Open Access Article
Open Access ArticleOxidant-free, three-component synthesis of 7-amino-6H-benzo[c]chromen-6-ones under green conditions†
B. S. Vachana,
Aishwarya Ramesha,
Muthu Karuppasamya,
Isravel Muthukrishnana,
Subbiah Nagarajan b,
J. Carlos Menéndez
b,
J. Carlos Menéndez c,
C. Uma Maheswari
c,
C. Uma Maheswari *a and
Vellaisamy Sridharan
*a and
Vellaisamy Sridharan *ad
*ad
aDepartment of Chemistry, School of Chemical and Biotechnology, SASTRA Deemed University, Thanjavur-613401, Tamil Nadu, India. E-mail: umamaheswaric@scbt.sastra.edu; uma.cchem@gmail.com
bDepartment of Chemistry, National Institute of Technology, Warangal, Warangal-506004, Telangana, India
cUnidad de Química Orgánica y Farmacéutica, Departamento de Química en Ciencias Farmacéuticas, Facultad de Farmacia, Universidad Complutense, 28040 Madrid, Spain
dDepartment of Chemistry and Chemical Sciences, Central University of Jammu, Rahya-Suchani (Bagla), District-Samba, Jammu-181143, J&K, India. E-mail: vesridharan@gmail.com; sridharan.che@cujammu.ac.in
First published on 16th October 2019
Abstract
An oxidant-free three-component synthesis of biologically significant 7-amino-6H-benzo[c]chromen-6-ones was established involving a Sc(OTf)3 catalyzed three-component reaction between primary amines, β-ketoesters and 2-hydroxychalcones under green conditions. In this strategy, both the B and C rings of 6H-benzo[c]chromen-6-ones were constructed simultaneously starting from acyclic precursors by generating four new bonds including two C–C, one C–N and one C–O in a single synthetic operation. The mechanism of this sequential cascade process involves the initial formation of a β-enaminone intermediate followed by Michael addition with 2-hydroxychalcone, intramolecular cyclization, dehydration, lactonization and aromatization steps. Unlike the related literature approaches, this reaction delivered the products without the addition of any external oxidants to achieve the key aromatization step.
Introduction
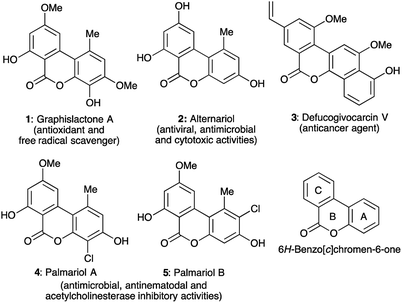
Among the many biologically valuable oxygen heterocycles, 6H-benzo[c]chromen-6-ones, which belong to an important group of heptaketide coumarin derivatives, are the core structure of a variety of biologically significant natural and synthetic compounds.1 These secondary metabolites, represented by graphislactone A 1,2 alternariol 2,3 defucogivocarcin V 3,4 and palmariol A and B (4 and 5)5 have predominantly been isolated from fungi, lichens, bacteria, plants, and animal waste, and are known to exhibit numerous potential biological activities including acting as an antioxidant via free radical scavenging, and antiviral, antimicrobial, cytotoxic, antinematodal and acetylcholinesterase inhibitory activities (Fig. 1). Other significant activities of 6H-benzo[c]chromen-6-one derivatives include anti-inflammatory, estrogenic activity, DNA gyrase inhibitory activity, casein kinase 2 (CK2) inhibitory activity, DNA intercalative activity, and many others.1,6Owing to the biological significance of 6H-benzo[c]chromen-6-ones, the synthesis of derivatives of this framework remain an attractive goal for synthetic chemists.7 Surprisingly, however, most of the existing approaches for the synthesis of 6H-benzo[c]chromen-6-ones involve the construction of the B ring (lactone ring) from biphenyl derivatives via lactonization reactions, CO insertion etc. Alternatively, the B ring was generated from two different aryl derivatives by simultaneous formation of the C–C bond between the A and C rings and lactonization reactions.8 The green protocol developed by Song and Chi involving a base mediated reaction between unsaturated aldehydes and coumarins, through a formal [4+2] process,9 and Bodwell's inverse electron demand Diels–Alder reaction strategy10 are significant approaches among the very few existing methods comprising the generation of the C ring. In addition, Fan and co-workers established the synthesis of aminonaphthochromenones starting from 2-[(2-hydroxyphenyl)ethynyl]benzonitriles and Reformatsky reagents by constructing both B and C rings.11 Poudel and Lee have also developed a base-promoted two-component reaction between 2-hydroxychalcones and β-ketoesters for the synthesis of 7-hydroxy-6H-benzo[c]chromen-6-ones in moderate yields.12 Almost simultaneously, five examples of 7-hydroxy-6H-benzo[c]chromen-6-ones were synthesized using a diethylamine-catalyzed approach in 51–59% yield.13 Despite these few methods, the synthesis of 6H-benzo[c]chromen-6-ones from readily available starting materials via multicomponent reactions remains almost unexplored. Consequently, we envisioned to develop a straightforward, oxidant-free, three-component, one-pot approach for the synthesis of 7-amino-6H-benzo[c]chromen-6-ones involving the simultaneous generation of both the B and C rings.
