Open Access Article
Open Access ArticleOptimization of metal–organic (citric acid) frameworks for controlled release of nutrients†
Ke
Wu
ab,
Changwen
Du
 *ab,
Fei
Ma
a,
Yazhen
Shen
a and
Jianmin
Zhou
a
*ab,
Fei
Ma
a,
Yazhen
Shen
a and
Jianmin
Zhou
a
aThe State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China. E-mail: chwdu@issas.ac.cn; Tel: +86-25-86881565
bCollege of Modern Agricultural Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 10th October 2019
Abstract
Although polymer-coated controlled-release fertilizers have been under development for decades, their high costs, complex production processes, and potential environmental hazards have limited their application. Therefore, it is necessary to design and develop new materials for controlled nutrient release. In this study, two novel MOFs, compounds I and II, were successfully fabricated and optimized using ferric chloride, phosphoric acid, citric acid, and urea under hydrothermal conditions. The N, P, and Fe contents in compound I were 9.05%, 14.92%, and 14.55%, respectively, while the corresponding values in compound II were 10.78%, 14.10%, and 16.68%. The soil incubation results revealed that both compounds showed good slow-release longevity (more than 100 days). This study provides a new strategy for the fabrication of novel controlled-release fertilizers.
Introduction
The world population currently exceeds 7 billion, and it is projected to reach 9.5 billion by 2050; thus, increased crop yields and food supplies are required. Consequently, the agricultural sector will require additional fertilizers to increase food production. In addition to the increasing fertilizer demand, the low utilization rate of fertilizers globally is a serious problem. In agricultural production, 45–55% of nitrogen fertilizers are lost due to volatilization, leaching, and runoff,1,2 while the utilization rate of phosphate fertilizers is typically about 10–25%.3 This not only leads to resource wastage but also to environmental problems, such as water pollution and increased emissions of harmful gases (e.g., NH3 and N2O).4,5Currently, many scientists are actively researching methods to improve nutrient utilization. Traditional methods include suitable fertilizer dosage, deep and fractional application of nitrogen fertilizer, fertilizer and water control technology, balanced fertilization, and soil testing and formula fertilization. New methods include real-time real-field nitrogen management (such as SPAD in situ determination of nitrogen content in plant leaves) and precise management of farmland nutrients. Traditional methods and new methods have their own advantages and disadvantages. First, reducing the amount of fertilizer can theoretically improve the utilization rate of fertilizer and reduce environmental risks; however, it is difficult to implement this technique in developing countries, which have rapidly increasing population that place significant strain on food demand. Second, fertilizer application can theoretically be guided more accurately using fertilizer and water control technology, soil testing formula fertilization technology, and balanced fertilization. However, these measures not only increase the human cost of fertilizer application but also make it difficult to popularize such technologies, particularly in China where the land is broadly distributed. Third, precise nutrient management technology in farmlands has broad prospects, but it is still in the research stage.
Controlled-release fertilizers provide a new method to solve low nutrient utilization rates.6,7 Currently, most of the controlled-release fertilizers used are coated with organic polymers, such as polyolefins, dicyclopentadiene, polystyrene, polysulfones, and glyceride.8–10 However, the high cost and complex production process of these materials limit their large-scale application in controlled-release fertilizers.11 In addition, these polymer materials can potentially harm the soil ecological environment. Relevant studies have shown that the accumulation of such materials in the soil not only reduces soil fertility but may also release toxic gases when they degrade.12 Some natural materials, such as chitosan, starch and lignin, have multiple advantages including easy availability, eco-friendly, a low-cost and biodegradability.13,14 Meanwhile, these materials also have many negative characteristics. For instance, chitosan is only soluble in diluted acid, making it difficult to prepare water-soluble chitosan derivatives; otherwise, chitosan is dissolved in organic solvents, which not only increases the costs, but also causes serious environmental concerns.15–17 Starch exhibits a poor water resistance and film-forming ability, and is easily degraded in soil.18,19 Lignin has complex components, making its fabrication difficult.20,21 Therefore, it is necessary to develop low-cost and environmentally friendly controlled-release fertilizers.
