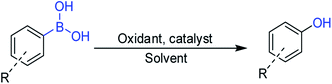
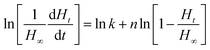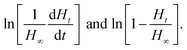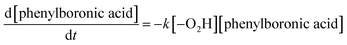Open Access Article
Open Access ArticleCatalyst- and solvent-free ipso-hydroxylation of arylboronic acids to phenols†
Xiufang Yangab,
Xulu Jianga,
Weitao Wang *ab,
Qi Yang
*ab,
Qi Yang c,
Yangmin Maab and
Kuan Wang*ab
c,
Yangmin Maab and
Kuan Wang*ab
aCollege of Chemistry & Chemical Engineering, Shaanxi University of Science & Technology, Xi'an, Shaanxi 710021, China. E-mail: wwt1806@163.com; wangkuan@sust.edu.cn
bShaanxi Key Laboratory of Chemical Additives for Industry, Shaanxi University of Science & Technology, Xi'an, Shaanxi 710021, China
cCollege of Chemistry and Materials Science, Northwest University, Xi'an, Shaanxi 710127, China
First published on 25th October 2019
Abstract
A catalyst-free method for the hydroxylation of arylboronic acids to form the corresponding phenols with sodium perborate as the oxidant was developed using water as the solvent. Under the reaction conditions, the yield of phenol reached 92% at only 5 min. Moreover, the reaction could be conducted without a catalyst under the solvent-free condition, the efficiency of which was as high as that of a liquid-phase reaction. Using a microcalorimeter, the reaction was found to be an exothermic reaction. The reaction mechanism was investigated and understood via DFT calculations, which revealed that it was a nucleophilic reaction.
Introduction
Phenol and its derivatives are important compounds used in industries, such as pharmaceuticals, agrochemicals, polymers, and natural antioxidants.1,2 Due to the important applications of phenols, a number of methods have been developed for the synthesis of phenol and its derivatives. Traditionally, phenol syntheses involves the hydroxylation of aryl halides using hydroxide salts,3 pyrolysis of sodium salts of benzene sulfonic acid,4 oxidation of cumene,5 hydrolysis of diazonium salts,6 and direct hydroxylation of benzene,7 which need harsh reaction conditions. Therefore, exploring new greener methodologies for the synthesis of phenol derivatives with better efficiency and less waste generation is an attractive research direction.Arylboronic acids are explored as a new source for the synthesis of phenol derivatives due to their easy availability and stability, and the synthetic route is known as the ipso-hydroxylation of arylboronic acids that occurs via C–B bond cleavage. An oxidant, such as O2,8 H2O2,9 NaClO2,10 NH2OH,11 (NH4)2S2O812 N-oxides,13 is essential for the ipso-hydroxylation reaction (Scheme 1). Catalysts, such as noble metal complexes,14 transition metal oxides,9,15 and noble metal catalysts,16 are often employed in the reaction system besides a base additive, such as NaOH17 and NaCO3.18 Moreover, some organic solvents, such as CH3OH,19 CH3CH2OH,18 CHCl3,20 DMF,21 THF,22 are used as the reaction solvents. The concept of green chemistry motivates researchers to develop a more efficient and cost-effective reaction system with environment benign solvents or a solvent-free reaction system for the ipso-hydroxylation of arylboronic acids to phenol and its derivatives via C–B bond cleavage.
Catalyst-free ipso-hydroxylation reaction has gained attraction in this regard. PEG-400,23 lactic acid,24 WERSA (Water Extract of Rice Straw Ashes)25 and dimethyl carbonate26 have been employed as reaction media for the catalyst-free ipso-hydroxylation reaction with H2O2 as the oxidant. However, the storage and transportation of H2O2 need additional safety measures. Hydrogen peroxide–solid adducts have received considerable attention in oxidation chemistry due to their storage stability, ready availability, and low cost.19,27 Sodium perborate (SPB), one such solid adducts, is an inexpensive large-scale industrial oxidant widely used in washing powder and bleaching. Moreover, the borate in SPB can help buffer, stabilize against the decomposition of H2O2 and activate nucleophilic oxidation.28 SPB has been widely used as a green oxidizing agent for organic oxidation reactions.29–32 It was employed as the oxidant in the oxidation of organoboranes and gave satisfying yields.33–35 Inspired by these, we expected SPB to act as an oxidant in the catalyst-free ipso-hydroxylation of aryl boronic acids to phenols through the release of nucleophilic species.