Results and discussion
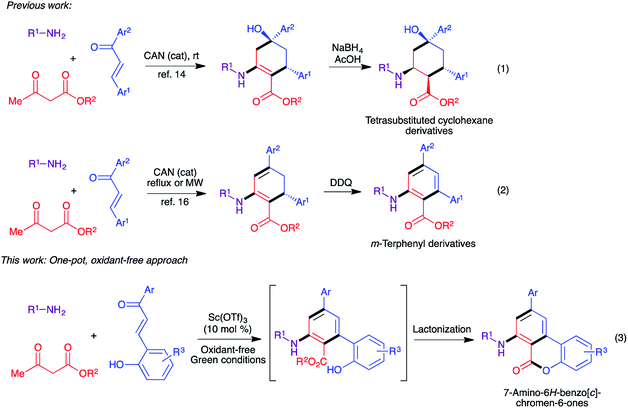
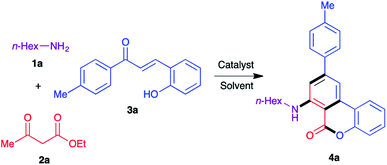
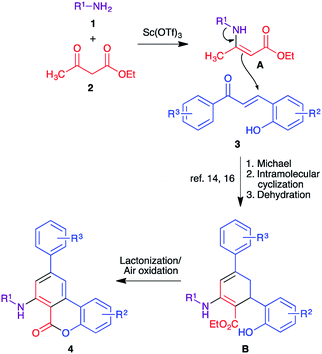
A decade ago we established a diastereoselective two-step synthesis of densely functionalized cyclohexane derivatives involving a cerium(IV) ammonium nitrate (CAN)-catalyzed three-component reaction between alkylamines, β-ketoesters, and chalcones (Scheme 1, eqn (1)).14,15 The key step in this reaction involves the formation of a β-enaminoester from alkylamines and β-ketoesters followed by a Michael addition to the chalcone component and an intramolecular cyclization to generate the cyclohexene ring from the acyclic precursors. Subsequently, we extended this approach to the synthesis of m-terphenyl derivatives by slightly modifying the reaction conditions (Scheme 1, eqn (2)).16 However, the final aromatization step required the use of one equivalent of DDQ. Yaragorla and Dada have also demonstrated a similar strategy for the synthesis of the related compounds, including 6H-benzo[c]chromen-6-ones, using Ca(OTf)2/n-Bu4NPF6 as catalyst and one equivalent of chloranil as oxidant.17 With these precedents, we herein describe a domino process that combines the three-component synthesis of cyclohexene derivatives and aromatization-lactonization steps for the synthesis of 7-amino-6H-benzo[c]chromen-6-ones starting from primary amines, β-ketoesters, and 2-hydroxychalcones in one-pot under oxidant-free green conditions by generating both B and C rings simultaneously (Scheme 1, eqn (3)).We began our optimization studies by using n-hexylamine 1a, ethyl acetoacetate 2a and (E)-3-(2-hydroxyphenyl)-1-(p-tolyl)prop-2-en-1-one 3a as model substrates to identify the suitable reaction conditions for the proposed three-component reaction. At the outset, we employed the previously established reaction conditions (10 mol% of CAN, ethanol, 80 °C) and 30% of the product 4a was isolated after 30 h (Table 1, entry 1). When the catalyst load was increased to 20 mol%, no significant improvement was observed (38%, entry 1). In order to increase the product yield we performed the reaction in t-BuOH and some green solvents such as PEG-200 and glycerol, but only 19–42% yield was observed (entries 2–4). Use of t-butyl acetoacetate as the starting material instead of ethyl acetoacetate failed to improve the yield significantly (39%, entry 5). We then tested other Lewis acids