Open Access Article
Open Access ArticleThe role of cation and anion dopant incorporated into a ZnO electron transporting layer for polymer bulk heterojunction solar cells†
Soyeon Kima,
Jaehoon Jeonga,
Quoc Viet Hoangb,
Joo Won Hanc,
Adi Prasetioac,
Muhammad Jahandar a,
Yong Hyun Kim
a,
Yong Hyun Kim c,
Shinuk Cho
c,
Shinuk Cho *d and
Dong Chan Lim*a
*d and
Dong Chan Lim*a
aSurface Technology Division, Korea Institute of Materials Science (KIMS), 797 Changwondaero, Seongsan-gu, Changwon, Gyeongnam 51508, Republic of Korea. E-mail: dclim@kims.re.kr
bVietnam–Korea Technological Innovation Center, Directorate for Standards, Metrology and Quality (STAMEQ), No. 8 Hoang Quoc Viet, Cau Giay, Ha Noi, Vietnam
cDepartment of Display Engineering, Pukyong National University, Busan 48513, Republic of Korea
dDepartment of Physics, EHSRC, University of Ulsan, Ulsan 44610, Republic of Korea. E-mail: sucho@ulsan.ac.kr
First published on 19th November 2019
Abstract
Doping is a widely-implemented strategy for enhancing the inherent electronic properties of charge transport layers in photovoltaic devices. A facile solution-processed zinc oxide (ZnO) and various cation and anion-doped ZnO layers were synthesized via the sol–gel method and employed as electron transport layers (ETLs) for inverted polymer solar cells (PSCs). The results indicated that all PSCs with doped ZnO ETLs exhibited better photovoltaic performance compared with the PSCs with a pristine ZnO ETL. By exploring the role of various anion and cation dopants (three compounds with the same Al3+ cation: Al(acac)3, Al(NO3)3, AlCl3 and three compounds with the same Cl− anion: NH4Cl, MgCl2, AlCl3), we found that the work function changed to favor electronic extraction only when the Cl anion was involved. In addition, the conductivity of ZnO was enhanced more with the Al3+ cation. Therefore, in inverted solar cells, doping with Al3+ and Cl− delivered the best power conversion efficiency (PCE). The maximum PCE of 10.38% was achieved from the device with ZnO doped with Al+ and Cl−.
1. Introduction
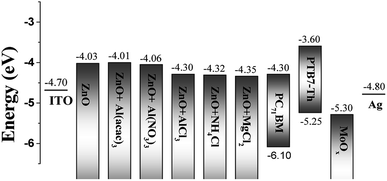
Bulk heterojunction (BHJ) polymer solar cells (PSCs) have attracted substantial attention for their potential applications in large-area flexible devices due to their unique advantages, such as being inexpensive and easy to fabricate, low weight, and possessing mechanical flexibility.1–4 After extensive studies on new conjugated polymers and new charge transport interlayers, single-junction PSCs have recently achieved an impressive power conversion efficiency (PCE) of over 15%.5,6In general, according to the hole and electron extraction direction, PSCs are classified as two types, conventional structure PSCs and inverted structure PSCs. Among them, the inverted structure has been preferred recently because it is known that inverted PSCs exhibit better stability.33,34 The inverted PSCs are basically composed of sequential stacking of the electron transport layer (ETL), photoactive layer, and hole transport layer (HTL) between the transparent indium tin oxide (ITO) and top metal electrode. In these inverted PSCs, since we utilize the ITO as a cathode electrode, a noble metal with a high work function (WF) such as Ag or Au must be used as a top anode electrode. Between Ag and Au, the use of silver is preferred because of its cheap price and easy processability. However, Ag has a similar WF to ITO (WF of ITO ∼ 4.7 eV, WF of Ag ∼ 4.6 eV). Thus, the inverted PSC with an Ag anode has an insufficient electromotive force for photocurrents. Increasing the potential difference of inverted PSCs is another role of the HTL and ETL layers. Therefore, it is very important that the charge transport layer not only has a good conductivity but also has a proper WF value capable of producing a sufficient driving potential.7,8,10
Various metal oxide materials have been utilized as charge transporting functional layers to date. Examples of metal oxide with good electron transport properties include ZnO,11–15 TiOx,16–18 SnO2,19–21 and Zr(acac)4.22,23 Examples metal oxides with good hole transport properties include MoOx,15,24–26 V2O5,26–28 NiOx,29,30 and WOx.31,32 Among ETLs, ZnO has been most widely used due to its high electron mobility, suitable work function, transparency, environmental stability, facile solution processing, low cost, and simple modification.35,36 In the fabrication of organic solar cells, ZnO is mainly prepared using the sol–gel method. However, wet-chemically prepared ZnO shows relatively low conductivity. In addition, it easily forms trap states associated with defects, which may induce a reduction of the PCE.35–37
To achieve higher performance by overcoming both the low conductivity and defect problem, either surface modification or doping with an ionic element have been applied. Several surface modification methods, including the insertion of self-assembled monolayers,9,38 treatment with either amine or an alcohol polar solvent,39,40 and the introduction of fullerene-based layers,41,42 have been employed between the active layer and the ZnO ETL.