Herein, we have reported a new methodology for quick and efficient synthesis of phenol derivatives with arylboronic acids and sodium perborate. The reaction could be conducted under a catalyst-free condition, as well as a solvent-free condition, at room temperature. The ΔH was determined by microcalorimetry, as well as thermokinetics analysis. In addition, the mechanism was revealed using DFT calculations.
Experimental section
Materials
Commercial reagents were used as received without additional purification.Characterization
The X-ray diffraction (XRD) patterns were obtained by an X-ray diffractometer (Rigaku IV) operated with Cu-Kα radiation at 40 kV and 40 mA, the scanning mode of 2 theta/theta, the scanning type of continuous scanning, and a scanning range from 3° to 90° at a scanning rate of 8° min−1. 1H NMR (400 MHz) was recorded with a Bruker spectrometer (ADVANCE III). The calorimetric experiment was performed in a Tian–Calvet type differential microcalorimeter Setaram C80 at a constant temperature of 20.00 °C. The phenylboronic acid solvent (60 mg, 1.0 mL H2O) was placed in a stainless steel sample cell. When it reached equilibrium, a container with sodium perborate (77 mg, 1.0 mL H2O) was pushed down . As a result, the solvents were mixed at 298.15 K, and the heat flow of the reaction was recorded with the increase of time.Catalyst-free ipso-hydroxylation of phenylboronic acid to phenol
Typically, in a 50 mL round-bottomed flask, a mixture of phenylboronic acid (5 mmol), Na2BO3·4H2O (5 mmol), and 10 mL of water was stirred at room temperature under an anaerobic condition. After the completion of the reaction, the reaction mixture was acidified with HCl solution. The solution was diluted to 25 mL in a volumetric flask. The concentration of phenol yielded in the solution was measured by high performance liquid chromatography (HPLC, WAYEE LC 3000-2 Series instrument), which calculated the HPLC yield of phenols.Catalyst-free ipso-hydroxylation of arylboronic acids to phenol derivatives
The synthesis procedure was the same as that for the ipso-hydroxylation of phenylboronic acid to phenol, except the substrate amounts were changed. Arylboronic acid (1 mmol) and SPB (2 mmol) were employed. The reaction was monitored by thin layer chromatography (TLC) using plates precoated with silica gel 60 GF-254. After the completion of the reaction, the reaction mixture was acidified with HCl solution. Then 30 mL diethylether was added and extracted with (3 × 50) mL of saturated ammonium chloride solution. The organic layer was dried over anhydrous Na2SO4. The solvent was removed in a rotary evaporator under reduced pressure. The crude product was purified by column chromatography (hexane/ethylacetate, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica (100–200 mesh) to get the desired product. The products were identified by 1H NMR.
1) on silica (100–200 mesh) to get the desired product. The products were identified by 1H NMR.
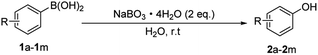
Catalyst- and solvent-free ipso-hydroxylation of arylboronic acids to phenol derivatives
The above reaction could be conducted under a catalyst- and solvent-free (solid state condition) reaction condition. Typically, arylboronic acid (1 mmol) and SPB (2 mmol) were added into a mortar and ground for 10 min. After the reaction, the solid mixture was dissolved in 5 mL H2O and then acidified. The following steps were the same as those in the ipso-hydroxylation of arylboronic acid to phenol derivatives.DFT computations
DFT computations were used to verify the proposed mechanism pathway. Geometry optimization and frequency analysis were performed in a water solvent with the conductor-like polarizable continuum model (CPCM)36,37 using M06-2X/6-311+G(2d,p). Intrinsic reaction coordinate (IRC) computations validated the connections between the reactants, transition states, and products. All calculations were performed in Gaussian 09,38 and the images of the optimized structures were generated and displayed using the CYLview software.39Results and discussion