such as InCl3, BiCl3, BF3·OEt2, AgOTf and Yb(OTf)3 in ethanol at 90 °C (entries 6–10). Encouragingly, AgOTf and Yb(OTf)3 afforded the product 4a in 59% and 65% yields respectively (entries 9 and 10). Next, Sc(OTf)3 was tested as catalyst and the reaction delivered 73% of the product 4a in 20 h (entry 11). Interestingly, when concentration was increased (1.5 mL of solvent at 0.5 mmol scale), the yield was increased to 78% (entry 12). Further attempts to improve the product yield by using other protic solvents including MeOH, i-PrOH and t-BuOH were not successful (entries 13–15). Finally, in order to achieve a green protocol for this three-component cascade process, the reaction was carried out in a set of common green solvents such as PEG-200, glycerol and water in the presence of Sc(OTf)3 (entries 16–18). Among the tested solvents, glycerol delivered a comparable yield to ethanol at 100 °C in the absence of any oxidant in 24 h (76%, entry 17) and the other two green solvents gave moderate yields of 42–49%. To understand the role of the catalyst in this transformation, we performed the reaction in the absence of any catalyst in glycerol at 100 °C, where only the intermediate β-enaminoester obtained from the amine and ethyl acetoacetate was observed in the crude reaction mixture (entry 19). The crucial role of oxygen (air) in this transformation was also demonstrated by performing the reaction under nitrogen atmosphere where only 22% of the product was isolated together with some unidentified mixture (entry 17). In conclusion, the use of 10 mol% of Sc(OTf)3 as catalyst in glycerol at 100 °C was selected as the optimized reaction condition for further experiments.
| Entry | Catalyst | Solvent | Temp. (°C) | Time (h) | Yield of 4ab (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1a (0.6 mmol), 2a (0.5 mmol) and 3a (0.5 mmol) with 10 mol% catalyst in 3 mL solvent.b Isolated yield.c 20 mol% of catalyst was used.d t-Butyl acetoacetate was used instead of ethyl acetoacetate.e 1.5 mL of solvent was used.f The reaction did not proceed at room temperature.g Optimized reaction conditions.h Under nitrogen atmosphere 22% of the product was obtained.i Formation of intermediate β-enaminoester was observed. | |||||
| 1 | CAN | EtOH | 80 | 30 | 30 (38)c |
| 2 | CAN | t-BuOH | 80 | 30 | 42 |
| 3 | CAN | PEG-200 | 80 | 30 | 22 |
| 4 | CAN | Glycerol | 80 | 30 | 19 |
| 5d | CAN | EtOH | 90 | 30 | 39 |
