Open Access Article
Open Access ArticleA bio-inspired synthesis of hybrid flavonoids from 2-hydroxychalcone driven by visible light†
Yu-Qi Gaoa,
Yi Houa,
Liming Zhub,
Guzhou Chena,
Dongyang Xua,
Sheng-Yong Zhang*c,
Yupeng He*b and
Weiqing Xie *ad
*ad
aShaanxi Key Laboratory of Natural Products & Chemical Biology, College of Chemistry & Pharmacy, Northwest A&F University, 22 Xinong Road, Yangling 712100, Shaanxi, China. E-mail: xiewq@nwafu.edu.cn
bCollege of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Dandong Lu West 1, Fushun 113001, China. E-mail: yupeng.he@lnpu.edu.cn
cDepartment of Chemistry, Fourth Military Medical University, Xi'an 710032, China. E-mail: syzhang@fmmu.edu.cn
dKey Laboratory of Botanical Pesticide R&D in Shaanxi Province, Yangling, Shaanxi 712100, China
First published on 16th September 2019
Abstract
A bio-inspired synthesis of hybrid flavonoids from 2-hydroxylchalcone is described. Under the irradiation of 24 W CFL, 2-hydroxychalcone reacts with various nucleophiles to deliver structurally diverse hybrid flavonoids in good to excellent yields in the presence of a catalytic Brønsted acid. Moreover, moderate enantioselectivities could be obtained using a catalytic chiral phosphoric acid via counter anion directed addition. Based on mechanistic studies, the reaction is proposed to proceed via tandem double-bond isomerization/dehydrated cyclization of 2-hydroxychalcone to form a transient flavylium cation, which is in situ captured by nucleophiles to afford hybrid flavonoids.
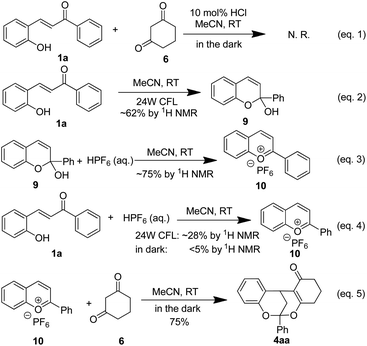
Nature elegantly utilizes hybridization of different natural products to construct structurally diverse natural products by merging different biosynthetic pathways.1 Inspired by nature's strategy, hybridization of natural products also provides new opportunities for the development of medicinal and agrichemical molecules.2 Hybrid flavonoids3 (Fig. 1, e.g. yuremamine4 and diinsininol5 A) are widely present in natural and pharmaceutical molecules, and exhibit wide biological activities including antitumor, antioxidant, antibacterial and anti-inflammatory properties. According to the biosynthetic pathway of flavonoids (Fig. 1, pathway 1),6 hybrid flavonoids (e.g. diinsininol5b) may be biogenetically generated via nucleophilic addition of flavanone IV to anthocyanidin salt II, which is derived from flavanol III catalyzed by anthocyanidin synthase. Flavanol III is in turn produced from chalcone 2 through a series of enzyme-promoted transformations. However, an alternative biosynthetic pathway has also been widely accepted, in which sunlight plays a pivotal role (Fig. 1, pathway 2),7 In this context, the photochemical interconversion of 2-hydroxychone 1 and flavylium salt II has been fully established, which reveals the equilibrium between those flavonoids in solution under the irradiation of UV or sunlight. As shown in Fig. 1, this process is initiated with the E/Z isomerization to deliver Z-enone I, which is transferred to flavylium cation II via dehydrative cyclization in the presence of Brønsted acid. The in situ capture of this flavylium cation has been proposed to constitute the key step for the biosynthesis of some type of hybrid flavonoids.7f
 | ||
| Fig. 1 Previous protocols for the synthesis of hybrid flavonoids and a bio-inspired synthesis of hybrid flavonoids form 2-hydrxoychalcone. | ||
Synthesis of hybrid flavonoids via nucleophilic addition to flavylium cation has been extensively explored,8 which relies on the high electrophilicity on the C4 of flavylium cation.9 However, those protocols usually need base to act as acid scavenger or heating in protic solvent. Furthermore, the flavylium cation is sensitive to light in solution.7 The other commonly employed strategy is the tandem Michael addition/dehydrated cyclization of 2-hydroxylchalcone 1 to deliver flavonoids 3 or polycyclic flavonoids 4.10 Different catalysts such as I2, TfOH and NbCl5 have been discovered to be effective for promoting this reaction. However, this strategy is limited by its harsh reaction conditions (heating) or need of precious metal catalysts. Drawn inspiration from the light-driven biosynthetic pathway of hybrid flavonoids (Fig. 1, pathway 3),6,7 we proposed that if a suitable nucleophile is exposed to 2-hydroxychalcone 1 in the presence of catalytic Brønsted acid under the irradiation of 24 CFL, hybrid flavonoids 3 or 4 could be biomimetically obtained via the in situ capture of the transient flavylium salt. Herein, we would like to describe our preliminary results on this bio-inspired synthesis of hybrid flavonoids driven by visible light.