Commercial phenylboronic acid and SPB were employed for ipso-hydroxylation reactions in various solvents and solvent-free reaction conditions. As shown in Table 1, the reaction could proceed in both protic and aprotic solvents, but the yield of phenol tended to vary with different solvents. Among the aprotic solvents, it was found that the yield of phenol was higher with tetrahydrofuran, acetone, and ethyl acetate than with acetonitrile (Table 1, entry 4–7). This indicated that solvents with large electron density atoms tended to favor the reaction. Therefore, it was postulated that the nucleophilicity of the solvent might affect the reaction. The protic solvents tended to be more efficient in the reaction (Table 1, entry 1–3). However, too many protons provided by inorganic acids disfavored the reaction (Table 1, entry 8). When NaHCO3 was employed as an additive in the reaction, the yield of phenol was not affected. This is because the SPB aqueous solution was basic with a pH of about 10.1.40 Therefore, the reaction did not require further base addition.| Entry | Solvents | Yieldb (%) |
|---|---|---|
| a Reaction conditions: phenylboronic acid, 5 mmol; SPB, 5 mmol; solvent, 5 mL; room temperature; 5 min.b HPLC yield of phenol.c With the addition of HCl aqueous solution.d With the addition of NaHCO3 as the base.e Solid phase reaction, grinding in mortar at room temperature for 5 min. | ||
| 1 | CH3OH | 91 |
| 2 | CH3CH2OH | 84 |
| 3 | H2O | 92 |
| 4 | CH3CN | 23 |
| 5 | THF | 77 |
| 6 | Acetone | 67 |
| 7 | Ethyl acetate | 72 |
| 8c | H2O | 62 |
| 9d | H2O | 92 |
| 10e | Solvent-free | 95 |
A white solid was found in the organic solvent when the reaction completed. After the reaction mixture was acidified, the white solid was dissolved, collected and identified as NaCl and H3BO3 by XRD (Fig. 1). These indicated that the white solid was sodium borate, which was easy to be separated in the solid form (Scheme S1†). Therefore, the reaction process was green from the reaction materials to the product. Moreover, it was interesting to find that the reaction could be carried out in the solid phase by just grinding the reactants at room temperature, which gave a yield as high as that of the liquid phase reaction (Table 1, entry 10).
With the optimized solvents in hand, the reaction condition was studied. The reaction could proceed from 0 °C to 35 °C with minimal changes in the yield (Table 2, entry 1–3). In this reaction system, the reaction was highly efficient with the yield of phenol as high as 92% at just 5 min. On prolonging the reaction time (Table 2, entry 2, 4 and 5), the yield did not vary, which indicated that the reaction was completed within 5 min. These implied that the reaction was kinetically favored. The solubility of phenylboronic acid was not good in water. However, it totally dissolved as the reaction proceeded. With an increase in the amount of SPB (Table 2, entry 2, 6–8), the yield of phenol increased. When the amount of SPB was 1.2 equivalent, the yield of phenol could reach 98%, which was higher than that obtained with 1.0 equivalent SPB. This indicated that the efficiency of SPB was not 100%.
To realize the reaction finish time, we employed microcalorimetry to monitor the reaction online (Fig. 2). The reaction started when the two liquids were mixed at 3.57500 h. At 3.64236 h, the exothermic maximum was reached. The result revealed that the reaction finished within 4.04 min. As shown in Fig. 2, the first exothermic peak was integrated to obtain the reaction heat ΔH of −71.7 kJ mol−1 at 298.15 K. The relatively high ΔH indicated that the reaction was thermodynamically favored. The second broad peak was ascribed to the reaction between the salts of different boron compounds since the yield of phenol did not change with time after the 5 minutes of reaction time.
The reaction order and rate constant were obtained by the thermokinetic equation:41,42
 is the heat production at time t. The linear relationship between
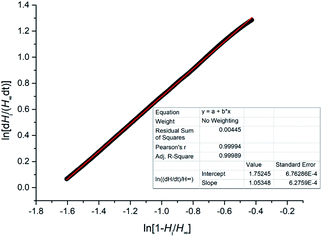
is the heat production at time t. The linear relationship between  is shown in Fig. 3. The reaction order was 1 (n = 1.05).
is shown in Fig. 3. The reaction order was 1 (n = 1.05).
