Open Access Article
Open Access ArticleRetracted Article: Long non-coding RNA NEAT1 accelerates cell progression in cervical cancer by regulating the miR-889-3p/E2F7 axis through the activation of the PI3K/AKT pathway
Hongmei Zhou *,
Ruixia Guo and
Chunfang Wang
*,
Ruixia Guo and
Chunfang Wang
Department of Gynecology, The First Affiliated Hospital of Zhengzhou University, No. 1, East Jianshe Road, Erqi District, Zhengzhou, 450052, Henan, China. E-mail: 13526528555@163.com; Tel: +86-13526528555
First published on 31st October 2019
Abstract
Cervical cancer (CC) is a common malignant gynecological cancer that is frequently diagnosed in women. Apparently, long noncoding RNA nuclear-enriched autosomal transcript 1 (NEAT1) has been identified as a tumor promoter in multiple cancers. Our research is focused on the effects of lncRNA NEAT1 on cell progression in cervical cancer and the potential molecular mechanism of NEAT1 for CC cell progression. The levels of NEAT1, MicroRNA (miR)-889-3p and E2F transcription factor 7 (E2F7) in CC tumors and cells were measured by a quantitative real-time polymerase chain reaction (qRT-PCR). The interaction of miR-889-3p with NEAT1 and E2F7 was validated by a luciferase reporter system and a RNA immunoprecipitation (RIP) assay, respectively. Cell counting kit-8 (CCK-8) and flow cytometry were used for cell proliferation and apoptosis evaluation. Cell migration and invasion were examined by a transwell assay. The protein expressions of E2F7, AKT, phospho-AKT (p-AKT), phosphatidylinositol 3-kinase (PI3K) and phosphorylated PI3K (p-PI3K) were analyzed by western blot assay. Animal models were established by subcutaneously injecting the Me180 cells stably transfected with sh-NEAT1 and sh-NC. The expressions of NEAT1 and E2F7 were up-regulated, whereas the expression of miR-889-3p was down-regulated in the CC tumors and cells when compared with those in normal tumors and cells. The interaction between miR-889-3p and NEAT1 or E2F7 was proved by luciferase reporter system and RIP assay. In addition, the miR-889-3p inhibitor attenuated the NEAT1 silencing-induced inhibition effects on cell proliferation, migration, and invasion and the promotion effects on apoptosis in CC. Consistently, E2F7 reversed the miR-889-3p-mediated inhibition on cell progression in CC. Moreover, NEAT1 modulated cell behavior by targeting the miR-889-3p/E2F7 axis through the PI3K/AKT pathway. Finally, the intervention of NEAT1 hindered tumor growth in vivo. Thus, NEAT1 contributes to cell progression in CC by targeting miR-889-3p to facilitate the E2F7 expression through the activation of the PI3K/AKT pathway, representing an alternative targeted therapy of CC.
1. Introduction
Cervical cancer (CC) is an aggressive gynecological cancer that seriously threatens the health of women worldwide.1,2 Typically, CC can invade adjacent organs such as parametria, vagina, and even bladder or rectum.3 The symptoms of CC normally occur at the advanced stage, leading to the vitiation of the optimum treatment time and high mortality, particularly in developing countries.4,5 In addition, smoking, age, race and secondary malignancies caused by invasiveness are critical risk factors of poor prognosis.6 Therefore, the discovery of novel biomarkers is imperative for the therapy of CC.Long noncoding RNAs (lncRNAs) are essential gene modulators that participate in cell cycle, metabolism, survival, inflammation, metastasis and drug resistance.7–9 Nuclear-enriched autosomal transcript 1 (NEAT1) located at 11q13.1 is a newly emerged oncogene that is involved in the tumorigenesis of a variety of cancers.10 The dysregulation of NEAT1 is implicated in the poor prognosis of many cancers. For example, the overexpression of NEAT1 has been reported to expedite colon cancer cell viability and migration; moreover, NEAT1 blocks apoptosis in vitro and in vivo by binding to miR-495-3p and up-regulating CDK6.11 The increased expression of NEAT1 accelerated cell proliferation and migration in hemangioma by sponging miR-361-5p to facilitate the VEGFA expression.12 Similarly, NEAT1 expedited tumor growth in vitro and in vivo by targeting miR-133a and altering the SOX4 expression.13 By contrast, the down-regulation of NEAT1 inhibited hepatocellular carcinoma cell growth by the activation of CD8+T cells through the regulation of miR-155.14 However, the regulatory mechanism of NEAT1 in CC is still unclear.