However, these methods affect only the surface of a ZnO layer. Thus, the intrinsic opto-electrical properties of ZnO are not substantially improved. As an alternative way to favorably change the properties of the bulk ZnO layer, element doping using aluminum,43,44 indium,45 magnesium,46 chlorine,47 and hydrogen48 have been applied, resulting in significantly improved photovoltaic performance and stability by eliminating the defect states.
There are two kinds of vacancy defects in solution-processed ZnO. One is a Zn vacancy, and the other one is an oxygen vacancy. The Zn vacancy can be filled by cation dopants. For example, small radius Al3+ ions (0.54 Å) better occupy interstitial sites in a ZnO lattice compared with larger Zn2+ ions (0.74 Å).49–51 On other hand, defects of oxygen vacancies in ZnO ETL possibly can be filled with small and medium-sized anions, such as fluoride, chloride, bromide, and iodide.52–59 Until now, studies on ZnO doping have focused more on cation doping than on anion doping. Rather, non-excessive oxygen vacancies were expected to help with conductivity enhancement because the oxygen vacancy generally acts as an electron donor in metal oxides. In a study on ZnO ETLs, the relationship between oxygen vacancies and electrical properties has rarely been studied, and little is known about how oxygen vacancies affect the electrical properties of a solution-processed ZnO ETL.
In this study, we have systematically investigated the influence of the cation and anion dopants on the electrical properties of a ZnO ETL through the simultaneous doping of both cations and anions. To investigate the influence of anions, Al(acac)3 (acac = acetylacetonate), Al(NO3)3, and AlCl3 were introduced as dopants. These three dopants (Cl−, NO3−, acac−) have the same number of different anions together with the same Al cation, thus they provide proper combinations to investigate the effects of different anions. The influence of cations was investigated using NH4Cl, AlCl3, and MgCl2. In addition, these three dopants (Al3+, Mg2+, NH4+) have different numbers of Cl anions, thereby we can collect information about the influence of the number of anions together. To eliminate concerns about the dependence on the photoactive material, the doped ZnO layer was applied on various solar cells fabricated with three different photoactive materials, PTB7-Th:PC71BM, PTB7:PC71BM, and P3HT:PC71BM.
2. Results and discussions
2.1. Optoelectronic properties and morphology
Chemically synthesized nanoparticles or sol–gel driven ZnO thin films are commonly used for the ETL of PCSs. The nanoparticle method allows a lower annealing temperature compared with the sol–gel method, but it is difficult to simultaneously dope cations and anions in ZnO because the anion can be possibly removed during the centrifugal step. Thus, in this work, we prepared doped ZnO by the simple sol–gel method using Zn(CH3COO)2 in 2-methoxyethanol. The details are provided in the Experimental section.| Layer | Mean resistancea (Ω) | Sheet resistancea (Ω per square) | Work functionb (eV) |
|---|---|---|---|
| a Mean and sheet resistances were characterized from 4-point measurements on 3 different points.b Work functions were characterized from the UPS. | |||
| ITO/ZnO | 1.99 ± 0.14 | 9.01 ± 0.63 | 4.03 |
| ITO/ZnO + Al(acac)3 | 1.85 ± 0.11 | 8.39 ± 0.50 | 4.01 |
| ITO/ZnO + Al(NO3)3 | 1.58 ± 0.15 | 7.15 ± 0.68 | 4.06 |
| ITO/ZnO + AlCl3 | 1.40 ± 0.12 | 6.33 ± 0.54 | 4.30 |
| ITO/ZnO + NH4Cl | 1.68 ± 0.15 | 7.61 ± 0.67 | 4.32 |
| ITO/ZnO + MgCl2 | 1.57 ± 0.11 | 7.10 ± 0.49 | 4.35 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O) in pristine ZnO and Al(acac)3 doped ZnO were 3.56 and 3.58, respectively. Just a small increase in the C of C–C and C–H indicated that there was a low presence of acetylacetonate in the ZnO layer, which means the influence of acetylacetonate on the ZnO layer seems to be negligible. A slight enhancement of the resistance in Al(acac)3-doped ZnO seems to be mainly due to Al.