The generality of this methodology was tested with different substituted arylboronic acids (Table 3). Despite the insolubility of the arylboronic acid substrates in water, the ipso-hydroxylation reactions could proceed with water as the solvent. Arylboronic acids with methyl and methoxyl substituents at ortho-, meta- and para-positions afforded the corresponding phenols with 81–87% yield (2a–2f). Halogen substituted phenols (2h–2j) were obtained with satisfactory yields. Substituents, such as nitrile, t-Bu, and i-Pr, also gave the desired products with excellent yield (2g, 2k–2m). In general, arylboronic acids with either electron-withdrawing or electron-donating substituents underwent the ipso-hydroxylation reaction, resulting in satisfactory yields. Interestingly, the reaction could be conducted in the solid phase (solvent-free) with only the reactants, namely arylboronic acids and SPB. The yields of the corresponding phenols from the solvent-free reaction condition were as high as the yields from the reactions in the water solvent (Table 3).
| Entry | Substrates | R | Products | Yieldb (%) | Yieldb,c (%) |
|---|---|---|---|---|---|
| a Reaction conditions: arylboronic acid, 1 mmol; SPB, 2 mmol; solvent, water 4 mL; room temperature; reaction time, 10 min.b Isolated yield.c Yield from solid phase reaction by grinding reactants in mortar at room temperature for 10 min.d Reaction time was 20 min. | |||||
| 1 | 1a | 2-Me |  |
87 | 84 |
| 2 | 1b | 3-Me |  |
82 | 98 |
| 3 | 1c | 4-Me |  |
82 | 80 |
| 4 | 1d | 2-OMe |  |
81 | 81 |
| 5 | 1e | 3-OMe |  |
82 | 86 |
| 6 | 1f | 4-OMe |  |
86 | 78 |
| 7 | 1g | 3-NO2 |  |
89 | 80d |
| 8 | 1h | 4-F |  |
80 | 81d |
| 9 | 1i | 4-Cl |  |
80 | 81d |
| 10 | 1j | 4-Br |  |
83 | 88d |
| 11 | 1k | 4-CN |  |
80 | 91 |
| 12 | 1l | 4-t-Bu |  |
80d | 77 |
| 13 | 1m | 4-i-Pr |  |
88d | 74 |
To reveal the reaction mechanism, radical scavengers were added in the reaction system to testify whether the reaction was a radical reaction (Table 4). When different radical scavengers were added into the reaction system, the yield of phenol did not change. This indicated that the reaction was not radical-involved.
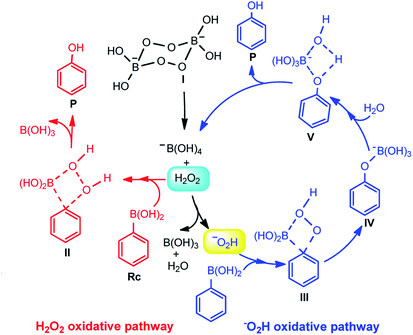
It is widely accepted that SPB can release H2O2 and sodium borate in dilute aqueous solutions, followed by an equilibrium state27 (Scheme 2). At alkaline pH, hydrogen peroxide or the perhydroxyl anion (HO2−) is responsible for the oxidization activity. At low pH, H2O2 is the main species, while the HO2− species dominant at high pH.40 The pH of the SPB aqueous solution was about 10.1, which indicated that the reaction had taken place by the nucleophilic attack of HO2−, which has very high nucleophilicity.
The mechanism underlying the ipso-hydroxylation of arylboronic acids with SPB was postulated, as shown in Scheme 3. At alkaline pH, hydrogen peroxide (H2O2) or the perhydroxyl anion (–O2H) is responsible for the oxidization activity. The possible mechanisms proposed are the H2O2 oxidative pathway or the –O2H oxidative pathway, which were analyzed via density functional theory (DFT) calculations. All geometry optimizations were performed in the water solvent with the conductor-like polarizable continuum model (CPCM) using the M06-2X functional with a basis set of 6-311+G(2d,p). A schematic depiction of the two pathways is shown in Scheme 3. Meanwhile, the optimized geometries and the corresponding relative energies are shown in Fig. 4 and S1.†
 | ||
| Fig. 4 DFT computed schematic energy diagram and the corresponding optimized geometries in the –O2H oxidative pathway. | ||
In the H2O2 oxidative pathway, phenylboronic acid (Rc) and H2O2 approach each other to reach a transition state TSH-1 with a very high activation free energy (ΔG≠) of 76.7 kcal mol−1 (Fig. S1†). Once the barrier is conquered, the hydroxylation product (P) is finally generated with an exergonicity of 94.7 kcal mol−1. However, the unusually high barrier indicates that the oxidation reaction of Rc with H2O2 is unlikely to occur.