Metal–organic frameworks (MOFs) are materials with adjustable pore sizes that are formed via the self-assembly of metal ions or metal ion clusters with organic ligands. MOFs have been applied in many fields, such as gas storage,22–24 catalysis,25 and drug carriers.26–28 In agricultural programs, studies on the application of Fe-based MOFs as Fe fertilizers in kidney bean crops have shown that they can improve the growth, chlorophyll content, protein, and enzyme activity of kidney beans.29 The impact of synthesized oxalate-phosphate-amine metal–organic frameworks (OPA-MOFs) (N content: 3.1%) on the growth, nutrient uptake, and grain yield of wheat has been investigated. The results indicated that OPA-MOF could be used as a novel efficiency-enhancing nitrogen fertilizer.30,31 Further, a MOF containing 5.16% N and 14.1% P was synthesized, and its degradation behavior in farmland soil was explored. The results revealed that the MOF material degraded by 50.9% throughout a wheat season, and exhibited a slow nutrient release rate.32 Although these materials show potential as controlled-release fertilizers, they are low in N content, which is likely to limit their application in agriculture. Therefore, in this work, we chose an eco-friendly organic ligand and developed two novel MOFs with relatively high nutrient contents.
The objective of this work was to synthesize two new MOF compounds using a hydrothermal synthetic route by optimizing the process conditions and components, using non-toxic ferric chloride and phosphoric acid as the inorganic reagents, citric acid as the organic ligand, and urea as the structure-directing agent (SDA), to achieve controlled release of nutrients, to improve nutrient utilization, and to reduce environmental risks.
Experimental section
Materials
Ferric chloride (FeCl3·6H2O), citric acid (H8C6O7·H2O), phosphoric acid (H3PO4), and urea (CO(NH2)2) were of analytical grade and purchased from Nanjing Ronghua Scientific Equipment Co., Ltd. (Jiangsu, China). Deionized water was used throughout the experiments (pH: 6.9; resistance: 18.2 MΩ cm−1). A KCF-2 autoclave was employed in the experiments (Beijing Century Senlang Experimental Apparatus Co., China).Synthesis of compound I and compound II
For compound I, 0.25 mol ferric chloride, 1.125 mol phosphoric acid, 0.375 mol citric acid, and 0.75 mol urea were dissolved in 292.5 mL of deionized water in a beaker, and mixed evenly. Therefore, the molar ratio of the components in solution was ferric chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphoric acid
phosphoric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid
citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea
urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 1
deionized water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65. The mixture was transferred to a KCF-2 autoclave with the stirring speed set to 80 rpm, and was held in the autoclave at 115 °C for 18 h. The obtained product was washed three times with deionized water, and subsequently dried at 60 °C. The molar ratio for synthesizing compound II was ferric chloride
65. The mixture was transferred to a KCF-2 autoclave with the stirring speed set to 80 rpm, and was held in the autoclave at 115 °C for 18 h. The obtained product was washed three times with deionized water, and subsequently dried at 60 °C. The molar ratio for synthesizing compound II was ferric chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphoric acid
phosphoric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid
citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea
urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 1
deionized water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65. The procedure was the same as that for compound I.