MicroRNAs (miRNAs) are highly conserved small-chain transcripts that are 16–25 nucleotides in length.15 Despite the lack of protein encoding capacity, they are negatively involved in gene regulation at the posttranscriptional level by targeting the messenger RNA (mRNA) and affecting RNA degradation and protein translation.16–18 Previous studies have shown that miRNAs are closely related to many pathological processes such as cell growth, infiltration, metastasis, invasion, differentiation, autophagy and apoptosis.19 The ectopic expression of miRNA is widely exhibited in different cancers. For instance, miR-889 is up-regulated in colorectal cancer, and miR-889 in high levels facilitates cell proliferation and invasion by targeting DAB2IP.20 By comparison, miR-889-3p acts as a tumor suppressor to inhibit cell survival and invasion in CC by interacting with FGFR2.21 Thus, the clarification of the exact function of miR-889-3p during the development of CC is of great clinical significance.
Herein, we attempted to illuminate the underlying mechanism of NEAT1/miR-889-3p axis for CC cell growth. We discovered that NEAT1 was capable of promoting cell growth and migration and blocking apoptosis in CC by targeting miR-889-3p and enhancing the E2F7 expression.
2. Materials and methods
2.1 Tissue samples
CC patients (n = 32) recruited from The First Affiliated Hospital of Zhengzhou University signed the informed consent. All the protocols were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Fresh CC tumors and the matched normal tissues were obtained from the participants by surgery. All experiments were performed in accordance with the Guidelines of “National Institutes of Health”. Experiments were approved by the ethics committee at “The First Affiliated Hospital of Zhengzhou University”. Informed consents were obtained from the human participants of this study.2.2 Cell transfection
CC cells such as the Me180, HeLa and normal ectocervical cells Ect1/E6E7 were purchased from ATCC (Manassas, VA, USA) and cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 0.05% penicillin/streptomycin. Small interfering/hairpin RNA (siRNA/shRNA) targeting NEAT1 (si-NEAT1, sh-NEAT1), negative control (si-NC, sh-NC), pcDNA, NEAT1 overexpression vector and E2F7 overexpression vector were synthesized by Genepharma (Shanghai, China). MiR-889-3p mimics, the miR-889-3p inhibitor (in-miR-889-3p), a negative control (miR-NC) and a negative control inhibitor (in-miR-NC) were purchased from RIBOBIO (Guangzhou, China). The plasmids were transfected in the Me180 and HeLa cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).2.3 Quantitative real-time polymerase chain reaction (qRT-PCR)
The CC tissues and cells were incubated with the TRIzol reagent (Invitrogen) to extract total RNA. cDNAs for NEAT1, miR-889-3p, and E2F7 were synthesized using the All-in-One™ First-Strand cDNA Synthesis Kit (FulenGen, Guangzhou, China). Lastly, the qRT-PCR was performed using SYBR Green (Applied Biosystems, Foster City, CA, USA). GAPDH and U6 were exploited as internal references. The primers for NEAT1, miR-889-3p, and E2F7 are listed as follows: NEAT1, (Forward, 5′-CTTCCTCCCTTTAACTTATCCATTCAC-3′; Reverse, 5′-CTCTTCCTCCACCATTACCAACAATAC-3′); miR-889-3p, (Forward, 5′-ACACTCCAGCTGGGTTAATATCGGACAAC-3′; Reverse, 5′-TGGTGTCGTGGAGTCG-3′); E2F7 (Forward, 5′-GGACACCCACAGCCAACCT-3′; Reverse, 5′-CGTGCCAACCATACTGATTCTTAG-3′); GAPDH, (Forward, 5′-AGGTCGGTGTGAACGGATTTG-3′; Reverse, 5′-GGGGTCGTTGATGGCAACA-3′); U6, (Forward, 5′-ACCCTGAGAAATACCCTCACAT-3′; Reverse, 5′-GACGACTGAGCCCCTGATG-3′).2.4 Luciferase reporter assay