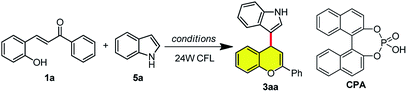
To verify our hypothesis, the reaction of hydroxyl chalcone 1a with indole 5a was initially examined to establish the optimal reaction conditions. To our delight, upon the irradiation of household 24 W compact fluorescent lamp (CFL) the desired flavonoid 3aa could be obtained in 73% yield in the presence of phosphoric acid CPA (Table 1, entry 1). Subsequent survey of different kind of Brønsted acids showed that biphenyl phosphate was the most efficient promoter, affording desired product in 98% yield (Table 1, entry 2–6 and ESI†). In sharp contrast, when the reaction was run in the absence of Brønsted acid or in the dark (Table 1, entry 7 and 8), no product could be detected, indicating that the light-driven formation of flavylium from 2-hydroxychalcone might be involved. Subsequently, survey of solvents revealed that THF was the optimal solvent, which gave comparable yield and showed better solubility for 2-hydroxylchalcone 1a (Table 1, entry 9 and ESI†).
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: to a mixture of hydroxyl chalcone 1a (0.1 mmol) and indole (0.12 mmol) was added solvent (3 mL). The reaction mixture was stirred with irradiation by household 24 W compact fluorescent lamp (CFL).b Isolated yields.c The reaction was carried out in the dark. | ||||
| 1 | CPA | MeCN | 18 | 73 |
| 2 | CSA | MeCN | 18 | 42 |
| 3 | HCl | MeCN | 18 | 40 |
| 4 | Biphenyl phosphate | MeCN | 30 | 98 |
| 5 | TsOH | MeCN | 42 | 45 |
| 6 | TFA | MeCN | 42 | 43 |
| 7 | — | MeCN | 42 | ND |
| 8c | Biphenyl phosphate | MeCN | 42 | ND |
| 9 | Biphenyl phosphate | THF | 19 | 98 |
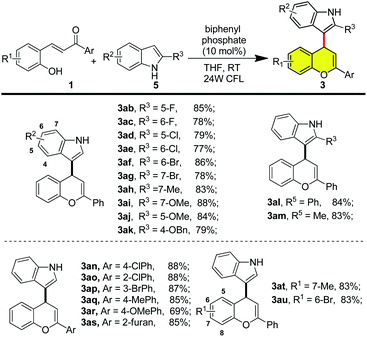
After establishing the optimal reaction conditions, the substrate scope was subsequently investigated. As shown in Scheme 1, electron-withdrawing group (e.g. F, Cl and Br) on the indole ring was compatible with the reaction conditions, providing corresponding products in good isolated yields (3ab to 3ag). Indoles with electron-donating groups (e.g. Me, methoxy) were also well tolerated, affording 3ah to 3ak in good yields. Substituents (e.g. phenyl and methyl) on the C-2 of indole did not affect the outcomes of this reaction (3al and 3am). Additionally, a variety of substituted 2-hydroxylchalcone was prepared and subjected to the standard reaction conditions. Satisfactorily, 2-hydroxylchalcones with electron-withdrawing groups (Cl, Br) and electron-donating group (Me, methoxy) could all be converted to hybrid flavonoids in good to excellent yields (3an to 3ar, 3at and 3au). Heterocycle such as furan was also compatible with the reaction conditions to afford flavonoid 3as in 85% yield.