O, C–O) in pristine ZnO and Al(acac)3 doped ZnO were 3.56 and 3.58, respectively. Just a small increase in the C of C–C and C–H indicated that there was a low presence of acetylacetonate in the ZnO layer, which means the influence of acetylacetonate on the ZnO layer seems to be negligible. A slight enhancement of the resistance in Al(acac)3-doped ZnO seems to be mainly due to Al.
For the ZnO layer doped with Al(NO3)3, the N 1s peak was revealed in the energy range of 390–410 eV (Fig. 1c and S6†). The N 1s signal of Al(NO3)3-doped ZnO consists of two peaks at 398.5 eV and 406.4 eV, belonging to residual ethanolamine and nitrate, respectively. For the NH4Cl-doped ZnO, the N 1s peaks were detected at 396.0 eV and 399.0 eV, which were attributed to ammonium and residual ethanolamine, respectively. The XPS spectra of AlCl3-doped ZnO, NH4Cl-doped ZnO and MgCl2-doped ZnO showed metal chloride-Cl 2p peaks at 198.7 eV, 199.0 eV, and 199.0 eV, and nonmetal chloride–Cl 2p peaks at 200.3 eV, 201.0 eV and 201.0 eV, respectively (Fig. 1d–f and S7†). A slight shifting of the Cl 2p peak to a lower binding energy in AlCl3-doped ZnO indicates that AlCl3-doped ZnO has a better metallic property compared with the ZnO doped with NH4Cl or MgCl2. This result was consistent with the 4-point probe results in Table 1. AlCl3-doped ZnO shows a better conductivity than NH4Cl-doped ZnO and MgCl2-doped ZnO.
 | ||
| Fig. 2 AFM images of ZnO ETLs. (a) Pristine ZnO, (b) ZnO doped with Al(acac)3, (c) ZnO doped with Al(NO3)3, (d) ZnO doped with AlCl3, (e) ZnO doped with NH4Cl, (f) ZnO doped with MgCl2. | ||
In comparing the three ZnO films doped with identical cations (Al3+) and different anions (Cl−, NO3−, acac−), the morphology of ZnO doped with Al(acac)3 or Al(NO3)3 showed a morphology similar to the pristine ZnO ripple. However, the ZnO film doped with AlCl3 showed a relatively homogeneous peak-to-peak structure and smoother surface, with a significantly reduced rms of 4.60 nm. These results clearly indicate that the Cl− anion affected the ZnO morphology more strongly than acac− and NO3−, which might due to the easier occupation of oxygen vacancies by the smaller Cl− anion relative to the larger acac− and NO3− anions. Comparing the ZnO films doped with the identical Cl− anion (Fig. 2d–f), NH4+- and Mg2+-doped ZnO films showed a more smooth and flat surface structure with rms values of 2.30 nm and 1.67 nm, respectively. Although the wrinkled structure of ZnO delivered some enhancement of the light absorption via light scattering, the current homogeneity can be significantly disturbed at the interface between the photoactive and ZnO layers. Therefore, it is necessary to derive an appropriate optimal point between the morphology change by doping and the improvement of electric characteristics.
 | ||
| Fig. 4 (a) SCLC characteristics of PTB7-Th:PC71BM electron only devices (device structures: Al/LiF/PTB7-Th:PC71BM/ETLs/ITO), (b) Nyquist plots of PTB7-Th:PC71BM with various ETLs. | ||
Fig. 4b shows the Nyquist plots of impedance spectroscopy (EIS) measurements for PTB7-Th:PC71BM solar cells with pristine ZnO or doped ZnO films measured at open circuit potentials (OCP) under the dark condition. All devices exhibited one semicircle without a transmission line. Series resistances (Rs) and shunt resistances (Rsh) are extracted from a fitting line and the results are summarized in Table S1.† In general, a low Rs and high Rsh are desired to obtain high-performance solar cells. The device with a pristine ZnO film and the devices with doped ZnO films all perform acceptably with a high Rsh of over 5.0 kΩ, which is adequate for solar cells. All doped ZnO devices exhibit a smaller Rs than a pristine undoped ZnO device and among them, the AlCl3-doped ZnO device shows the lowest Rs.