Herein, wild-type NEAT1 (NEAT1 WT) and E2F7 (E2F7 3′UTR WT) and mutant-type NEAT1 (NEAT1 MUT) and E2F7 (E2F7 3′UTR MUT) luciferase vectors were constructed. Immediately, the vectors were co-transfected with the miR-889-3p mimics or miR-NC in the Me180 and HeLa cells using the Lipofectamine 2000 transfection reagent (Invitrogen). Lastly, luciferase activities were determined by a dual-luciferase reporter system using a luminometer (Promega GloMax 20/20 Luminometer).2.5 RNA immunoprecipitation (RIP)
The Magna RNA immunoprecipitation kit (Millipore, Massachusetts, USA) was exploited for the RIP assay. In brief, the Me180 and HeLa cells transfected with the miR-889-3p mimics and miR-NC were lysed by RIP buffer. The magnetic beads were coated with the anti-Ago2 or IgG antibody, and the cell lysate was incubated with the magnetic beads; the enriched NEAT1 or E2F7 was subjected to the qRT-PCR.2.6 Western blot
The protein expressions of E2F7, p-AKT, AKT, p-PI3K and PI3K extracted from the Me180 and HeLa cells were assessed by the western blot assay. In brief, the protein samples were separated by electrophoresis, transferred onto polyvinylidene fluoride membranes (Millipore), and blocked by 5% nonfat milk. Then, the membranes were incubated with primary antibodies against E2F7, p-AKT, AKT, p-PI3K and PI3K (Abcam, Cambridge, MA, USA) and HRP-conjugated secondary antibody (Sangon, Shanghai, China).2.7 Cell counting kit-8 (CCK-8) assay
The transfected Me180 and HeLa cells were placed on 96-well plates and allowed to grow for 24 h, 48 h and 72 h. After this, 10 μL CCK-8 (Beyotime, Shanghai, China) was added to each well and allowed to react with the cells for 2 h. Subsequently, the OD value at 450 nm was detected by a spectrophotometer.2.8 Transwell assay
For the migration assay, the transfected Me180 and HeLa cells were placed on the upper chamber of transwell for 48 h. Then, the migrated cells at the lower chamber of transwell were stained with 0.1% crystal violet (Sigma, St. Louis, MO, USA). For the invasion assay, the upper chamber of transwell was pre-coated with Matrigel. The following procedure was the same as that of the migration assay. Then, the migration and invasion rates were determined using a microscope.2.9 Flow cytometry
The transfected Me180 and HeLa cells were placed on 24-well plates for 48 h. Then, the cells were harvested and co-stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) using the Apoptosis Detection Kit (Vazyme, Nanjing, China). The apoptotic rate was measured by a flow cytometer.2.10 Animal models
Male nude mice (4–5 weeks old, n = 6) were purchased from the National Rodent Laboratory Animal Resources, Shanghai Branch (China). The Me180 cells were stably transfected with sh-NEAT1 and sh-NC. The stably transfected Me180 cells were subcutaneously injected into the mice to obtain xenograft mice. Tumor volumes were measured till day 28. The mice were sacrificed, and the tumors were obtained and weighed. All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of “National Institutes of Health” and approved by the Animal Ethics Committee of “The First Affiliated Hospital of Zhengzhou University”.2.11 Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed by the SPSS software and GraphPad Prism 7. The correlation of miR-889-3p with NEAT1 and E2F7 was analyzed by the Pearson's correlation coefficient. A P value less than 0.05 (P < 0.05) was considered statistically significant.3. Results