| 6 | InCl3 | EtOH | 90 | 24 | 42 |
| 7 | BiCl3 | EtOH | 90 | 24 | 21 |
| 8 | BF3·OEt2 | EtOH | 90 | 24 | 14 |
| 9 | AgOTf | EtOH | 90 | 20 | 59 |
| 10 | Yb(OTf)3 | EtOH | 90 | 24 | 65 |
| 11 | Sc(OTf)3 | EtOH | 90 | 20 | 73 |
| 12e | Sc(OTf)3 | EtOH | 90 | 20 | 78 |
| 13e | Sc(OTf)3 | MeOH | 70 | 20 | 67 |
| 14e | Sc(OTf)3 | i-PrOH | 100 | 20 | 59 |
| 15e | Sc(OTf)3 | t-BuOH | 100 | 20 | 58 |
| 16 | Sc(OTf)3 | PEG-200 | 100 | 20 | 49 |
| 17f,g | Sc(OTf)3 | Glycerol | 100 | 24 | 76 (72)d,h |
| 18 | Sc(OTf)3 | Water | 100 | 20 | 42 |
| 19 | — | Glycerol | 100 | 30 | Tracesi |
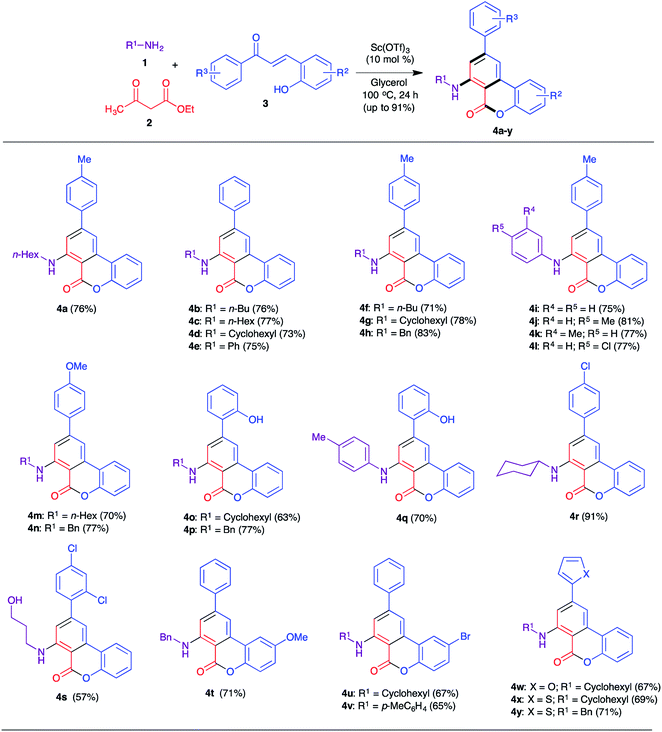
After having optimized the reaction conditions, we turned to explore the scope and limitations of the three-component reaction by employing a variety of primary amines, β-ketoesters and 2-hydroxychalcones bearing a large number of substituents on both the aryl rings (Scheme 2). At the outset, the effect of the primary amines, including alkyl and arylamines, was investigated. The combination of ethyl acetoacetate, unsubstituted 2-hydroxychalcones and alkyl amines including n-butylamine, n-hexylamine, cyclohexylamine and aniline delivered the corresponding 6H-benzo[c]chromen-6-ones 4b–4e in good yields (73–77%). Likewise, n-butylamine, cyclohexylamine and benzylamine were also reacted with chalcone 3a to furnish the products 4f–4h in 71–83% yields. Arylamines including aniline and its p-Me, m-Me and p-Cl derivatives also reacted smoothly under the optimized conditions to afford the corresponding diarylamine derivatives 4i–4l in comparable yields (75–81%). Subsequently, we varied the substituents in the 2-hydroxychalcone component, where substituents including 4′-OMe (4m, 4n), 2′-OH (4o–4q) and 4′-Cl (4r) were well tolerated, and delivered the products in good yields (63–91%). A maximum yield of 91% was observed for the combination of cyclohexylamine and the 4′-Cl substituted chalcone (4r). 3-Aminopropanol and 2′,4′-dichlorochalcone were also reacted with ethyl acetoacetate and furnished the product 4s in 57% yield. Both electron-donating (OMe, 4t) and withdrawing (Br, 4u and 4v) substituents were introduced in the 2-hydroxy aryl ring and these chalcones also reacted well to give the respective 6H-benzo[c]chromen-6-ones in 65–71% yields. Finally, chalcones bearing heteroaryl rings such as 2-furyl and 2-thienyl were also tested and 67–71% yields of products (4w–4y) were obtained.