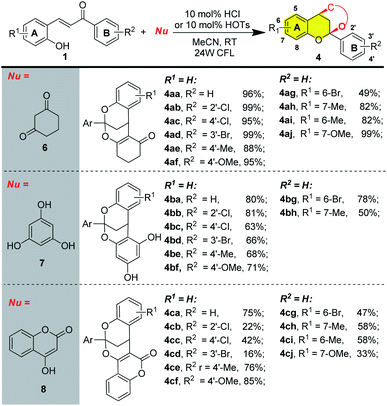
Furthermore, this protocol was also applicable to other type of bifunctional nucleophiles to give rise to polycyclic flavonoids resembling diinsininol. As listed in Scheme 2, cyclohexa-1,3-dione, phloroglucinol, 4-hydroxycoumarin could all engage in this type of reaction to give dioxabicyclo[3.3.1]nonane scaffold via tandem cycloisomerization/nucleophilic addition/cyclization process. For those substrates, employment of HCl (for cyclohexa-1,3-dione and 4-hydroxycoumarin) or TsOH (for phloroglucinol) was more beneficial (see ESI† for details). When cyclohexa-1,3-dione was employed as nucleophile, various substituents on the aromatic ring of 2-hydroxyl chalcone were well compatible excepting the Br on the aromatic ring A (4ag), delivering the desired product in good to excellent yields (Scheme 2, 4aa to 4aj). Phloroglucinol was also a suitable nucleophile for this reaction, generating polycyclic flavonoids in acceptable yields. Different type of substitutes were tolerated on the aromatic ring B of 2-hydryxoychalcone (Scheme 2, 4ba to 4bg), while the electron-donating groups on the aromatic ring A were unfavorable for this reaction, resulting in much lower yields (Scheme 2, 4bh). Finally, 4-hydroxycoumarin was evaluated as the nucleophile to give access to coumarin hybrid flavonoids. For this specific substrate, the yields depended on the substituents on the aromatic rings of 2-hydryxoychalcone. Electron-withdrawing groups on the aromatic ring B of 2-hydryxoychalcone were detrimental for reactions (4cb to 4cd), affording much lower isolated yields than those with electron-donating substituents (4ce and 4cf) and substituents on the ring A were not well compatible with the reaction conditions (4cg to 4cj).
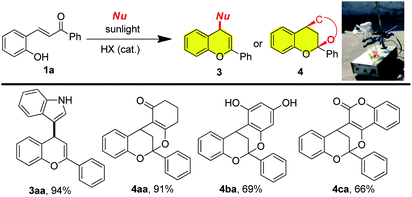
To mimic the biosynthetic conditions, the sunlight-driven coupling of 2-hydroxychalcone with different nucleophiles was also carried out. As shown in Scheme 3, indole, cyclohexa-1,3-dione, phloroglucinol and 4-hydroxycoumarin could all engage in the tandem process under the irradiation of sunlight using the same reaction conditions shown in Scheme 1 or 2, providing correspondent hybrid flavonoids 3aa to 4ca in comparable isolated yields with those using 24 W CFL as light source.