3.1 Up-regulation of NEAT1 and E2F7 while down-regulation of miR-889-3p in CC
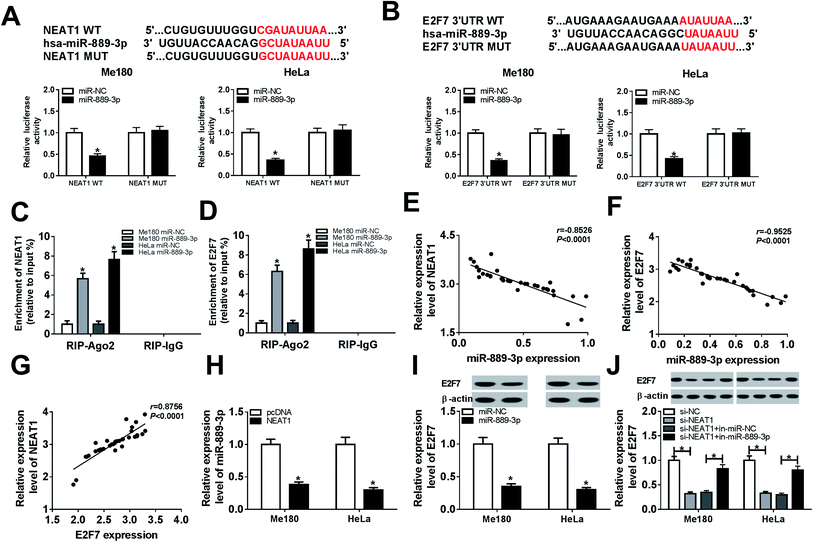
The potential roles of NEAT1, miR-889-3p and E2F7 during CC progression were evaluated by the qRT-PCR. As illustrated in Fig. 1A and B, the NEAT1 expression was extremely higher in the CC tumors and cells (Me180 and HeLa) than that in normal tissues and cells (Ect1/E6E7). Consistently, the E2F7 expression was up-regulated in the CC tumors and cells when compared with that in the corresponding normal tissues and cells (Fig. 1E and F). Conversely, a decreased expression of miR-889-3p was noticed in the CC tumors and cells when compared with that in the normal counterparts (Fig. 1C and D). These findings implied that NEAT1 and E2F7 might function as promoters, whereas miR-889-3p might function as a suppressor in CC.3.2 The interaction of miR-889-3p with NEAT1 and E2F7
Bioinformatics analysis by DianaTools-lncbasev2 has exhibited that putative binding sites exist between miR-889-3p and NEAT1. Declined luciferase activity was observed in the Me180 and HeLa cells co-transfected with NEAT1 WT and miR-889-3p, whereas the luciferase activity remained unchanged in the case of the NEAT1 MUT transfection group; this confirmed the interaction between miR-889-3p and NEAT1 (Fig. 2A). Similarly, by searching through Diana Tools-microT CDS, we discovered that miR-889-3p could bind to the 3′ untranslated regions (3′UTR) of E2F7. The luciferase activity was decreased dramatically in the Me180 and HeLa cells co-transfected with E2F7 3′UTR WT and miR-889-3p; this validated the interaction between E2F7 and miR-889-3p (Fig. 2B). In addition, increased enrichment of NEAT1 and E2F7 was observed in the Me180 and HeLa cells transfected with miR-889-3p, as examined by the RIP assay (Fig. 2C and D). Through analysis using the Pearson's correlation coefficient, we proved that miR-889-3p was negatively correlated with NEAT1 (r = −0.8526, P < 0.0001) and E2F7 (r = −0.9525, P < 0.0001) (Fig. 2E and F, respectively). However, NEAT1 was positively correlated with E2F7 (r = 0.8756, P < 0.0001) (Fig. 2G). More importantly, the expression of miR-889-3p was inhibited by NEAT1 (Fig. 2H). Similarly, the E2F7 protein expression was repressed by miR-889-3p (Fig. 2I). As expected, the miR-889-3p inhibitor inversed the NEAT1 silencing-mediated inhibition effects on the E2F7 protein production (Fig. 2J). Overall, NEAT1 could regulate the E2F7 expression by interacting with miR-889-3p in CC.3.3 NEAT1 regulated cell proliferation, migration, invasion and apoptosis in CC by sponging miR-889-3p
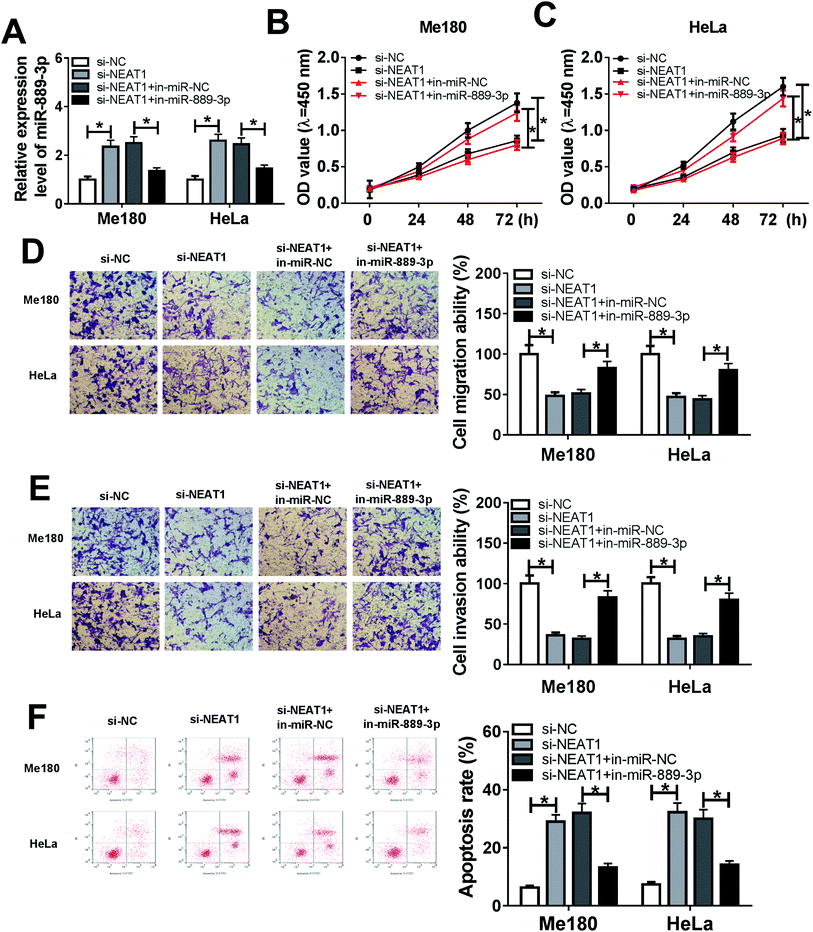