2.2. Photovoltaic performance
 | ||
| Fig. 5 (a and b) J–V curves and (c and d) EQE spectra of devices with different electron transporting layers. | ||
| ETL | PCE best (%) | PCE avg.a (%) | FF (%) | Voc (V) | Jsc (mA cm−2) | Jsc b (mA cm−2) | Rsc (Ω) |
|---|---|---|---|---|---|---|---|
| a The average PCE from more than 12 devices.b Calculated Jsc from EQE spectra.c Extraction from solar simulation program.d Surface modifying ETLs with PEIE. | |||||||
| ZnO | 9.16 | 8.89 | 67.7 | 0.790 | 17.10 | 16.57 | 32.8 |
| ZnO + Al(acac)3 | 9.71 | 9.44 | 68.8 | 0.796 | 17.68 | 17.15 | 25.8 |
| ZnO + Al(NO3)3 | 9.79 | 9.57 | 69.0 | 0.799 | 17.74 | 17.32 | 26.5 |
| ZnO + AlCl3 | 10.38 | 10.15 | 71.3 | 0.807 | 18.01 | 17.64 | 22.4 |
| ZnO + NH4Cl | 9.73 | 9.43 | 69.3 | 0.802 | 17.47 | 17.09 | 28.0 |
| ZnO + MgCl2 | 9.83 | 9.68 | 69.1 | 0.803 | 17.70 | 17.23 | 28.2 |
| ZnO/PEIEd | 10.14 | 9.76 | 70.2 | 0.804 | 17.94 | 26.1 | |
| ZnO + AlCl3/PEIEd | 10.48 | 10.18 | 71.8 | 0.809 | 18.03 | 25.8 | |
Consequently, the combination of Al3+ and Cl− dopants boost the doping effect, thereby enhancing the PCE from 9.16% to 10.38%. In the case of solar cells based on P3HT or PTB7 as a donor polymer, the highest PCE was also obtained in the PSC with an AlCl3-doped ZnO ETL, as shown in Fig. S12, S13, Table S2 and S3.† Note that optimization details for a proper AlCl3 doping concentration and an optimal thickness of the AlCl3-doped ZnO ETL are presented in Fig. S14 and S15.† The optimal doping concentration and thickness are 2.5 mM and ∼120 nm, respectively.
In our solar cells results, all the solar cell parameters such as Jsc, FF, and Voc were improved with doped ZnO. The increase in Jsc was considered to be a result of the improvement of the charge transport property of the doped ZnO ETL (Fig. 4a). In case of FF, it might be attributed to reduced charge recombination and reduced resistance (Fig. 4b). For the Voc, slightly increase open circuit voltage was observed with doped ZnO ETL because of Fermi level shift. Generally, Voc change is also related to the photon energy loss. Therefore, higher Voc of the device with doped ZnO ETL was attributed to the reduction of both interfacial recombination and energy loss.
| ETL | PCE (%) | FF (%) | Voc (V) | Jsc (mA cm−2) | Rs (ohm) |
|---|---|---|---|---|---|
| ZnO | 8.00 | 58.7 | 6.914 | 1.973 | 35.688 |
| ZnO + AlCl3 | 9.34 | 66.4 | 7.213 | 1.950 | 24.319 |
| ZnO/PEIE | 8.66 | 66.9 | 7.027 | 1.843 | 22.818 |
| ZnO + AlCl3/PEIE | 9.36 | 67.6 | 7.207 | 1.922 | 21.965 |
3. Conclusions
We have successfully demonstrated an enhanced PCE of inverted solar cells by introducing a doped ZnO electron transport layer. Because of the synergistic effect of dual Al3+ and Cl− doping in the ZnO layer, the photovoltaic efficiency of inverted PSCs were significantly enhanced from 9.16% (with a pristine ZnO layer) to 10.48% (together with PEIE). The role of Al3+ was tied to the improvement of conductivity and electron mobility of ZnO. Meanwhile, Cl− induced the elimination of defects and a reduction in the work function, thereby improving the conductivity, electron mobility, and surface morphology. Comparing the various possible dopant utilized in this study, Al3+ shows better optoelectronic properties compared with other larger cations (Mg2+, NH4+ and Cl−) also allows better optoelectronic properties compared with other larger anions (NO3−, acac−). Consequently, the combination of Al3+ and Cl-dopants boost the doping effect, thereby enhancing the PCE.4. Experimental section
4.1. Materials and device fabrication
The polymer PTB7-Th (code PCE-10), polymer PTB7, polymer P3HT and PC71BM (purity >99% by HPLC) were purchased from 1-material and utilized without further purification. The high-grade doping precursors, Al(acac)3, Al(NO3)3·9H2O, AlCl3·6H2O, NH4Cl, MgCl2, and other chemicals and solvents were purchased from Sigma Aldrich. The sol–gel ZnO precursor solution (0.5 M) was prepared using Zn(CH3COOH)2·2H2O (1.09 g, 5 mmol) and 2-ethanolamine (500 μL) were dissolved in 2-methoxyethanol (10 mL). The solution was stirred at 60 °C for 2 hours then filtered using a 0.2 μm PTFE filter. Similar to the ZnO precursor solution, the sol–gel doped ZnO precursor solutions were obtained by adding salt sources (Al(acac)3: 2.5 mM, Al(NO3)3: 2.5 mM, AlCl3: 2.5 mM, NH4Cl: 7.5 mM, MgCl2: 3.7 mM) together with Zn(CH3COO)2·2H2O into 2-methoxyethanol. A 0.05% w/w PEIE solution was prepared with anhydrous ethanol. Solutions of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1.0