A mechanism is proposed involving the formation of β-enaminone A, starting from primary amines 1 and ethyl acetoacetate 2, based on our previous reports14,16 (Scheme 3). The in situ generated β-enaminone A, in the presence of the Lewis acid catalyst,18,19 undergoes a Michael addition with 2-hydroxychalcone 3 followed by intramolecular cyclization and dehydration to deliver the cyclohexene derivative B. Final lactonization and aromatization reactions under the experimental conditions, in the absence of an oxidant different from air, furnished the final products 4.
Conclusions
In conclusion, we have demonstrated a simple three-component protocol for the synthesis of biologically relevant 7-amino-6H-benzo[c]chromen-6-ones under green conditions. The Sc(OTf)3 catalyzed three-component reaction between primary amines, β-ketoesters and 2-hydroxychalcones afforded the title compounds in good yields by generating four new bonds including two C–C, one C–N and one C–O bonds, and two rings (one carbo- and one heterocycle) in a single synthetic operation. The reaction was found to be general and a variety of substituents were tolerated on both the primary amines and 2-hydroxychalcones. The mechanism of this cascade reaction involves the initial reaction between primary amines and β-ketoesters in the presence of Sc(OTf)3 to deliver the β-enaminone intermediate followed by Michael addition with α,β-unsaturated ketones to furnish the products via intramolecular cyclization, dehydration, lactonization and aromatization steps in one-pot. Notably, the reaction delivered the products with no external oxidants to achieve the key aromatization reaction unlike the related reports available in literature.Experimental section
General information
All reagents and solvents were purchased from commercial suppliers (Avra, Alfa Aesar, Sigma-Aldrich, CDH, Merck) and used without further purification. All reactions were carried out in oven-dried glassware under air atmosphere. The reactions were monitored by thin layer chromatography using Merck silica gel 60 F254 and visualized by UV detection or using p-anisaldehyde stain or molecular iodine. Silica gel (230–400 mesh) was used for flash column chromatography. Melting points were recorded on a melting point apparatus in capillaries and are uncorrected. 1H and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 at room temperature on a Bruker Avance 300 spectrometer operating at 300 MHz for 1H and 75 MHz for 13C. Chemical shifts (δ) are expressed in ppm using TMS as an internal standard and coupling constants (J) are given in Hz. Infrared (IR) spectra were obtained using an Agilent Cary630 FTIR Spectrometer with a diamond ATR accessory for solid and liquid samples, requiring no sample preparation and the major frequencies were reported in cm−1. Elemental analyses were determined at the CAI de Microanálisis Elemental, Universidad Complutense, by using a Leco 932 CHNS combustion microanalyzer.General procedure for the synthesis of 6H-benzo[c]chromen-6-ones 4a–y
To a stirred solution of primary amine 1 (1.2 mmol, 1.2 equiv.) in glycerol (6 mL) was added β-ketoester 2 (1.0 mmol, 1.0 equiv.) and Sc(OTf)3 (10 mol%). The reaction mixture was stirred at room temperature for 15 minutes and then chalcone 3 (1.0 mmol, 1.0 equiv.) was added, and the reaction mixture was stirred at 100 °C for 24 h. After completion of the reaction, the mixture was diluted with ethyl acetate (20 mL) and washed with water followed by brine. The solvent was evaporated under reduced pressure and the crude product was purified through silica column chromatography using petroleum ether-ethyl acetate mixture (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 90
4 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) as eluent to obtain compounds 4.
10, v/v) as eluent to obtain compounds 4.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Department of Science and Technology-Science and Engineering Research Board, DST-SERB (No. EMR/2016/001619 & EMR/2016/002485) and MICINN (RTI2018-097662-B-I00) is gratefully acknowledged.Notes and references
- For reviews on natural 6H-benzo[c]chromen-6-ones and their biological activities, see: (a) Ya. L. Garazd and M. M. Garazd, Chem. Nat. Compd., 2016, 52, 1–18 CrossRef CAS; (b) Z. Mao, W. Sun, L. Fu, H. Luo, D. Lai and L. Zhou, Molecules, 2014, 19, 5088–5108 CrossRef.
- (a) T. Tanahashi, M. Kuroishi, A. Kuwahara, N. Nagakura and N. Hamada, Chem. Pharm. Bull., 1997, 45, 1183–1185 CrossRef CAS; (b) E. Hormazabal, G. Schmeda-Hirschmann, L. Astudillo, J. Rodriguez and C. Theoduloz, Z. Naturforsch., C: J. Biosci., 2005, 60, 11–21 CAS.