The Me180 and HeLa cells were transfected with si-NC, si-NEAT1, si-NEAT1+in-miR-NC and si-NEAT1+in-miR-889-3p to investigate the regulatory effects of the NEAT1/miR-889-3p axis on the CC progression. As displayed in Fig. 3A, the expression of miR-889-3p was increased by NEAT1 silencing and decreased by the miR-889-3p inhibitor. Moreover, cell proliferation was suppressed by NEAT1 silencing, and the miR-889-3p inhibitor reversed these effects (Fig. 3B and C). Similarly, the miR-889-3p inhibitor restored the NEAT1 silencing-induced inhibition effects on cell migration and invasion (Fig. 3D and E). By contrast, cell apoptosis was accelerated by NEAT1 silencing. However, the miR-889-3p inhibitor reversed the promotion effects (Fig. 3F). All these data indicated that NEAT1 could regulate cell proliferation, migration, invasion and apoptosis in CC by sponging miR-889-3p.3.4 Restoration of E2F7 counteracted the inhibition effects of miR-889-3p on cell proliferation, migration, and invasion and the promotion effects on apoptosis in CC
To explore the regulatory effects of the miR-889-3p/E2F7 axis on the CC cell development, the Me180 and HeLa cells were transfected with miR-NC, miR-889-3p, miR-889-3p+pcDNA and miR-889-3p+E2F7. As shown in Fig. 4A, the E2F7 protein expression was reduced in the CC cells transfected with miR-889-3p and enhanced after E2F7 transfection. The CCK-8 results revealed that miR-889-3p markedly inhibited cell growth, whereas E2F7 evidently contributed to the CC cell growth (Fig. 4B and C). Similarly, cell migration and invasion were restricted by miR-889-3p, and E2F7 reversed these suppressive effects (Fig. 4D and E). On the other hand, the restoration of E2F7 abolished the accelerating effects of miR-889-3p on cell apoptosis (Fig. 4F); collectively, the restoration of E2F7 neutralized the inhibition effects of miR-889-3p on cell proliferation, migration, and invasion and the promotion effects on apoptosis in CC.3.5 NEAT1 contributed to the activation of the PI3K/AKT pathway in CC by targeting the miR-889-3p/E2F7 axis
Previous studies have clarified that the PI3K/AKT signaling pathway is widely implicated in cell proliferation, migration and apoptosis. Thus, the molecular mechanism of NEAT1 for CC cell progression was studied by the western blot assay after the transfection of si-NC, si-NEAT1, si-NEAT1+in-miR-NC, si-NEAT1+in-miR-889-3p, miR-NC, miR-889-3p, miR-889-3p+pcDNA and miR-889-3p+E2F7 in the Me180 and HeLa cells. The expressions of p-AKT and p-PI3K were blocked by NEAT1 silencing and boosted by the miR-889-3p inhibitor (Fig. 5A). By calculation, we discovered that the miR-889-3p inhibitor recovered the inhibition effects of NEAT1 silencing on relative protein expressions (p-AKT/AKT, p-PI3K/PI3K) (Fig. 5B and C). Moreover, miR-889-3p restrained, whereas E2F7 facilitated the p-AKT and p-PI3K protein expressions in the CC cells (Fig. 5D). Similarly, the relative protein expressions (p-AKT/AKT and p-PI3K/PI3K) were reduced in the miR-889-3p-transfected cells and enhanced in the E2F7-transfected cells (Fig. 5E and F). In short, NEAT1 regulated cell progression by targeting the miR-889-3p/E2F7 axis through the activation of the PI3K/AKT pathway in CC.3.6 NEAT1 inversed the LY-induced inhibition of the activation of the PI3K/AKT pathway
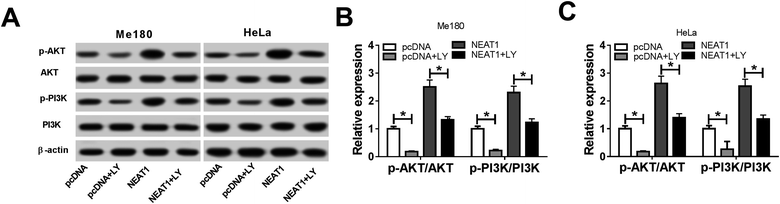