In organoboronic acids, the boron atoms adopt sp2 hybridization.16 As shown in Fig. 4, when the nucleophile –O2H approaches the boron atom in Rc, a boron “ate” complex is generated with rehybridization to form sp3 boron (Int-1) in the –O2H oxidative pathway. Starting with Int-1, the C–B bond is dissociated due to the high electron density on the boron, followed by aryl migration to the adjacent acceptor atom of oxygen with unchanged configuration to generate intermediate Int-2. The ΔG≠ in this step (Int-1 → Int-2) is 28.8 kcal mol−1 via a transition state TS-1. Next, the C–B bond in Int-2 is elongated to form intermediate Int-3 via a transition state TS-2 with a ΔG≠ of 25.1 kcal mol−1. With an H2O molecule approaching Int-3, the hydrolysis step occurs from intermediate Int-4 through TS-3 to form the P⋯−B(OH)4 complex (Int-5) (ΔG≠ = 8.8 kcal mol−1). Consequently, the target product P is released with 104.1 kcal mol−1 relative to the zero-point surface of exergonicity.
The calculated ΔG≠ of the rate-limiting step in the –O2H oxidative pathway (Int-1 → Int-2) is much lower than that in the H2O2 oxidative pathway (IntH-1 → IntH-2). Therefore, the oxidation reaction of arylboronic acids through –O2H is more favourable both kinetically and thermodynamically, which is in very good agreement with the experimental observations. From the mechanism, it can be found that too much H+ would lead to low pH, which would result in the low concentration of –O2H and a relatively low yield of phenol. From Scheme 3, the reaction rate could be expressed as the following equation, according to the rate-determination-step approximation:
The phenylboronic acid reaction was a first order reaction, which was consistent with the thermokinetics analysis.
Conclusions
A convenient and safe method for the ipso-hydroxylation of arylboronic acids to corresponding phenols was established with SPB as the oxidant. With water as the solvent and without solvents, the yields of phenols were satisfactory. Moreover, the reaction was both thermodynamically and kinetically favored. The mechanism of the reaction was found to be nucleophilic attack in the presence of –O2H.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank The Project Supported by Natural Science Basic Research Plan in Shaanxi Province of China (No. 2019JM-080), Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 18JK0117), the National Natural Science Foundation of China (No. 21673181) for financial support.Notes and references
- Y. Zhou, Z. Ma, J. Tang, N. Yan, Y. Du, S. Xi, K. Wang, W. Zhang, H. Wen and J. Wang, Nat. Commun., 2018, 9, 2931 CrossRef.
- W. Wang, N. Li, L. Shi, Y. Ma and X. Yang, Appl. Catal., A, 2018, 553, 117–125 CrossRef CAS.
- A. Tlili, N. Xia, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2010, 48, 8725–8728 CrossRef.
- M. C. Boswell and J. V. Dickson, J. Am. Chem. Soc., 1918, 40, 1786–1793 CrossRef CAS.
- V. M. Zakoshansky, Pet. Chem., 2007, 47, 273–284 CrossRef.
- J. P. Lambooy, J. Am. Chem. Soc., 1950, 72, 5327–5328 CrossRef CAS.
- W. Wang, N. Li, H. Tang, Y. Ma and X. Yang, Mol. Catal., 2018, 453, 113–120 CrossRef CAS.
- I. Saikia, M. Hazarika, N. Hussian, M. R. Das and C. Tamuly, Tetrahedron Lett., 2017, 58, 4255–4259 CrossRef CAS.
- P. Shreemoyee, M. Abhijit and R. M. Harunar, Appl. Catal., A, 2018, 562, 58–66 CrossRef.
- P. Gogoi, P. Bezboruah, J. Gogoi and R. C. Boruah, Eur. J. Org. Chem., 2013, 7291–7294 CrossRef CAS.
- E. Kianmehr, M. Yahyaee and K. Tabatabai, Tetrahedron Lett., 2007, 48, 2713–2715 CrossRef CAS.
- C. A. Contreras-Celedón, L. Chacón-García and N. J. Lira-Corral, J. Chem., 2014, 5 Search PubMed.
- C. Zhu, R. Wang and J. R. Falck, Org. Lett., 2012, 14, 3494–3497 CrossRef CAS.
- Y. Zou, J. Chen, X. Liu, L. Lu, R. L. Davis, K. A. Jørgensen and W. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef CAS.