The enantioselective coupling of 2-hydroxychalcone was also explored using chiral phosphoric acid as organocatalyst via counter anion directed addition (Scheme 4).11 To our delight, flavonoid 3aa could be obtained in 99% yield with 45% ee employing catalytic 8H-(R)-TRIP. Additionally, the enantioselective coupling of cyclohexa-1,3-dione to 2-hydroxy chalcone 1a could also be realized using (R)-TRIP as catalyst albeit in 67% yield with 27% ee. When phloroglucinol was employed reaction partner, flavonoid 4ba could be obtained in 52% yield with 70% ee using CPA1 as promoter (Scheme 4). Those initial results paved a new way for the enantioselective synthesis of hybrid flavonoids via counter-anion directed enantioselective addition.11
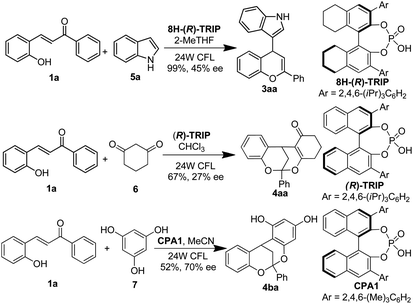 | ||
| Scheme 4 Chiral phosphoric acid catalyzed enantioselective addition of nucleophile to 2-hydroxychalcone. | ||
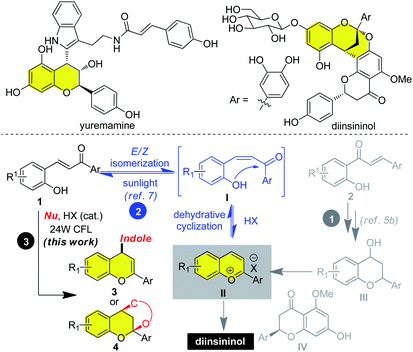
To gain in insights into the reaction pathway, a series of experimental were executed (Scheme 5). When the reaction was run in the dark, no product was detected with most of the starting material being recovered (Scheme 4, eqn (1)). The hemi-ketal 9 could be obtained in 62% yield when 2-hydroxylchalcone was irradiated by visible-light (Scheme 4, eqn (2)), which was in accordance with previous report.7 Treatment hemi-ketal 9 with stoichiometric amount of HPF6, flavylium salt 10 could be obtained in 62% yield (Scheme 4, eqn (3)). Additionally, when presence of stoichiometric amount of HPF6, 2-hydroxyl chalcone could also be converted to flavylium salt 10 in 28% yield. The low yield was ascribed to the instability of flavylium cation (Scheme 4, eqn (4)). In sharp contrast, only trace of product could be observed when this reaction was run in the dark. We also found that addition of cyclohexa-1,3-dione 6 to flavylium 10 salt could smoothly proceeded in the dark, affording the flavonoid 4aa in 75% yield (Scheme 4, eqn (5)). All these observations strongly supported the reaction proceeded through the tandem isomerization/cyclization/dehydration of 2-hydroxychalcone to produce a transient flavylium ion, which was in situ captured by the nucleophile to afford hybrid flavonoids (Fig. 1).
Conclusions
In conclusion, a bio-inspired synthesis of hybrid flavonoids from 2-hydroxychalcone was developed. This protocol provided a facile entry to structurally diverse hybrid flavonoids under the irradiation of 24 W CFL. Based on the mechanistic studies, this reaction was proposed to proceed via the cascade double bond isomerization/dehydrated cyclization to afford a transient flavylium ion, which is in situ incepted by nucleophile. Furthermore, this protocol also provides novel strategy for the enantioselective synthesis of hybrid flavonoids.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (grants. 21722206, 21672171) and Shaanxi government. Financial support from Chinese Universities Scientific Fund and the Scientific Fund of Northwest A&F University are also acknowledged.Notes and references
- (a) K. Suzuki, in Natural Products in Medicinal Chemistry V60, ed. S. Hanessian, Wiley-VCH, Verlag GmbH & Co. KGaA, 2014 Search PubMed; (b) K. Suzuki, Chem. Rec., 2010, 10, 291–307 CrossRef CAS PubMed.