The Me180 and HeLa cells were treated with LY (a PI3K/AKT pathway inhibitor) for 6 h to block the PI3K/AKT pathway and transfected with pcDNA and NEAT1 to further illuminate the function of NEAT1 during the CC cell progression. As expected, reduced expressions of the p-AKT and p-PI3K protein were found after the LY treatment. However, the expressions of the p-AKT and p-PI3K proteins recovered after the NEAT1 transfection (Fig. 6A). After normalization, we found that NEAT1 attenuated the LY-induced inhibition of the relative protein expressions (p-AKT/AKT, p-PI3K/PI3K) (Fig. 6B and C). Altogether, we demonstrated that NEAT1 could reverse the induced inhibition effects on the activation of the PI3K/AKT pathway.3.7 Elimination of NEAT1 hindered tumor growth in vivo
Xenograft mice were established by subcutaneously injecting the Me180 cells stably transfected with sh-NEAT1 and sh-NC to explore the influences of NEAT1 on tumor development in vivo. Obviously, tumor growth was attenuated in the sh-NEAT1-transfected mice (Fig. 7A). Tumor weight was significantly lower in the mice transfected with sh-NEAT1 when compared with the case of the sh-NC group (Fig. 7B). The biological analysis results indicated that NEAT1 was inhibited, whereas miR-889-3p was promoted in the tumors obtained from the sh-NEAT1-transfected mice (Fig. 7C and D). Moreover, the expression of the E2F7 protein was reduced in the tumors of the sh-NEAT1 group when compared with the case of the sh-NC group (Fig. 7E). Therefore, we concluded that the elimination of NEAT1 inhibited tumor growth in vivo.4. Discussion
Emerging evidences indicate that NEAT1 is a significant prognostic biomarker for multiple medical conditions such as cardiovascular disease, myocardial ischemia-reperfusion injury and cancers.22,23 For example, NEAT1 positively contributes to myocardial ischemia-reperfusion injury by interacting with miR-495-3p and activating MAPK6.24 In addition, when NEAT1 is present in high levels, it promotes cell proliferation and migration by absorbing miR-302a-3p to regulate RELA in pancreatic ductal adenocarcinoma. Consistently, the up-regulation of NEAT1 accelerates cell progression in melanoma by improving E2F3 via miR-495-3p sponging.25 Moreover, the NEAT1/miR-23a-3p/SMC1A feedback loop has been reported to facilitate cell cycle and survival and attenuate apoptosis in acute myeloid leukemia.26 Furthermore, NEAT1 is closely associated with radio-sensitivity. The suppression of NEAT1 lowers the radioactive iodine resistance of the cells by targeting miR-101-3p to regulate FN1 in papillary thyroid carcinoma through the alteration of PI3K-AKT signaling.27 Therefore, we suggested that NEAT1 could modulate cell behavior by interacting with a specific target gene in CC.According to the bioinformatics analysis and prediction by the DianaTools-lncbasev2, we have discovered that miR-889-3p contains the binding sites for NEAT1. The luciferase reporter system and RIP assay further confirmed the relationship between NEAT1 and the miR-889-3p axis. Previous studies have shown that miR-889 typically acts as tumor promoter, whereas miR-889-3p functions as a suppressor. For example, Wu et al. reported that miR-889 accelerated non-small cell lung cancer cell proliferation and invasion by binding to KLF9.28 Consistently, miR-889 in high levels facilitates tumor development in vitro and in vivo by targeting DAB2IP in esophageal squamous cell carcinomas.29 Furthermore, histone deacetylase inhibitors are capable of reducing the expression of miR-889 in hepatocellular carcinoma, causing improved resistance to natural killer cytotoxicity.30 However, the regulatory effects of miR-889-3p in CC progression are poorly understood.