PC71BM (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 wt) or PTB7
1.5 wt) or PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1.0
PC71BM (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 wt), both with a total concentration of 20 mg mL−1, were dissolved in chlorobenzene
1.5 wt), both with a total concentration of 20 mg mL−1, were dissolved in chlorobenzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,8-diiodooctane (97
1,8-diiodooctane (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) and stirred at 70 °C overnight. P3HT
3 v/v) and stirred at 70 °C overnight. P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1.0
PC71BM (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 wt), with a total concentration of 40 mg mL−1 dissolved in 1,2-dichlorobenzene (ODCB). Then solutions were filtered through a 0.45 μm pore size PTFE and stored in an Argon-filled glovebox.
0.8 wt), with a total concentration of 40 mg mL−1 dissolved in 1,2-dichlorobenzene (ODCB). Then solutions were filtered through a 0.45 μm pore size PTFE and stored in an Argon-filled glovebox.
Inverted polymer solar cells were fabricated by spin-coating ZnO or the doped ZnO precursor on the UV-pretreated ITO (140 nm, 15 Ω sq−1) and annealing at 180 °C in air for 10 minutes to form a ∼50 nm thick ZnO layer. In the case using PEIE as a surface-modified layer, the PEIE solution was spun at 3000 rpm on the ZnO layers and then annealed at 100 °C in air for 10 minutes. The devices were transferred into the glovebox for spin-coating the active layer with controlled thickness. The active layer thicknesses of PTB7-Th:PC71BM, PTB7:PC71BM and P3HT:PC71BM were 120 ± 5 nm, 100 ± 5 nm and 240 ± 8 nm, respectively. In the case of P3HT, devices were annealed at 140 °C for 10 minutes. The resulting devices were then dried under high vacuum (<5 × 10−6 mbar) for 3 hours. Finally, MoO3 (5 nm) and Ag (100 nm) electrodes were sequentially deposited on the active layer through a shadow mask by thermal evaporation (<5 × 10−6 mbar), which defines the active area of 0.13 cm2. Module devices for out-of-lab testing were fabricated with the same procedures as described in the unit cell fabrication. The areas of the photoactive layers were 18.63 cm2. A module consists of a monolithic interconnection of individual stripe pattern sub-cells in series by covering the negative contact of one ITO substrate with the positive contact of the lateral top electrode.
4.2. Device characterization
The J–V curves of devices were characterized using a Keithley 2400 source with MODUSYS solar simulator (PS-KS2C) under AM 1.5G simulated illumination (100 mW cm−2). The EQE spectra were characterized using a QE/IPCE/spectra response measurement system (ABET Technologies). The resistance/square of ITO and ITO/ETLs were characterized by a 4-point probe set (MODUSYS). The transmittance spectra were measured using a UV-vis spectrometer (Varian Co., Carry 5000 model). Ultraviolet photoelectron spectroscopy (UPS) measurements of ITO/ETLs were carried out by a Multilab-2000 (Thermo Scientific) instrument using He I photons (hν = 21.22 eV). X-ray photoelectron spectroscopy (XPS) measurements of the Si wafer/ETLs were carried out by the same instrument, with the UPS using monochromatized Al Kα X-ray photons (hν = 1486.6 eV). Atomic force microscopy (AFM) measurements of ITO/ETLs were performed by a Dimension Icon Scanning Probe Microscope (Bruker). The electrochemical impedance spectroscopy (EIS) measurements were performed on a ZIVE SP5 (ZIVE LAB, Korea) in the frequency range from 1 MHz to 1 Hz, with a perturbation amplitude of 0.1 V. The impedance spectra were characterized at their open-circuit potentials (OCP) under the dark condition.To characterize the vertical electron mobility, a space charge limited current (SCLC) model was applied. The device structures of Al (100 nm)/LiF (0.5 nm)/PTB7-Th:PC71BM (120 nm)/ZnO or doped ZnO (40 nm)/ITO were used and J–V curves over the range 0–5 V were characterized in the dark condition. The electron mobilities were calculated using the SCLC model, where the SCLC is described by J = 9ε0εrμV2/8L3, where J is the current density, L is the film thickness of the active layer, μ is the electron mobility, εr is the relative dielectric constant of the transport medium, ε0 is the permittivity of free space (8.85 × 10−12 F m−1), V is the internal voltage in the device, and V = Vappl − Vbi, where Vappl is the applied voltage to the device and Vbi is the built-in voltage due to the relative work function difference of the two electrodes.
Author contributions
S. K., A. P., M. J. and Q. V. H. designed the fabricated solar cells and conducted the characterization. S. K., S. C. and D. C. L. wrote the manuscript. J. H. J. and Q. V. H. provided supporting materials and contacted the analysis center. J. W. H. and Y. H. K. gave valuable suggestions during the writing of the manuscript. D. C. L. supervised the whole project. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This research was mainly supported by a grant from the Korea Institute of Materials Science (KIMS) and the Korea Institute of Energy Technology Evaluation and Planning (KETEP, No. 20173030014180, No. 2018201010636A). The portion of this research conducted at the University of Ulsan was supported by a National Research Foundation of Korea grant (2017R1A2B4003583, 2017M2A2A6A01018599, 2019R1A6A1A11053838).References
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS.
- C. Wang, C. Li, R. C. I. Mackenzie, S. Wen, Y. Liu, P. Ma, G. Wang, W. Tian and S. Ruan, J. Mater. Chem. A, 2018, 6, 17662–17670 RSC.
- J. Subbiah, V. D. Mitchell, N. K. C. Hui, D. J. Jones and W. W. H. Wong, Angew. Chem., Int. Ed., 2017, 56, 8431–8434 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Nat. Commun., 2019, 10, 1–8 CrossRef CAS PubMed.
- V. Gupta, A. K. K. Kyaw, D. H. Wang, S. Chand, G. C. Bazan and A. J. Heeger, Sci. Rep., 2013, 3, 1965 CrossRef PubMed.
- C. Y. Jiang, X. W. Sun, D. W. Zhao, A. K. K. Kyaw and Y. N. Li, Sol. Energy Mater. Sol. Cells, 2010, 94, 1618–1621 CrossRef CAS.
- H.-L. Yip, S. K. Hau, N. S. Baek, H. Ma and A. K. Y. Jen, Adv. Mater., 2008, 20, 2376–2382 CrossRef CAS.
- D. W. Zhao, P. Liu, X. W. Sun, S. T. Tan, L. Ke and A. K. K. Kyaw, Appl. Phys. Lett., 2009, 95, 153304 CrossRef.
- X. Bulliard, S.-G. Ihn, S. Yun, Y. Kim, D. Choi, J.-Y. Choi, M. Kim, M. Sim, J.-H. Park, W. Choi and K. Cho, Adv. Funct. Mater., 2010, 20, 4381–4387 CrossRef CAS.
- J. Huang, Z. Yin and Q. Zheng, Energy Environ. Sci., 2011, 4, 3861–3877 RSC.
- Z. Liang, Q. Zhang, O. Wiranwetchayan, J. Xi, Z. Yang, K. Park, C. Li and G. Cao, Adv. Funct. Mater., 2012, 22, 2194–2201 CrossRef CAS.
- K.-D. Kim, D. C. Lim, J. Hu, J.-D. Kwon, M.-G. Jeong, H. O. Seo, J. Y. Lee, K.-Y. Jang, J.-H. Lim, K. H. Lee, Y. Jeong, Y. D. Kim and S. Cho, ACS Appl. Mater. Interfaces, 2013, 5, 8718–8723 CrossRef CAS PubMed.
- S. Nho, G. Baek, S. Park, B. R. Lee, M. J. Cha, D. C. Lim, J. H. Seo, S.-H. Oh, M. H. Song and S. Cho, Energy Environ. Sci., 2016, 9, 240–246 RSC.
- K. Lee, J. Y. Kim, S. H. Park, S. H. Kim, S. Cho and A. J. Heeger, Adv. Mater., 2007, 19, 2445–2449 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297–302 CrossRef CAS.
- X. Bao, L. Sun, W. Shen, C. Yang, W. Chen and R. Yang, J. Mater. Chem. A, 2014, 2, 1732–1737 RSC.
- S. Trost, K. Zilberberg, A. Behrendt and T. Riedl, J. Mater. Chem., 2012, 22, 16224–16229 RSC.
- B. Bob, T.-B. Song, C.-C. Chen, Z. Xu and Y. Yang, Chem. Mater., 2013, 25, 4725–4730 CrossRef CAS.
- S. Trost, A. Behrendt, T. Becker, A. Polywka, P. Görrn and T. Riedl, Adv. Energy Mater., 2015, 5, 1500277 CrossRef.
- Z. a. Tan, S. Li, F. Wang, D. Qian, J. Lin, J. Hou and Y. Li, Sci. Rep., 2014, 4, 4691 CrossRef PubMed.
- H. Fan and X. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 33856–33862 CrossRef CAS PubMed.
- J. J. Jasieniak, J. Seifter, J. Jo, T. Mates and A. J. Heeger, Adv. Funct. Mater., 2012, 22, 2594–2605 CrossRef CAS.
- B. J. Tremolet de Villers, R. C. I. MacKenzie, J. J. Jasieniak, N. D. Treat and M. L. Chabinyc, Adv. Energy Mater., 2014, 4, 1301290 CrossRef.
- F. Xie, W. C. H. Choy, C. Wang, X. Li, S. Zhang and J. Hou, Adv. Mater., 2013, 25, 2051–2055 CrossRef CAS PubMed.
- K. Zilberberg, S. Trost, J. Meyer, A. Kahn, A. Behrendt, D. Lützenkirchen-Hecht, R. Frahm and T. Riedl, Adv. Funct. Mater., 2011, 21, 4776–4783 CrossRef CAS.
- G. Teran-Escobar, J. Pampel, J. M. Caicedo and M. Lira-Cantu, Energy Environ. Sci., 2013, 6, 3088–3098 RSC.
- S. Bai, M. Cao, Y. Jin, X. Dai, X. Liang, Z. Ye, M. Li, J. Cheng, X. Xiao, Z. Wu, Z. Xia, B. Sun, E. Wang, Y. Mo, F. Gao and F. Zhang, Adv. Energy Mater., 2014, 4, 1301460 CrossRef.
- F. Jiang, W. C. H. Choy, X. Li, D. Zhang and J. Cheng, Adv. Mater., 2015, 27, 2930–2937 CrossRef CAS.
- T. Stubhan, N. Li, N. A. Luechinger, S. C. Halim, G. J. Matt and C. J. Brabec, Adv. Energy Mater., 2012, 2, 1433–1438 CrossRef CAS.
- M. Vasilopoulou, A. Soultati, D. G. Georgiadou, T. Stergiopoulos, L. C. Palilis, S. Kennou, N. A. Stathopoulos, D. Davazoglou and P. Argitis, J. Mater. Chem. A, 2014, 2, 1738–1749 RSC.
- M. Jørgensen, K. Norrman and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2008, 92, 686–714 CrossRef.
- Z. Yin and Q. Zheng, Adv. Energy Mater., 2012, 2, 179–218 CrossRef CAS.
- Z. Yin, J. Wei and Q. Zheng, Adv. Sci., 2016, 3, 1500362 CrossRef PubMed.
- Z. Liang, Q. Zhang, L. Jiang and G. Cao, Energy Environ. Sci., 2015, 8, 3442–3476 RSC.
- W. Zhongwei, S. Tao, X. Zhouhui, W. Huaixin and S. Baoquan, Nanotechnology, 2013, 24, 484012 CrossRef PubMed.
- T. Stubhan, M. Salinas, A. Ebel, F. C. Krebs, A. Hirsch, M. Halik and C. J. Brabec, Adv. Energy Mater., 2012, 2, 532–535 CrossRef CAS.
- J. Jo, J.-R. Pouliot, D. Wynands, S. D. Collins, J. Y. Kim, T. L. Nguyen, H. Y. Woo, Y. Sun, M. Leclerc and A. J. Heeger, Adv. Mater., 2013, 25, 4783–4788 CrossRef CAS PubMed.
- S. Shao, K. Zheng, T. Pullerits and F. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 380–385 CrossRef CAS PubMed.
- C.-H. Hsieh, Y.-J. Cheng, P.-J. Li, C.-H. Chen, M. Dubosc, R.-M. Liang and C.-S. Hsu, J. Am. Chem. Soc, 2010, 132, 4887–4893 CrossRef CAS PubMed.