- for review, see: (a) L. F. Tietze, H. P. Bell and S. Chandrasekhar, Angew. Chem., Int. Ed., 2003, 42, 3996–4028 CrossRef CAS PubMed; for selected recent examples, see: (b) K. Gademann, Chimia, 2006, 60, 841–845 CrossRef CAS; (c) B. Dasari, R. Jimmidi and P. Arya, Eur. J. Med. Chem., 2015, 94, 497–508 CrossRef CAS PubMed; (d) S. Christou, A. C. Edwards, S. Kaskun, R. G. Pritchard, P. Quayle, Y. W. Song, I. J. Stratford, K. F. Williams and R. C. Whitehead, Tetrahedron, 2017, 73, 1295 CrossRef CAS; (e) S. Choudhary, P. K. Singh, H. Verma, H. Singh and O. Silakari, Eur. J. Med. Chem., 2018, 151, 62–97 CrossRef CAS PubMed.
- (a) B. Harborne and T. J. Mabry, The Flavonoids: advances in research, Chapman and Hall, London, New York, 1982 CrossRef; (b) J. B. Harborne, The Flavonoids: advances in research since 1980, Chapman and Hall, London, New York, 1988 CrossRef; (c) B. Harborne, The Flavonoids: advances in research since 1986, Chapman & Hall, London, New York, 1st edn, 1994 CrossRef; (d) C. A. Williams and R. J. Grayer, Nat. Prod. Rep., 2004, 21, 539–573 RSC; (e) .M. Andersen and K. R. Markham, Flavonoids: chemistry, biochemistry, and applications, CRC, Taylor & Francis, Boca Raton, FL, 2006 Search PubMed; (f) K. Gould, K. M. Davies and C. Winefield, Anthocyanins: biosynthesis, functions, and applications, Springer, New York, 2009 Search PubMed; (g) S. Kumar and A. K. Pandey, Sci. World J., 2013, 2013, 16 Search PubMed; (h) N. C. Veitch, Nat. Prod. Rep., 2013, 30, 988–1027 RSC.
- (a) J. J. Vepsalainen, S. Auriola, M. Tukiainen, N. Ropponen and J. C. Callaway, Planta Med., 2005, 71, 1053–1057 CrossRef CAS PubMed; (b) M. B. Calvert and J. Sperry, Chem. Commun., 2015, 51, 6202–6205 RSC.
- (a) A. Ogundaini, M. Farah, P. Perera, G. Samuelsson and L. Bohlin, J. Nat. Prod., 1996, 59, 587–590 CrossRef CAS PubMed; for the biomimetic synthesis, see: (b) C. Selenski and T. R. R. Pettus, Tetrahedron, 2006, 62, 5298–5307 CrossRef CAS PubMed.
- (a) L. A. Weston and U. Mathesius, J. Chem. Ecol., 2013, 39, 283–297 CrossRef CAS; (b) B. Winkel-Shirley, Plant Physiol., 2001, 126, 485–493 CrossRef CAS PubMed.
- For selected reviews: (a) F. Pina, M. J. Melo, C. A. T. Laia, A. J. Parola and J. C. Lima, Chem. Soc. Rev., 2012, 41, 869–908 RSC; (b) F. Pina, V. Petrov and C. A. T. Laia, Dyes Pigm., 2012, 92, 877–889 CrossRef CAS; for selected examples: (c) L. Jurd, Tetrahedron, 1969, 25, 2367–2380 CrossRef CAS PubMed; (d) D. Dewar and R. G. Sutherland, J. Chem. Soc. D, 1970, 272–273 RSC; (e) H. Hiroaki, Y. Akinobu, O. Tetsuo and H. Hiroshi, Bull. Chem. Soc. Jpn., 1999, 72, 2429–2435 CrossRef; (f) V. Petrov, A. M. Diniz, L. Cunha-Silva, A. J. Parola and F. Pina, RSC Adv., 2013, 3, 10786–10794 RSC.