We hypothesized that NEAT1 accelerated cell development by interacting with miR-889-3p in CC. Initially, we discovered that the expressions of NEAT1 and E2F7 were up-regulated, whereas the expression of miR-889-3p was down-regulated in the CC tumors and cells when compared with the case of the corresponding normal counterparts; this suggested the oncogenic role of NEAT1 and E2F7 and suppressive role of miR-889-3p in CC. Subsequently, the interaction of miR-889-3p with NEAT1 and E2F7 was also determined by the luciferase reporter system and RIP assay. The Pearson's correlation coefficient analysis revealed that miR-889-3p was negatively correlated with NEAT1 and E2F7. The rescue experiments exhibited that the miR-889-3p inhibitor attenuated the NEAT1 silencing-induced inhibition effects on cell proliferation, migration, and invasion and the promotion effects on apoptosis in CC. Similarly, the restoration of E2F7 rescued the miR-889-3p-mediated inhibition of cell progression in CC. As is well-known, the PI3K/AKT pathway is involved in the progression of multiple cancers. To disclose the underlying biological mechanism, the western blot assay was exploited to analyze the relative protein expressions. The results demonstrated that NEAT1 modulated cell behavior by targeting the miR-889-3p/E2F7 axis and activating the PI3K/AKT pathway. Moreover, animal experiments showed that the elimination of NEAT1 inhibited tumor growth in vivo, further validating the oncogenic role of NEAT1.
In conclusion, we revealed that NEAT1 contributed to cell viability, migration, and invasion and suppressed apoptosis in CC by sponging miR-889-3p to improve the E2F7 expression. In addition, cell progression regulation was exerted by the activation of the PI3K/AKT pathway. Our study provides promising biomarkers for the diagnosis and therapy of CC.
Funding
None.Conflicts of interest
There is no conflict of interest regarding the publication of this paper.References
- Y. Fan, Y. Nan, J. Huang, H. Zhong and W. Zhou, Biosci. Rep., 2018, 38(3), BSR20180483 CrossRef CAS.
- W. Small Jr, M. A. Bacon, A. Bajaj, L. T. Chuang, B. J. Fisher, M. M. Harkenrider, A. Jhingran, H. C. Kitchener, L. R. Mileshkin, A. N. Viswanathan and D. K. Gaffney, Cancer, 2017, 123, 2404–2412 CrossRef.
- K. Matsuo, H. Machida, E. A. Blake, T. Takiuchi, M. Mikami and L. D. Roman, Eur. J. Surg. Oncol., 2017, 43, 725–734 CrossRef CAS.
- M. Saei Ghare Naz, N. Kariman, A. Ebadi, G. Ozgoli, V. Ghasemi and F. Rashidi Fakari, Asian Pac. J. Cancer Prev., 2018, 19, 875–884 Search PubMed.
- J. Li, Z. P. Chu, H. Han, Y. Zhang, F. Tian, J. Q. Zhang and X.-H. Huang, Hum. Cell, 2019, 32, 160–171 CrossRef PubMed.
- B. A. Quinn, X. Deng, A. Colton, D. Bandyopadhyay, J. S. Carter and E. C. Fields, Brachytherapy, 2019, 18, 29–37 CrossRef.
- Y. Li, G. Liu, X. Li, H. Dong, W. Xiao and S. Lu, Biochem. Biophys. Res. Commun., 2018, 503, 2826–2832 CrossRef CAS.
- Y. Li, C. Zeng, J. Hu, Y. Pan, Y. Shan, B. Liu and L. Jia, J. Hematol. Oncol., 2018, 11, 89 CrossRef PubMed.