- R. Borah, E. Saikia, S. J. Bora and B. Chetia, Tetrahedron Lett., 2017, 58, 1211–1215 CrossRef CAS.
- C. Zhu and J. R. Falck, Adv. Synth. Catal., 2014, 356, 2395–2410 CrossRef CAS.
- S. Zhao and Z. Wang, Chin. J. Org. Chem., 2016, 32, 862–866 CrossRef.
- G. Silveira-Dorta, D. M. Monzón, F. P. Crisóstomo, T. Martín, V. S. Martín and R. Carrillo, Chem. Commun., 2015, 51, 7027–7030 RSC.
- S. Gupta, P. Chaudhary, V. Srivastava and J. Kandasamy, Tetrahedron Lett., 2016, 57, 2506–2510 CrossRef CAS.
- I. Kumar, R. Sharma, R. Kumar and U. Sharma, Adv. Synth. Catal., 2018, 360, 2013–2019 CrossRef CAS.
- J. M. Tobin, T. J. D. McCabe, A. W. Prentice, S. Holzer, G. O. Lloyd, M. J. Paterson, V. Arrighi, P. A. G. Cormack and F. Vilela, ACS Catal., 2017, 7, 4602–4612 CrossRef CAS.
- W. Ding, J.-R. Chen, Y.-Q. Zou, S.-W. Duan, L.-Q. Lu and W.-J. Xiao, Org. Chem. Front., 2014, 1, 151–154 RSC.
- M. Gohain, M. d. Plessis, J. H. v. Tonder and B. C. B. Bezuidenhoudt, Tetrahedron Lett., 2014, 55, 2082–2084 CrossRef CAS.
- S. Gupta, P. Chaudhary, L. Seva, S. Sabiah and J. Kandasamy, RSC Adv., 2015, 5, 89133–89138 RSC.
- E. Saikia, S. J. Bora and B. Chetia, RSC Adv., 2015, 5, 102723–102726 RSC.
- R. B. Wagh and J. M. Nagarkar, Tetrahedron Lett., 2017, 58, 4572–4575 CrossRef CAS.
- A. McKillop and W. R. Sanderson, Tetrahedron, 1995, 51, 6145–6166 CrossRef CAS.
- A. McKillop and W. R. Sanderson, J. Chem. Soc., Perkin Trans. 1, 2000, 4, 471–476 RSC.
- M. M. Hashemi, B. Eftekhari-Sis, B. Khalili and Z. Karimi-Jaberi, J. Braz. Chem. Soc., 2005, 16, 1082–1084 CrossRef CAS.
- M. V. Gómez, R. Caballero, E. Vázquez, A. Moreno, A. de la Hoz and Á. Díaz-Ortiz, Green Chem., 2007, 9, 331–336 RSC.
- A. Podgoršek, M. Zupan and J. Iskra, Angew. Chem., Int. Ed., 2009, 48, 8424–8450 CrossRef.
- L. T. Pilarski, P. G. Janson and K. J. Szabó, J. Org. Chem., 2011, 76, 1503–1506 CrossRef CAS.
- C. Sandford and V. K. Aggarwal, Chem. Commun., 2017, 53, 5481–5494 RSC.
- G. W. Kabalka, T. M. Shoup and N. M. Goudgaon, Tetrahedron Lett., 1989, 30, 1483–1486 CrossRef CAS.
- G. W. Kabalka, P. P. Wadgaonkar and T. M. Shoup, Organometallics, 1990, 9, 1316–1320 CrossRef CAS.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc.Wallingford, CT, 2010 Search PubMed.
- C. Y. Legault. CYLview, Canada, 2009, http://www.cylview.org Search PubMed.
- J. Burgess and C. D. Hubbard, Chapter Six-Catalysis or Convenience Perborate in Context, Advances in Inorganic Chemistry, Academic Press, 1st edn, 2013, vol. 65, pp. 217–310 Search PubMed.
- N. Li, F. Zhao, H. Gao, R. Hu, L. Xiao, E. Yao, X. Wang and P. Chang, Acta Phys.-Chim. Sin., 2013, 29, 2101–2106 CAS.
- L. Xiao, F. Zhao, X. Xing, H. Huang, Z. Zhou, T. An, Q. Pei and Y. Tan, Thermochim. Acta, 2012, 546, 138–142 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07201b |
| This journal is © The Royal Society of Chemistry 2019 |