- (a) F. Kröhnke and K. Dickoré, Chem. Ber., 1959, 92, 46–62 CrossRef; (b) L. Jurd and B. J. Bergot, Tetrahedron, 1965, 21, 3697–3705 CrossRef CAS; (c) L. Jurd and A. C. Waiss, Tetrahedron, 1965, 21, 1471–1483 CrossRef CAS; (d) L. Jurd and R. Lundin, Tetrahedron, 1968, 24, 2653–2661 CrossRef CAS; (e) G. Lee, K. Ishimaru, H. Iwasaki, K. Ohkata and K. Akiba, J. Org. Chem., 1991, 56, 2058–2066 CrossRef; (f) G. A. Kraus, Y. Yuan and A. Kempema, Molecules, 2009, 14, 807–815 CrossRef CAS PubMed; (g) F. Benfatti, E. Benedetto and P. G. Cozzi, Chem.–Asian J., 2010, 5, 2047–2052 CrossRef CAS PubMed; (h) Z. Y. Yang, Y. He and F. D. Toste, J. Am. Chem. Soc., 2016, 138, 9775–9778 CrossRef CAS PubMed; (i) A. Alejo-Armijo, N. Glibota, M. P. Frías, J. Altarejos, A. Gálvez, S. Salido and E. Ortega-Morente, J. Agric. Food Chem., 2018, 66, 2151–2158 CrossRef CAS PubMed.
- C. Fichtner, G. Remennikov and H. Mayr, Eur. J. Org. Chem., 2001, 4451–4456 CrossRef CAS.
- (a) T. Nishikata, Y. Yamamoto and N. Miyaura, Adv. Synth. Catal., 2007, 349, 1759–1764 CrossRef CAS; (b) G. Yin, L. Fan, T. Ren, C. Zheng, Q. Tao, A. Wu and N. She, Org. Biomol. Chem., 2012, 10, 8877–8883 RSC; (c) N. C. Ganguly, P. Mondal and S. Roy, Tetrahedron Lett., 2013, 54, 2386–2390 CrossRef CAS; (d) Y. Rao and G. D. Yin, Org. Biomol. Chem., 2013, 11, 6029–6035 RSC; (e) F. J. Wang, F. Chen, M. L. Qu, T. Li, Y. L. Liu and M. Shi, Chem. Commun., 2013, 49, 3360–3362 RSC; (f) Y. Rao, M. L. Liu, L. Wu and G. D. Yin, RSC Adv., 2014, 4, 64551–64558 RSC; (g) C. Bingi, N. R. Emmadi, M. Chennapuram, Y. Poornachandra, C. G. Kumar, J. B. Nanubolu and K. Atmakur, Bioorg. Med. Chem. Lett., 2015, 25, 1915–1919 CrossRef CAS PubMed; (h) A. Ganesan, J. Kothandapani and S. G. Subramaniapillai, RSC Adv., 2016, 6, 20582–20587 RSC; (i) J. M. Guo, X. G. Bai, Q. L. Wang and Z. W. Bu, J. Org. Chem., 2018, 83, 3679–3687 CrossRef CAS PubMed; (j) Y. N. Zhu, Z. G. Yao and F. Xu, Tetrahedron, 2018, 74, 4211–4219 CrossRef CAS; (k) Y. S. Zhu, J. Guo, S. J. Jin, J. M. Guo, X. G. Bai, Q. L. Wang and Z. W. Bu, Org. Biomol. Chem., 2018, 16, 1751–1759 RSC; (l) J.-M. Guo, W.-B. Wang, J. Guo, Y.-S. Zhu, X.-G. Bai, S.-J. Jin, Q.-L. Wang and Z.-W. Bu, RSC Adv., 2018, 8, 15641–15651 RSC.
- (a) R. J. Phipps, G. L. Hamilton and F. D. Toste, Nat. Chem., 2012, 4, 603–614 CrossRef CAS PubMed; (b) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518–533 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, and spectroscopic date for the all new compounds. See DOI: 10.1039/c9ra07198a |
| This journal is © The Royal Society of Chemistry 2019 |