- S.-H. Liao, H.-J. Jhuo, Y.-S. Cheng and S.-A. Chen, Adv. Mater., 2013, 25, 4766–4771 CrossRef CAS PubMed.
- L. K. Jagadamma, M. Al-Senani, A. El-Labban, I. Gereige, G. O. Ngongang Ndjawa, J. C. D. Faria, T. Kim, K. Zhao, F. Cruciani, D. H. Anjum, M. A. McLachlan, P. M. Beaujuge and A. Amassian, Adv. Energy Mater., 2015, 5, 1500204 CrossRef.
- X. Liu, X. Li, Y. Li, C. Song, L. Zhu, W. Zhang, H.-Q. Wang and J. Fang, Adv. Mater., 2016, 28, 7405–7412 CrossRef CAS PubMed.
- A. Puetz, T. Stubhan, M. Reinhard, O. Loesch, E. Hammarberg, S. Wolf, C. Feldmann, H. Kalt, A. Colsmann and U. Lemmer, Sol. Energy Mater. Sol. Cells, 2011, 95, 579–585 CrossRef CAS.
- Z. Yin, Q. Zheng, S.-C. Chen, D. Cai and Y. Ma, Adv. Energy Mater., 2016, 6, 1501494 Search PubMed.
- J. Jae Hoon, H. Kihyon, K. Se-Hun and L. Dong Chan, J. Korean Inst. Surf. Eng., 2016, 49, 490–497 CrossRef.
- E. Polydorou, I. Sakellis, A. Soultati, A. Kaltzoglou, T. A. Papadopoulos, J. Briscoe, D. Tsikritzis, M. Fakis, L. C. Palilis, S. Kennou, P. Argitis, P. Falaras, D. Davazoglou and M. Vasilopoulou, Nano Energy, 2017, 34, 500–514 CrossRef CAS.
- Q. Zhu, X. Bao, J. Yu, D. Zhu, Q. Zhang, C. Gu, H. Dong, R. Yang and L. Dong, Thin Solid Films, 2016, 605, 202–207 CrossRef CAS.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- A. A. Al-Ghamdi, O. A. Al-Hartomy, M. El Okr, A. M. Nawar, S. El-Gazzar, F. El-Tantawy and F. Yakuphanoglu, Spectrochim. Acta, Part A, 2014, 131, 512–517 CrossRef CAS PubMed.
- J. Hu and R. G. Gordon, Sol. Cells, 1991, 30, 437–450 CrossRef CAS.
- F. Wang, J.-H. Seo, Z. Li, A. V. Kvit, Z. Ma and X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 1288–1293 CrossRef CAS PubMed.
- Y. Zhang, C. Liu, J. Liu, J. Xiong, J. Liu, K. Zhang, Y. Liu, M. Peng, A. Yu, A. Zhang, Y. Zhang, Z. Wang, J. Zhai and Z. L. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 1381–1387 CrossRef CAS PubMed.
- W.-Q. Wu, Q. Wang, Y. Fang, Y. Shao, S. Tang, Y. Deng, H. Lu, Y. Liu, T. Li, Z. Yang, A. Gruverman and J. Huang, Nat. Commun., 2018, 9, 1625 CrossRef PubMed.
- W. Q. Wu, J. F. Liao, Y. Jiang, L. Wang and D. B. Kuang, Small, 2019, 15, 1900606 CrossRef PubMed.
- J.-F. Liao, W.-Q. Wu, J.-X. Zhong, Y. Jiang, L. Wang and D.-B. Kuang, J. Mater. Chem. A, 2019, 7, 9025–9033 RSC.
- M. J. Kim and T. G. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 5453–5457 CrossRef CAS PubMed.
- C. A. Hoel, T. O. Mason, J.-F. Gaillard and K. R. Poeppelmeier, Chem. Mater., 2010, 22, 3569–3579 CrossRef CAS.
- Q. V. Hoang, C. E. Song, I.-N. Kang, S.-J. Moon, S. K. Lee, J.-C. Lee and W. S. Shin, RSC Adv., 2016, 6, 28658–28665 RSC.
- A. K. K. Kyaw, D. H. Wang, V. Gupta, J. Zhang, S. Chand, G. C. Bazan and A. J. Heeger, Adv. Mater., 2013, 25, 2397–2402 CrossRef CAS PubMed.
- B. A. E. Courtright and S. A. Jenekhe, ACS Appl. Mater. Interfaces, 2015, 7, 26167–26175 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: High-resolution XPS spectra, AFM height images, XRD, and UPS spectra. See DOI: 10.1039/c9ra06974g |
| This journal is © The Royal Society of Chemistry 2019 |