65. The procedure was the same as that for compound I.
Characterization
The structures of the compounds were determined using a Fourier-transform infrared spectroscopy Nicolet 6700 spectrometer (Thermo Electron Scientific Instruments Corporation, Madison, WI, USA) equipped with a model 300 photoacoustic cell (FTIR-PAS) (MTEC Photoacoustics, Inc., Oakland, California, USA) and an attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) instrument (4300 Handheld FTIR, Agilent Technologies, USA). The surface morphologies were obtained using a MERLIN field emission-scanning electron microscope (FE-SEM) (Carl Zeiss Microscopy GmbH, Jena, Germany), and the surface elemental compositions and distribution were analyzed using a Thermo Fisher 250Xi X-ray photoelectron spectrometer (XPS) and an energy-dispersive spectroscopy (EDS) detector attached to the SEM. The powder X-ray diffraction (PXRD) data were collected in the 5–80° range using an ARL X'TRA diffractometer (Thermo Electron Corporation, Switzerland). The elemental compositions were measured by laser-induced breakdown spectroscopy (LIBS) with a MobiLIBS system (IVEA, France), an iCAP 7000 inductively-coupled plasma optical emission spectrometer (ICP-OES, Thermo Fisher Scientific, USA), and a CHN-O-Rapid elemental analyzer (Heraeus, Germany).Nutrient release behavior in water
A 3 g sample of each compound was immersed in 100 mL of deionized water at 25 °C. The solution (100 mL) was removed after 20, 40, 60, 80, and 100 days and replaced with 100 mL of deionized water for each of the three replicates. The NH4+-N and NO3−-N contents were measured using a SmartChem 200 discrete auto analyzer (AMS Alliance, Frepillon, France). The urea content was determined by the para-dimethylaminobenzaldehyde colorimetric method (Epoch microplate spectrophotometer, BioTek). The P content was determined using an iCAP 7000 ICP-OES spectrometer (Thermo Fisher Scientific, USA). The total Fe, Fe2+, and Fe3+ contents were measured by O-phenanthroline colorimetry. The pH values of the soil samples were determined using a pH meter (Orion Star A211, Thermo Fisher, USA). The nutrient release behaviors were estimated via the nutrient cumulative release rates.Soil incubation
Soil incubation experiments were conducted to examine the nutrient release behaviors of both compounds using paddy soil. The soil samples were air-dried to 20% moisture content and sieved to 3 mm, and then dried to constant weights at 50 °C. The soil physicochemical properties were as follows: organic matter, 18.25 g kg−1; total-N, 1.23 g kg−1; NH4+-N, 12.15 mg kg−1; NO3−-N, 14.33 mg kg−1; available P, 17.65 mg kg−1; available Fe, 7.95 mg kg−1; and pH, 6.58. The soil samples (100 g) were placed in 8 cm-diameter pots, and the moisture was adjusted to 38% (w/w) in each pot. Three treatments were prepared: (1) control treatment (with no fertilizer); (2) compound I treatment, which involved an application of 75 kg N ha−1 (compound I N content: 9.5%); and (3) compound II treatment, which also involved an application of 75 kg N ha−1 (compound II N content: 10.78%). Three replicates were conducted for each treatment. All the pots were placed in a shade house and were loosely covered with plastic wrap to reduce soil water evaporation. The soil samples were collected at different time intervals (20, 40, 60, 80, and 100 days) for a total of 45 pots. The mineral N (NH4+-N and NO3−-N) contents were determined using a SmartChem 200 discrete auto analyzer (AMS Alliance, Frepillon, France). The available P and available Fe were measured using an iCAP 7000 ICP-OES spectrometer (Thermo Fisher Scientific, USA). The soil pH was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific, USA). The N, P, and Fe cumulative release rates from the MOF compounds were assessed by the ratios of mineral N in the soil (in the NH4+-N and NO3−-N fractions), available P, and available Fe contents, to the corresponding initial nutrient applications.Results and discussion
To confirm the formation of compounds I and II during the hydrothermal reaction, the ATR-FTIR (Fig. 1A) and FTIR-PAS (Fig. 1B) spectra of the two compounds were recorded. As shown in the spectra, both compounds show the same characteristic peaks. The broad peak at approximately 3365 cm−1 was associated with the N–H stretching vibration, indicating that the urea group was involved in the formation of the two compounds. The peaks at 2925, 1654, 1431, and 1022 cm−1 were assigned to C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C, and C–O stretching vibrations, respectively, revealing that citric acid groups were successfully introduced into the two compounds. In the fingerprint region, the peak at 902 cm−1 was attributed to the P–O stretching vibration, implying that phosphoric acid was involved in the hydrothermal reaction and was also a part of the two compounds.
O, C–C, and C–O stretching vibrations, respectively, revealing that citric acid groups were successfully introduced into the two compounds. In the fingerprint region, the peak at 902 cm−1 was attributed to the P–O stretching vibration, implying that phosphoric acid was involved in the hydrothermal reaction and was also a part of the two compounds.