- Y. Li, C. Li, D. Li, L. Yang, J. Jin and B. Zhang, OncoTargets Ther., 2019, 12, 2649–2660 CrossRef.
- W. Zhong, J. Yang, M. Li, L. Li and A. Li, J. Cell. Physiol., 2019, 234, 11567–11576 CrossRef CAS PubMed.
- Z. He, J. Dang, A. Song, X. Cui, Z. Ma and Z. Zhang, J. Cell. Physiol., 2019, 234, 19582–19591 CrossRef CAS PubMed.
- X. Yu, X. Liu, R. Wang and L. Wang, Biochem. Biophys. Res. Commun., 2019, 512, 825–831 CrossRef CAS.
- L. Y. Yuan, M. Zhou, H. Lv, X. Qin, J. Zhou, X. Mao, X. Li, Y. Xu, Y. Liu and H. Xing, J. Cell. Physiol., 2019, 234, 18985–18993 CAS.
- K. Yan, Y. Fu, N. Zhu, Z. Wang, J.-l. Hong, Y. Li, W.-j. Li, H.-b. Zhang and J.-h. Song, Int. J. Biochem. Cell Biol., 2019, 110, 1–8 CrossRef CAS.
- D. Zhao, Y. Sui and X. Zheng, Oncol. Rep., 2016, 35, 1075–1082 CrossRef CAS.
- M. Yang, X. Zhai, T. Ge, C. Yang and G. Lou, Oncol. Res., 2018, 26, 703–712 CrossRef.
- Y. Yin, B. Zhang, W. Wang, B. Fei, C. Quan, J. Zhang, M. Song, Z. Bian, Q. Wang, S. Ni, Y. Hu, Y. Mao, L. Zhou, Y. Wang, J. Yu, X. Du, D. Hua and Z. Huang, Clin. Cancer Res., 2014, 20, 6187–6199 CrossRef CAS.
- Y. Zhang, H. Cai, S. Chen, D. Sun, D. Zhang and Y. He, J. Cell. Biochem., 2019, 13187 CrossRef CAS.
- Q. Zhang, Y. Li, M. Zhao, H. Lin, W. Wang, D. Li, W. Cui, C. Zhou, J. Zhong and C. Huang, Sci. Rep., 2019, 9, 3008 CrossRef.
- Z.-H. L. Y. Xiao and Y.-H. Bi, Eur. Rev. Med. Pharmacol. Sci., 2019, 23, 3326–3334 Search PubMed.
- Y. Sun, Y. Cheng, Y. Zhang and K. Han, Exp. Ther. Med., 2019, 18, 1440–1448 CAS.
- Z. Liu, K. Wu, J. Wu, D. Tian, Y. Chen, Z. Yang and A. Wu, J. Cell. Biochem., 2019, 120, 9831–9838 CrossRef CAS.
- M. Zhang, X. Wang, J. Yao and Z. Qiu, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 3129–3137 CrossRef CAS.
- M. Luo, Q. Sun, H. Zhao, J. Tao and D. Yan, J. Cell. Physiol., 2019, 105 Search PubMed.
- Y. Xia, Y. Zhou, H. Han, P. Li, W. Wei and N. Lin, J. Cell. Physiol., 2019, 234, 19592–19601 CrossRef CAS.
- C. Zhao, S. Wang, Y. Zhao, F. Du, W. Wang, P. Lv and L. Qi, J. Cell. Physiol., 2019, 234, 6161–6172 CrossRef CAS.
- C. Liu, Z. Feng, T. Chen, J. Lv, P. Liu, L. Jia, J. Zhu, F. Chen, C. Yang and Z. Deng, Cell Cycle, 2019, 18, 167–203 CrossRef CAS.
- X. Han, Y. Tang, Y. Dai, S. Hu, J. Zhou, X. Liu, J. Zhu and Y. Wu, Gene, 2019, 699, 94–101 CrossRef CAS.
- Y. Xu, J. He, Y. Wang, X. Zhu, Q. Pan, Q. Xie and F. Sun, FEBS Lett., 2015, 589, 1127–1135 CrossRef CAS.
- H. Xie, Q. Zhang, H. Zhou, J. Zhou, J. Zhang, Y. Jiang, J. Wang, X. Meng, L. Zeng and X. Jiang, Cytotechnology, 2018, 70, 513–521 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |