.4, 139.6, 139.4, 137.0, 135.3, 132.9, 131.7, 129.9 (2C), 129.1, 128.8 (2C), 128.7, 127.8, 127.3 (4C), 127.3, 127.2, 127.0 (2C), 123.6, 109.4, 81.0, 68.7, 61.2, 44.8, 44.2, 41.0, 28.4 (3C), 21.0. HRMS (ESI) m/z: 582.2720 [M + Na]+; calcd for C36H37N3NaO3+, 582.2727.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (34% yield, unseparable diastereoisomers, 1
3). Yellow foam (34% yield, unseparable diastereoisomers, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). 1H NMR (400 MHz, CDCl3, 7
1 dr). 1H NMR (400 MHz, CDCl3, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of diastereoisomers) δ 7.67–7.20 (m, 16H), 7.13 (d, J = 2.1 Hz, 0.7H), 6.85 (m, br, 0.3H), 6.61 (d, J = 8.4 Hz, 0.7H), 6.50 (d, J = 8.4 Hz, 0.3H), 5.07–4.93 (m, br, 1.3H), 4.85–4.64 (m, br, 1.7H), 3.21 (dd, J = 13.2 and 5.0 Hz, 0.7H), 3.29 (dd, J = 13.1 and 4.4 Hz, 0.3H), 3.14 (dd, J = 13.1 and 6.7 Hz, 0.3H), 2.97 (dd, J = 13.2 and 8.8 Hz, 0.7H), 2.61 (dd, J = 13.2 and 8.2 Hz, 0.3H), 2.42 (dd, J = 12.6 and 8.2 Hz, 0.7H), 2.37 (dd, J = 12.6 and 7.6 Hz, 0.7H), 2.21 (dd, J = 13.2 and 7.6 Hz, 0.3H), 1.56 (m, 9H). 13C NMR (100 MHz, CDCl3, 7
3 mixture of diastereoisomers) δ 7.67–7.20 (m, 16H), 7.13 (d, J = 2.1 Hz, 0.7H), 6.85 (m, br, 0.3H), 6.61 (d, J = 8.4 Hz, 0.7H), 6.50 (d, J = 8.4 Hz, 0.3H), 5.07–4.93 (m, br, 1.3H), 4.85–4.64 (m, br, 1.7H), 3.21 (dd, J = 13.2 and 5.0 Hz, 0.7H), 3.29 (dd, J = 13.1 and 4.4 Hz, 0.3H), 3.14 (dd, J = 13.1 and 6.7 Hz, 0.3H), 2.97 (dd, J = 13.2 and 8.8 Hz, 0.7H), 2.61 (dd, J = 13.2 and 8.2 Hz, 0.3H), 2.42 (dd, J = 12.6 and 8.2 Hz, 0.7H), 2.37 (dd, J = 12.6 and 7.6 Hz, 0.7H), 2.21 (dd, J = 13.2 and 7.6 Hz, 0.3H), 1.56 (m, 9H). 13C NMR (100 MHz, CDCl3, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of diastereoisomers) δ 175.7 and 173.7 (1C), 159.0 (br), 146.1 and 142.2 (1C), 140.9 and 140.2 (1C), 139.7 and 138.7 (1C), 137.0 and 136.8 (1C), 136.0 and 135.1 (1C), 134.6 and 134.2 (1C), 133.7 and 120.7 (1C), 132.7–126.0 (16C), 112.2 and 110.8 (1C), 81.5, 68.3 and 68.1 (1C), 61.1 and 60.1 (1C), 44.4 and 43.5 (1C), 43.9 and 41.3 (1C), 43.5 and 41.0 (1C), 28.4 and 28.1 (3C). HRMS (ESI) m/z: 646.1676 [M + Na]+; calcd for C35H34BrN3NaO3+, 646.1676.
3 mixture of diastereoisomers) δ 175.7 and 173.7 (1C), 159.0 (br), 146.1 and 142.2 (1C), 140.9 and 140.2 (1C), 139.7 and 138.7 (1C), 137.0 and 136.8 (1C), 136.0 and 135.1 (1C), 134.6 and 134.2 (1C), 133.7 and 120.7 (1C), 132.7–126.0 (16C), 112.2 and 110.8 (1C), 81.5, 68.3 and 68.1 (1C), 61.1 and 60.1 (1C), 44.4 and 43.5 (1C), 43.9 and 41.3 (1C), 43.5 and 41.0 (1C), 28.4 and 28.1 (3C). HRMS (ESI) m/z: 646.1676 [M + Na]+; calcd for C35H34BrN3NaO3+, 646.1676.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (72% yield, separated diastereoisomers, 1.5
3). Yellow foam (72% yield, separated diastereoisomers, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).Diastereoisomer 6m. 1H NMR (400 MHz, CDCl3) δ 7.62 (dd, br, J = 7.9 and 1.5 Hz, 4H), 7.48 (t, J = 7.8 Hz, 2H), 7.44–7.23 (m, 9H), 6.90 (dd, br, J = 7.8 and 1.2 Hz, 1H), 6.75–6.65 (m, br, 1H), 6.65 (d, br, J = 1.5 Hz, 1H), 5.02 (m, br, 1H), 4,91–4.68 (m, br, 2H), 3.21 (dd, J = 13.5 and 4.4 Hz, 1H), 3.16 (dd, J = 13.5 and 7.0 Hz, 1H), 2.59 (dd, J = 12.9 and 7.9 Hz, 1H), 2.20 (dd, J = 12.9 and 7.9 Hz, 1H), 1.57 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.1, 157.3, 145.6, 141.4, 140.5, 137.4 (2C), 136.4, 135.8, 131.1 (2C), 129.6 (2C), 129.5 (2C), 128.5, 128.0 (3C), 127.8 (2C), 127.7 (2C), 124.3, 123.3, 110.6, 81.6, 66.9, 60.9, 44.2, 41.1 (2C), 29.1 (3C). HRMS (ESI) m/z: 602.2189 [M + Na]+; calcd for C35H34ClN3NaO3+, 602.2181. Diastereoisomer 6m′. 1H NMR (300 MHz, CDCl3) δ 7.54 (t, br, J = 7.9 Hz, 4H), 7.42 (t, br, J = 7.8 Hz, 2H), 7.37–7.20 (m, 9H), 6.95 (d, br, J = 7.8 Hz, 1H), 6.91 (t, br, J = 7.8 Hz, 1H), 6.70 (s, br, 1H), 4,94 (d, J = 15.6 Hz, 1H), 4,75 (d, J = 15.6 Hz, 1H), 4.69 (m, br, 1H), 3.39 (dd, J = 12.7 and 4.9 Hz, 1H), 2.91 (dd, J = 12.7 and 8.8 Hz, 1H), 2.39 (dd, J = 12.7 and 8.8 Hz, 1H), 2.29 (dd, J = 12.7 and 7.8 Hz, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 174.2, 156.0, 143.2, 140.9, 139.7, 136.8 (2C), 134.8, 134.6, 129.9 (2C), 129.1 (2C), 128.8 (2C), 128.1, 127.4 (2C), 127.3 (3C), 127.1 (2C), 123.7, 123.2, 110.4, 81.2, 67.8, 61.1, 44.6, 44.4, 40.8, 28.4 (3C). HRMS (ESI) m/z: 602.2188 [M + Na]+; calcd for C35H34ClN3NaO3+, 602.2181.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yellow foam (47% yield, separated diastereoisomers, 1.4
3). Yellow foam (47% yield, separated diastereoisomers, 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).Diastereoisomer 6n. 1H NMR (300 MHz, CDCl3) δ 7.66–7.54 (m, 4H), 7.54–7.33 (m, 6H), 7.31–6.93 (m, 7H), 6.83 (m, br, 1H), 5.15 (d, br, J = 16.6 Hz, 1H), 5.10–4.94 (m, 2H), 3.24–3.08 (m, 2H), 2.59 (dd, J = 12.7 and 7.8 Hz, 1H), 2.20 (dd, J = 12.7 and 7.8 Hz, 1H), 1.47 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 177.7, 156.9 (br), 142.0, 140.8, 136.7, 136.2, 132.6, 130.6 (2C), 128.9 (2C), 128.4 (2C), 128.0 (q, J = 5.9 Hz), 127.4 (4C), 127.0 (2C), 126.9, 126.2, 125.7 (2C), 123.0 (q, J = 272.8 Hz), 122.3, 113.2 (q, J = 33.9 Hz), 81.0, 64.7, 60.1, 45.7, 45.0, 40.2, 28.3 (3C). HRMS (ESI) m/z: 636.2448 [M + Na]+; calcd for C36H34F3N3NaO3+, 636.2444. Diastereoisomer 6n′. 1H NMR (300 MHz, CDCl3) δ 7.60–7.52 (m, 5H), 7.43 (t, br, J = 7.8 Hz, 2H), 7.37–7.02 (m, 11H), 5.22 (d, J = 16.6 Hz, 1H), 5.17 (d, J = 16.6 Hz, 1H), 4.77 (m, br, 1H), 3.40 (dd, J = 12.7 and 4.9 Hz, 1H), 2.94 (dd, J = 12.7 and 8.8 Hz, 1H), 2.45 (dd, J = 12.7 and 7.8 Hz, 1H), 2.37 (dd, J = 12.7 and 7.8 Hz, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 175.9, 150.5 (br), 140.9, 140.1, 139.7, 136.7, 135.6, 134.8, 129.9 (2C), 128.8 (2C), 128.5 (2C), 127.4–127.0 (7C), 126.5, 125.6 (2C), 123.2 (q, J = 272.8 Hz), 123.1, 113.6 (q, J = 33.9 Hz), 81.3, 66.3, 61.2, 46.0, 44.9, 40.7, 28.4 (3C). HRMS (ESI) m/z: 636.2449 [M + Na]+; calcd for C36H34F3N3NaO3+, 636.2444.
Analytical data of other new compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans 7
trans 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 diastereoisomeric mixture) δ 7.64–7.11 (m, 16H), 7.01 (t, br, J = 7.8 Hz, 0.3H), 6.98 (t, br, J = 7.8 Hz, 0.7H), 6.65 (d, J = 7.8 Hz, 0.7H), 6.61 (d, J = 7.8 Hz, 0.3H), 5.27–5.02 (m, 2H), 4.81 (m, 0.3H), 4.77 (dd, J = 10.7 and 3.9 Hz, 0.7H), 4.66 (d, br, J = 15.6 Hz, 0.3H), 4.46 (d, br, J = 15.6 Hz, 0.7H), 3.69 (dd, J = 13.7 and 3.9 Hz, 0.7H), 2.86 (dd, J = 13.7 and 10.7 Hz, 0.7H), 2.74–2.56 (m, 0.6H), 1.57 (s, 6.3H), 1.55 (s, 0.9H), 1.50 (s, 0.9H), 1.38 (s, 2.1H), 1.31 (s, 2.7H), 1.25 (s, 2.1H). 13C NMR (75 MHz, CDCl3) δ 176.5 and 169.6 (1C), 157.8 and 156.0 (1C), 144.6 and 143.3 (1C), 140.8, 139.1, 137.7 and 136.7 (1C), 135.8 and 123.1 (2C), 129.8–125.2 (xC), 122.6 and 121.9 (1C), 109.3 and 109.0 (1C), 81.1 and 80.4 (1C), 73.2 and 69.4 (1C), 67.0, 49.3, 43.7 and 43.6 (1C), 36.4 and 34.1 (1C), 28.5 and 28.1 (3C), 25.9 and 20.4 (1C), 22.2 and 18.0 (1C). HRMS (ESI) m/z: 596.2888 [M + Na]+; calcd for C37H39N3NaO3+, 596.2884.
3 diastereoisomeric mixture) δ 7.64–7.11 (m, 16H), 7.01 (t, br, J = 7.8 Hz, 0.3H), 6.98 (t, br, J = 7.8 Hz, 0.7H), 6.65 (d, J = 7.8 Hz, 0.7H), 6.61 (d, J = 7.8 Hz, 0.3H), 5.27–5.02 (m, 2H), 4.81 (m, 0.3H), 4.77 (dd, J = 10.7 and 3.9 Hz, 0.7H), 4.66 (d, br, J = 15.6 Hz, 0.3H), 4.46 (d, br, J = 15.6 Hz, 0.7H), 3.69 (dd, J = 13.7 and 3.9 Hz, 0.7H), 2.86 (dd, J = 13.7 and 10.7 Hz, 0.7H), 2.74–2.56 (m, 0.6H), 1.57 (s, 6.3H), 1.55 (s, 0.9H), 1.50 (s, 0.9H), 1.38 (s, 2.1H), 1.31 (s, 2.7H), 1.25 (s, 2.1H). 13C NMR (75 MHz, CDCl3) δ 176.5 and 169.6 (1C), 157.8 and 156.0 (1C), 144.6 and 143.3 (1C), 140.8, 139.1, 137.7 and 136.7 (1C), 135.8 and 123.1 (2C), 129.8–125.2 (xC), 122.6 and 121.9 (1C), 109.3 and 109.0 (1C), 81.1 and 80.4 (1C), 73.2 and 69.4 (1C), 67.0, 49.3, 43.7 and 43.6 (1C), 36.4 and 34.1 (1C), 28.5 and 28.1 (3C), 25.9 and 20.4 (1C), 22.2 and 18.0 (1C). HRMS (ESI) m/z: 596.2888 [M + Na]+; calcd for C37H39N3NaO3+, 596.2884.Conflicts of interest
The authors declare no competing financial interest.References
- L. M. Blair and J. Sperry, Natural Products Containing a Nitrogen–Nitrogen Bond, J. Nat. Prod., 2013, 76, 794–812 CrossRef CAS PubMed.
- M. S. Ali, F. Ahmad, V. U. Ahmad, I. Azhar and K. Usmanghani, Unusual Chemical Constituents of Lotus garcinia (Fabaceae), Turk. J. Chem., 2001, 25, 107–112 CAS.
- D. Secci, A. Bolasco, P. Chimenti and S. Carradori, The State of the Art of Pyrazole Derivatives as Monoamine Oxidase Inhibitors and Antidepressant/Anticonvulsant Agents, Curr. Med. Chem., 2011, 18, 5114–5144 CrossRef CAS PubMed.
- B. Parashar, A. Jain, S. Bharadwaj and V. K. Sharma, Synthesis and pharmacological properties of some novel pyrazolidine and pyrazole derivatives, Med. Chem. Res., 2012, 21, 1692–1699 CrossRef CAS.
- E. Frank, Z. Mucsi, I. Zupko, B. Rethy, G. Falkay, G. Schneider and J. Wolfling, Efficient Approach to Androstene-Fused Arylpyrazolines as Potent Antiproliferative Agents. Experimental and Theoretical Studies of Substituent Effects on BF3-Catalyzed Intramolecular [3+2] Cycloadditions of Olefinic Phenylhydrazones, J. Am. Chem. Soc., 2009, 131, 3894–3904 CrossRef CAS PubMed.
- M. J. Kornet and R. J. Garrett, Synthesis of 1-phenyl-2-(phenylcarbamoyl)pyrazolidines as potential anticonvulsant agents, J. Pharm. Sci., 1979, 68, 377–378 CrossRef CAS PubMed.
- (a) J. H. Ahn, J. A. Kim, H. M. Kim, H. M. Kwon, S. C. Huh, S. D. Rhee, K. R. Kim, S. D. Yang, S. D. Park, J. M. Lee, S. S. Kim and H. G. Cheon, Synthesis and evaluation of pyrazolidine derivatives as dipeptidyl peptidase IV (DP-IV) inhibitors, Bioorg. Med. Chem. Lett., 2005, 15, 1337–1340 CrossRef CAS PubMed; (b) H. G. Cheon, S. S. Kim, K. R. Kim, S. D. Rhee, S. D. Yang, J. H. Ahn, S. D. Park, J. M. Lee, W. H. Jung, H. S. Lee and H. Y. Kim, Inhibition of dipeptidyl peptidase IV by novel inhibitors with pyrazolidine scaffold, Biochem. Pharmacol., 2005, 70, 22–29 CrossRef CAS PubMed.
- G. X. Yang, L. L. Chang, Q. Truong, G. A. Doherty, P. A. Magriotis, S. E. de Laszlo, B. Li, M. MacCoss, U. Kidambi, L. A. Egger, E. McCauley, G. V. Riper, R. A. Mumford, J. A. Schmidt and W. K. Hagmann, N-Tetrahydrofuroyl-(L)-phenylalanine derivatives as potent VLA-4 antagonists, Bioorg. Med. Chem. Lett., 2002, 12, 1497–1500 CrossRef CAS PubMed.
- S. H. Ahmed, H. Ahmed, S. H. Ali, S. Fatima, S. Hyder and A. Pasha, Synthesis and anti-bacterial activity of some novel pyrazolidinedione substituted derivatives of 2-quinolones, Indo Am. J. Pharm. Res., 2017, 7, 801–823 CAS.
- K. A. Koo, N. D. Kim, Y. S. Chon, M. S. Jung, B. J. Lee, J. H. Kim and W. J. Song, QSAR analysis of pyrazolidine-3,5-diones derivatives as Dyrk1A inhibitors, Bioorg. Med. Chem. Lett., 2009, 19, 2324–2328 CrossRef CAS PubMed.
- K. Singh, R. S. N. Munuganti, E. Leblanc, Y. L. Lin, E. Leung, N. Lallous, M. Butler, A. Cherkasov and P. S. Rennie, In silico discovery and validation of potent small-molecule inhibitors targeting the activation function 2 site of human oestrogen receptor α, Breast Cancer Res., 2015, 17, 1–17 CrossRef CAS PubMed.
- J. Velcicky, U. Bodendorf, P. Rigollier, R. Epple, D. R. Beisner and D. Guerini, et al., Discovery of the First Potent, Selective, and Orally Bioavailable Signal Peptide Peptidase-Like 2a (SPPL2a) Inhibitor Displaying Pronounced Immunomodulatory Effects In Vivo, J. Med. Chem., 2018, 61, 865–880 CrossRef CAS PubMed.
- Y. Yamashita and S. Kobayashi, Zirconium-Catalyzed Enantioselective [3+2] Cycloaddition of Hydrazones to Olefins Leading to Optically Active Pyrazolidine, Pyrazoline, and 1,3-Diamine Derivatives, J. Am. Chem. Soc., 2004, 126, 11279–11282 CrossRef CAS PubMed.
- (a) F. Požgan, H. AlMamari, U. Grošelj, J. Svete and B. Štefane, Synthesis of Non-Racemic Pyrazolines and Pyrazolidines by [3+2] Cycloadditions of Azomethine Imines, Molecules, 2018, 23, 3 CrossRef PubMed; (b) U. Grošelj, J. Svete, H. Al Mamari, F. Požgan and B. Štefane, Metal-catalyzed [3+2] cycloadditions of azomethine imines, Chem. Heterocycl. Compd., 2018, 54, 214–240 CrossRef.
- (a) B. R. Rosen, J. E. Ney and J. P. Wolfe, Use of Aryl Chlorides as Electrophiles in Pd-Catalyzed Alkene Difunctionalization Reactions, J. Org. Chem., 2010, 75, 2756–2759 CrossRef CAS PubMed; (b) J. P. Wolfe and N. C. Giampietro, Stereoselective Synthesis of cis- or trans-3,5-Disubstituted Pyrazolidines via Pd-Catalyzed Carboamination Reactions: Use of Allylic Strain to Control Product Stereochemistry Through N-Substituent Manipulation, J. Am. Chem. Soc., 2008, 130, 12907–12911 CrossRef PubMed.
- L. O. Davis, Recent Developments in the Synthesis and Applications of Pyrazolidines. A Review, Org. Prep. Proced. Int., 2013, 45, 437–464 CrossRef CAS.
- (a) G. Rainoldi, F. Begnini, M. de Munnik, L. Lo Presti, C. M. L. Vande Velde, R. Orru, G. Lesma, E. Ruijter and A. Silvani, Sequential Multicomponent Strategy for the Diastereoselective Synthesis of Densely Functionalized Spirooxindole-Fused Thiazolidines, ACS Comb. Sci., 2018, 20, 98–105 CrossRef CAS PubMed; (b) G. Rainoldi, M. Faltracco, C. Spatti, A. Silvani and G. Lesma, Organocatalytic Access to Enantioenriched Spirooxindole-Based 4-Methyleneazetidines, Molecules, 2017, 22, 2016 CrossRef PubMed; (c) G. Rainoldi, M. Faltracco, L. Lo Presti, A. Silvani and G. Lesma, Highly diastereoselective entry into chiral spirooxindole-based 4-methyleneazetidines via formal [2 + 2] annulation reaction, Chem. Commun., 2016, 52, 11575–11578 RSC; (d) G. Rainoldi, F. Begnini, A. Silvani and G. Lesma, Efficient Synthesis of Spirooxindole-fused 3-Thiazoline Derivatives by a One-Pot Asinger-Type Reaction, Synlett, 2016, 27, 2831–2835 CrossRef CAS; (e) G. Rainoldi, A. Sacchetti, A. Silvani and G. Lesma, Organocatalytic vinylogous Mannich reaction of trimethylsiloxyfuran with isatin-derived benzhydryl-ketimines, Org. Biomol. Chem., 2016, 14, 7768–7776 RSC; (f) G. Lesma, F. Meneghetti, A. Sadcchetti, M. Stucchi and A. Silvani, Asymmetric Ugi 3CR on isatin-derived ketimine: synthesis of chiral 3,3-disubstituted 3-aminooxindole derivatives, Beilstein J. Org. Chem., 2014, 10, 1383–1389 CrossRef PubMed; (g) G. Lesma, N. Landoni, T. Pilati, A. Sacchetti and A. Silvani, Grignard Addition to Imines Derived from Isatine: A Method for the Asymmetric Synthesis of Quaternary 3-Aminooxindoles, J. Org. Chem., 2009, 74, 4537–4541 CrossRef CAS PubMed.