- (a) J. Xiao, Q. Zhang, Y.-Q. Gao, J.-J. Tang, A.-L. Zhang and J.-M. Gao, J. Agric. Food Chem., 2014, 62, 3584–3590 CrossRef CAS; (b) M. H. Tao, Y.-C. Chen, X.-Y. Wei, J.-W. Tan and W.-M. Zhang, Helv. Chim. Acta, 2014, 97, 426–430 CrossRef CAS; (c) S. Liu, Y. Dong, Y. Li, L. Bao, H. Liu and H. Li, Fitoterapia, 2014, 91, 9–14 CrossRef; (d) H. Raistrick, C. E. Stickings and R. Thomas, Biochem. J., 1953, 55, 421–433 CAS.
- (a) D. J. Hart and A. Mannino, Tetrahedron, 1996, 52, 3841–3856 CrossRef CAS; (b) A. D. Patten, N. H. Nguyen and S. J. Danishefsky, J. Org. Chem., 1988, 53, 1003–1007 CrossRef CAS; (c) D. M. Balitz, F. A. O'Herron, J. Bush, D. M. Vyas, D. E. Nettleton, R. E. Grulich, W. T. Bradner, T. W. Doyle, E. Arnold and J. J. Clardy, Antibiotics, 1981, 34, 1544–1555 CrossRef CAS.
- (a) X. Meng, Z. Mao, J. Lou, L. Xu, L. Zong, Y. Peng, L. Zhou and M. Wang, Molecules, 2012, 17, 11303–11314 CrossRef CAS; (b) T. Matumoto, T. Hosoya and H. Shigemori, Heterocycles, 2010, 81, 1231–1237 CrossRef CAS.
- P. Bhattacharya, S. M. Mandal and A. Basak, Eur. J. Org. Chem., 2016, 1439–1448 CrossRef CAS.
- For a review, see: O. Mazimba, Turk. J. Chem., 2016, 40, 1–27 CrossRef CAS.
- For selected recent examples of the synthesis of 6H-benzo[c]chromen-6-ones involving the generation of the lactone ring, see: (a) T. Morack, J. B. Metternich and R. Gilmour, Org. Lett., 2018, 20, 1316–1319 CrossRef CAS; (b) Q. Yang, Z. Jia, L. Li, L. Zhang and S. Luo, Org. Chem. Front., 2018, 5, 237–241 RSC; (c) K. Subramanian, S. L. Yedagea and B. M. Bhanagea, Adv. Synth. Catal., 2018, 360, 2511–2521 CrossRef CAS; (d) X.-Z. Tao, J.-J. Dai, J. Z. J. Xu and H.-J. Xu, Chem.–Eur. J., 2018, 24, 6932–6935 CrossRef CAS; (e) L. Zhang, Z. Zhang, J. Hong, J. Yu, J. Zhang and F. Mo, J. Org. Chem., 2018, 83, 3200–3207 CrossRef CAS; (f) A. Shao, J. Zhan, N. Li, C.-W. Chiang and A. Lei, J. Org. Chem., 2018, 83, 3582–3589 CrossRef CAS; (g) P. D. Q. Dao, S. L. Ho, H.-J. Lim and C. S. Cho, J. Org. Chem., 2018, 83, 4140–4146 CrossRef CAS; (h) L. Lia, Q. Yang, Z. Jia and S. Luo, Synthesis, 2018, 50, 2924–2929 CrossRef; (i) Y. Wang, J.-Y. Gu and Z.-J. Shi, Org. Lett., 2017, 19, 1326–1329 CrossRef CAS; (j) S. L. Yedage and B. M. Bhanage, J. Org. Chem., 2017, 82, 5769–5781 CrossRef CAS; (k) J. Zhang, D. Shi, H. Zhang, Z. Xu, H. Bao, H. Jin and Y. Liu, Tetrahedron, 2017, 73, 154–163 CrossRef CAS; (l) L. Huang and D. J. Weix, Org. Lett., 2016, 18, 5432–5435 CrossRef CAS; (m) R. Oketani, T. Miyake, S. Jinnai, T. Fukui, H. Tsujimoto, M. Matsumura and S. Higashida, Chem. Lett., 2016, 45, 801–803 CrossRef CAS; (n) P. Bhattacharya, S. M. Mandal and A. Basak, Eur. J. Org. Chem., 2016, 1439–1448 CrossRef CAS; (o) K. Kudo and N. Yamamoto, Org. Process Res. Dev., 2015, 19, 309–314 CrossRef CAS; (p) S. Luo, F.-X. Luo, X.-S. Zhang and Z.-J. Shi, Angew. Chem., Int. Ed., 2013, 52, 10598–10601 CrossRef CAS.