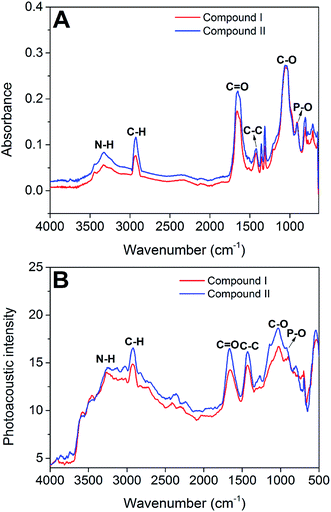 | ||
| Fig. 1 Structural characterizations of the two compounds by ATR-FTIR spectra (A) and FTIR-PAS spectra (B). | ||
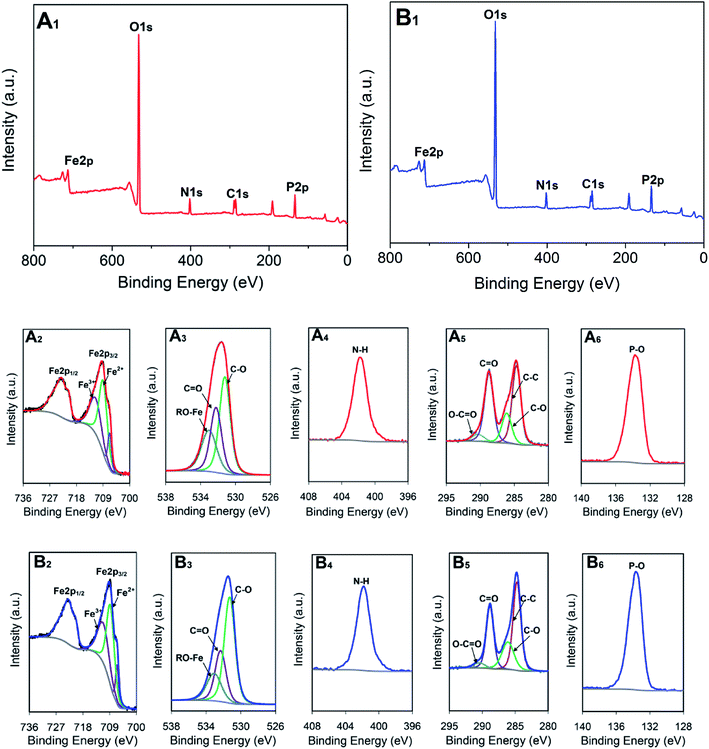
XPS was used to investigate the elemental compositions and bonding configurations of the compounds, as shown in Fig. 2A1. Five peaks at approximately 724, 532, 402, 285, and 134 eV, respectively corresponding to Fe 2p1/2, O 1s, N 1s, C 1s, and P 2p, were observed, indicating that the ferric chloride, citric acid, urea, and phosphoric acid reagents were involved in the formation of the compounds. Moreover, to further study the chemical bond configuration of compounds, the high-resolution characteristic peaks of both compounds were deconvoluted. Fe3+ (712 eV), Fe2+ (709.2 eV), and RO-Fe (533.2 eV) revealed the valence state and chemical bonding of Fe in compound I (Fig. 2A2 and A3), and mixed-valence Fe3+/Fe2+ MOFs have been previously reported.33,34 The peak at 401.98 eV was assigned to NH4+ (Fig. 2A4), implying that the decomposition of urea occurred during the hydrothermal reaction.
In this work, urea was used as a SDA, which influenced the structure of the target products, and also the resulting stability of the obtained compounds. For most MOFs synthesized using amine-based SDAs, the SDAs usually remain essentially unchanged and exist as guests in the framework pores. In some cases, however, the SDAs fully or partially decompose to more stable secondary structures, as shown in previous studies.30,35,36 The O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (290.38 eV), C
O (290.38 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.78 eV), C–O (286.18 eV), and C–C (284.68 eV) peaks in the spectra suggested the successful introduction of citric acid groups (Fig. 2A5), while the peak at 133.7 eV was associated with P–O (Fig. 2A6), suggesting that phosphoric acid participated in the MOF formation and became part of its skeleton. The XPS spectra of compound II shows essentially the same features (Fig. 2B1–B6).
O (288.78 eV), C–O (286.18 eV), and C–C (284.68 eV) peaks in the spectra suggested the successful introduction of citric acid groups (Fig. 2A5), while the peak at 133.7 eV was associated with P–O (Fig. 2A6), suggesting that phosphoric acid participated in the MOF formation and became part of its skeleton. The XPS spectra of compound II shows essentially the same features (Fig. 2B1–B6).