- Design of Hybrid Molecules for Drug Development. ed. M. Decker, Elsevier Ed, 2017 Search PubMed.
- (a) G.-J. Mei and F. Shi, Catalytic asymmetric synthesis of spirooxindoles: recent developments, Chem. Commun., 2018, 54, 6607–6621 RSC; (b) G. Meller, T. Berkenbosch, J. C. J. Benningshof, D. Stumpfe and J. Bajorath, Charting Biologically Relevant Spirocyclic Compound Space, Chem.–Eur. J., 2017, 23, 703–71 CrossRef PubMed; (c) V. Pace, L. Castoldi, A. D. Mamuye, T. Langer and W. Holzera, Chemoselective Addition of Halomethyllithiums to Functionalized Isatins: A Straightforward Access to Spiro-Epoxyoxindoles, Adv. Synth. Catal., 2016, 358, 172–177 CrossRef CAS.
- B. Wagner, W. Hiller, H. Ohno and N. Krause, Gold-catalyzed three-component spirocyclization: a one-pot approach to functionalized pyrazolidines, Org. Biomol. Chem., 2016, 14, 1579–1583 RSC.
- (a) N. A. Aslam, S. A. Babu, S. Rani, S. Mahajan, J. Solanki, M. Yasuda and A. Baba, Diastereoselective Construction of 3-Aminooxindoles with Adjacent Stereocenters: Stereocontrolled Addition of γ-Substituted Allylindiums to Isatin Ketimines, Eur. J. Org. Chem., 2015, 4168–4189 CrossRef CAS; (b) B. Alcaide, P. Almendros and C. Aragoncillo, Indium-Promoted Allylation Reaction of Imino-Isatins in Aqueous Media: Synthesis of Quaternary 3-Aminooxindoles, Eur. J. Org. Chem., 2010, 2845–2848 CrossRef CAS.
- P. D. Chaudhari, B. C. Hong and G. H. Lee, Organocatalytic Enantioselective Michael-Michael-Michael-Aldol Condensation Reactions: Control of Six Stereocenters in a Quadruple-Cascade Asymmetric Synthesis of Polysubstituted Spirocyclic Oxindoles, Org. Lett., 2017, 19, 6112–6115 CrossRef CAS PubMed.
- D. Ganggang, H. Danfeng, W. Ke-Hu, C. Xiaowei, X. Yanli, M. Junyan, S. Yingpeng, F. Ying and H. Yulai, One-pot preparation of trifluoromethylated homoallylic N-acylhydrazines or α-methylene-γ-lactams from acylhydrazines, trifluoroacetaldehyde methyl hemiacetal, allyl bromide and tin, Org. Biomol. Chem., 2016, 14, 1492–1500 RSC.
- J. E. Thomson, A. F. Kyle, K. B. Ling, S. R. Smith, A. M. Z. Slawin and A. D. Smith, Applications of NHC-mediated O- to C-carboxyl transfer: synthesis of (±)-N-benzyl-coerulescine and (±)-horsfiline, Tetrahedron, 2010, 66, 3801–3813 CrossRef CAS.
- L. Firmansjah and G. C. Fu, Intramolecular Heck Reactions of Unactivated Alkyl Halides, J. Am. Chem. Soc., 2007, 129, 11340–11341 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all new compounds; 1H–1H ROESY NMR for compounds 6a and 6a′; full discussion of the crystallographic results, including information on crystal packing, for compound 6f′ (PDF). CCDC 1938731. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra07712j |
| This journal is © The Royal Society of Chemistry 2019 |