- C. Mou, T. Zhu, P. Zheng, S. Yang, B.-A. Song and Y. R. Chi, Adv. Synth. Catal., 2016, 358, 707–712 CrossRef CAS.
- I. R. Pottie, P. R. Nandaluru, W. L. Benoit, D. O. Miller, L. N. Dawe and G. J. Bodwell, J. Org. Chem., 2011, 76, 9015–9030 CrossRef CAS.
- Y. He, X. Zhang and X. Fan, Asian J. Org. Chem., 2014, 3, 1284–1291 CrossRef CAS.
- T. N. Poudel and Y. R. Lee, Org. Biomol. Chem., 2014, 12, 919–930 RSC.
- I. B. Masesane and O. Mazimba, Bull. Chem. Soc. Ethiop., 2014, 28, 289–294 CrossRef.
- V. Sridharan and J. C. Menéndez, Org. Lett., 2008, 10, 4303–4306 CrossRef CAS.
- For a review on cerium(IV) ammonium nitrate as a catalyst in organic synthesis, see: V. Sridharan and J. C. Menéndez, Chem. Rev., 2010, 110, 3805–3849 CrossRef CAS.
- D. Rocchi, J. F. González, J. Gómez-Carpintero, V. González-Ruiz, M. A. Martín, V. Sridharan and J. C. Menéndez, ACS Comb. Sci., 2018, 20, 722–731 CrossRef CAS PubMed.
- S. Yaragorla and R. Dada, ACS Omega, 2017, 2, 4859–4869 CrossRef CAS PubMed.
- For the generation of β-enaminones via CAN catalyzed reaction, see: V. Sridharan, C. Avendaño and J. C. Menéndez, Synlett, 2007, 0881–0884 CAS.
- For selected examples of β-enaminone formation-initiated multicomponent reactions from our group, see: (a) V. Estévez, V. Sridharan, S. Sabaté, M. Villacampa and J. C. Menéndez, Asian J. Org. Chem., 2016, 5, 652–662 CrossRef; (b) T. Vivekanand, P. Vinoth, B. Agieshkumar, N. Sampath, A. Sudalai, J. C. Menéndez and V. Sridharan, Green Chem., 2015, 17, 3415–3423 RSC; (c) P. Vinoth, P. S. R. Prasad, T. Vivekanand, C. U. Maheswari, S. Nagarajan, J. C. Menéndez and V. Sridharan, RSC Adv., 2015, 5, 81881–81888 RSC; (d) G. Tenti, E. Parada, R. León, J. Egea, S. Martínez-Revelles, A. M. Briones, V. Sridharan, M. G. López, M. T. Ramos and J. C. Menéndez, J. Med. Chem., 2014, 57, 4313–4323 CrossRef CAS; (e) P. A. Suryavanshi, V. Sridharan, S. Maiti and J. C. Menéndez, Chem.–Eur. J., 2014, 20, 8791–8799 CrossRef CAS; (f) P. A. Suryavanshi, V. Sridharan and J. C. Menéndez, Chem.–Eur. J., 2013, 19, 13207–13215 CrossRef CAS; (g) P. A. Suryavanshi, V. Sridharan and J. C. Menéndez, Tetrahedron, 2013, 69, 5401–5406 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of new products. See DOI: 10.1039/c9ra07108c |
| This journal is © The Royal Society of Chemistry 2019 |