The SEM images with different magnifications were used to observe the surface morphology of the compounds. As shown in Fig. 3A1–A3, the surface of compound I was rough and uneven, and contains many massive aggregated crystallites of different sizes. There is a clear difference between the surface structures of the two compounds, and many regular crystal plate-like structures with sharp edges and smooth surfaces are observed in compound II (Fig. 3B1–B3), indicating that the amount of added urea greatly influenced the compounds' structures. Furthermore, EDS maps were employed to observe the surface elemental compositions and distributions of the compounds. As shown in Fig. 3A4 and B4, Fe, P, N, C, and O were uniformly distributed on the compounds' surfaces, which directly verified that all the reagents were involved in the formation of the two compounds.
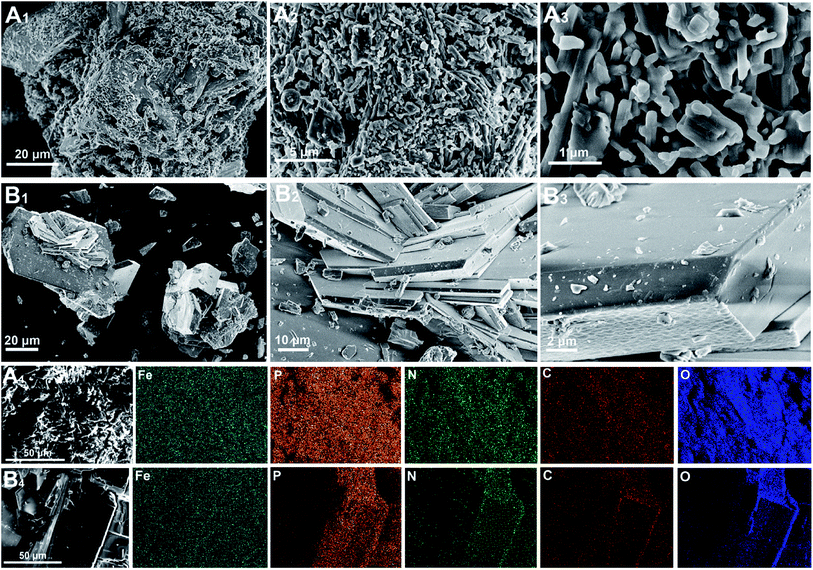 | ||
| Fig. 3 SEM images of compound I (A1–A3) and compound II (B1–B3). EDS elemental maps corresponding to the SEM images of compound I (A4) and compound II (B4). | ||
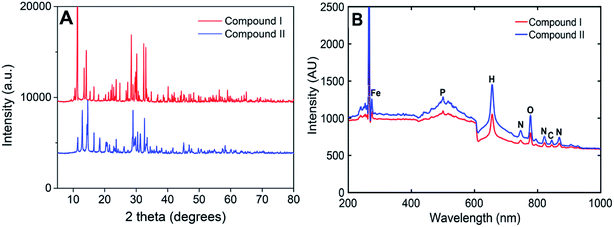
PXRD and LIBS were employed to investigate the phase and elemental composition of the two compounds. As shown in Fig. 4A, the characteristic peaks of the two compounds were clearly different, indicating that the urea (SDA) concentration in the reaction solution affected the crystal structure of the product, which is consistent with the results from a previous study.30 The PXRD data of the compounds were compared to the International Centre for Diffraction Data (ICDD). Since no match was found, it was suggested that our synthesized compounds are novel MOFs. The LIBS spectra of both compounds showed similar spectral shapes and characteristic peaks (Fig. 4B). The peaks at 274.6 nm, 500.3 nm, and 844.8 nm, 777.3 nm, and 655.6 nm were attributed to the characteristic bands of Fe, P, C, O, and H, respectively, while the peaks at 746.8, 819.2, and 868.3 nm were assigned to N, this shows that all reagents participated in the formation of the MOFs during the hydrothermal synthesis.
To better understand the elemental compositions and content of both compounds, CHN-O-Rapid elemental analysis and ICP-OES were used to determine the C, H, and N contents of the compounds and the elemental content of the solution after the hydrothermal reaction, respectively. As shown in Table 1, the yield of compound I was slightly higher than that of compound II. Compound II contained more Fe, N, and H than compound I, and less P and C. These results imply that the concentration of urea (SDA) in the hydrothermal reaction can affect the elemental composition of the products.
| Compound I | Compound II | |
|---|---|---|
| Yields | 35.8 ± 1.6 | 32.1 ± 2.2 |
| Fe | 14.55 ± 1.1 | 16.68 ± 1.9 |
| C | 8.25 ± 0.26 | 7.72 ± 0.18 |
| N | 9.05 ± 0.11 | 10.78 ± 0.20 |
| P | 14.92 ± 1.05 | 14.10 ± 1.28 |
| H | 2.56 | 3.12 |
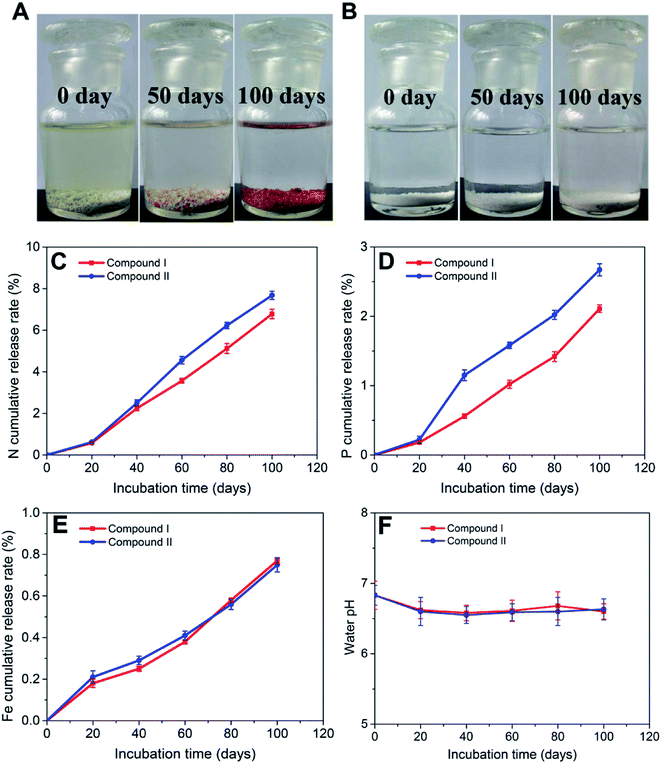
The compounds were incubated in water at 25 °C to study their nutrient release behaviors. As shown in Fig. 5A, compound I gradually changed color from yellow to brownish-red during incubation, while the color change in compound II was not noticeable (Fig. 5B). The N cumulative release rates of both compounds at 100 days were 6.78% and 7.66%, respectively (Fig. 5C), which are both significantly lower than that reported for coated controlled-release fertilizers (N cumulative release rate over 80% within 70 days).37,38 Interestingly, the N in the water was mostly present as NH4+-N (Fig. S1†), while urea and NO3−-N were undetected. One reason may be that the water pH is under acidic conditions, resulting in the predominance of NH4+-N species; it may also be possible that N is present in the compound materials as NH4+-N, which is consistent with a previous study.30 However, the cumulative release rates of P and Fe were lower than that of N (Fig. 5D and E), which is likely due to the structure of the compounds. Fe and P constituted the framework of the compounds, while SDA (urea) was embedded in the crystal channels as NH4+-N. Therefore, the P and Fe nutrient release rates may be inhibited by the strong P and Fe bonds in the framework. Furthermore, Fe3+ and Fe2+ were detected in the water (Fig. S2†). Besides, the water pH slightly declined during the incubation process (Fig. 5F), which is likely attributed to the hydrolysis of NH4+-N. These results suggest that the two compounds have slow nutrient release rates, and may be unable to meet the nutrient requirements of crops when used as controlled-release fertilizers.
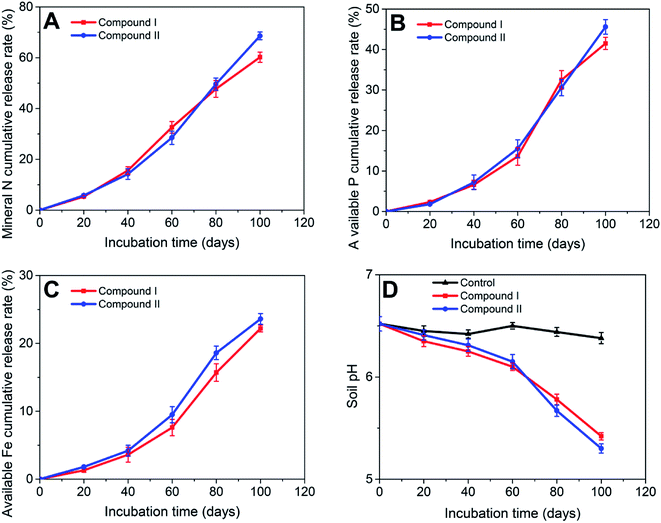
To further explore the potential of using the two compounds as controlled-release fertilizers, a soil incubation experiment was conducted to investigate their nutrient release behaviors. For both compounds, the mineral N (NH4+-N and NO3−-N) cumulative release rates over 100 days were 60.2% and 68.6%, respectively (Fig. 6A). The available P cumulative release rates of compounds I and II were 42.2% and 46.8%, respectively (Fig. 6B), while the available Fe cumulative release rates were 22.2% and 23.6%, respectively (Fig. 6C). Compared to previously reported nutrient release rates (in compounds using oxalic acid as ligands),32 the compounds reported here showed higher nutrient release rates. This result is likely due to the difference between ligands. The citric acid used in this study is a low molecular weight organic acid widely found in soil, which exhibits a lower sorption in soil and higher biodegradability than that of oxalic acid.39–42 Therefore, the MOF compounds using citric acid as organic ligands were more susceptible to mineralization by microorganisms. Additionally, in comparison to that of the control group, the pH of the soil treated with the compounds decreased during incubation (Fig. 6D), which was likely due to the redox-induced oxidation of the structural Fe2+ in the compounds. The XPS, water incubation, and soil incubation results all indicated that a part of Fe in the compounds was present as Fe2+, which is supported by previous studies.30,33,43 Therefore, Fe2+ oxidation is the preferred reaction because Fe2+/Fe3+ has a higher redox-potential compared to that of NH4+ nitrification, resulting in the release of 2H+ for each oxidized Fe atom.44 Moreover, this oxidation process limits the nitrification.
Conclusions
Two novel MOF compounds rich in N, P, and Fe nutrients were successfully fabricated via hydrothermal synthesis. The characterization results showed that urea (SDA) affected the structure of the products, and all the reagents participated in the formation of the compounds. Water incubation and soil incubation were employed to investigate the nutrient release behaviors of the compounds. Consequently, the compounds showed much better release performance when placed in soil than when placed in water, and both compounds lasted longer than 100 days. Therefore, these novel MOF materials can provide new strategies for the development of controlled-release fertilizers in the future.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFD0200704), the Key Research and Development Program of Jiangsu Province (BE2017388) and the “STS” project from Chinese Academy of Sciences (KFJ-STS-QYZX-046, KFJ-PTXM-003).References
- Y. C. Yang, M. Zhang, Y. C. Li, X. H. Fan and Y. Q. Geng, Soil Sci. Soc. Am. J., 2012, 76, 2307–2317 CrossRef CAS.
- U. Shavit, A. Shaviv, G. Shalit and D. Zaslavsky, J. Controlled Release, 1997, 43, 131–138 CrossRef.
- J. Shen, R. Li, F. Zhang, C. Tang and Z. Rengel, Field Crop. Res., 2004, 86, 225–238 CrossRef.
- X. Yan, J. Y. Jin, P. He and M. Z. Liang, Agric. Sci. China, 2008, 7, 469–479 CrossRef.
- A. T. M. A. Choudhury and I. R. Kennedy, Commun. Soil Sci. Plant Anal., 2005, 36, 1625–1639 CrossRef CAS.
- M. Zhang, Y. C. Yang and Y. X. Shi, Chem. Fert. Ind., 2001, 32, 25–27 Search PubMed.
- S. P. Jin, G. R. Yue, L. Feng, Y. Q. Han, X. H. Yu and Z. H. Zhang, J. Agric. Food Chem., 2011, 59, 322–327 CrossRef CAS.
- S. K. Jain, G. P. Agrawal, M. Kumar and N. Anande, Pharma Rev., 2007, 5, 1–7 Search PubMed.
- A. Shaviv, Proc. - Int. Fert. Soc., 2001, 469, 1–23 CAS.
- M. Tomaszewska and A. Jarosiewicz, J. Agric. Food Chem., 2002, 50, 4634–4639 CrossRef CAS.
- Y. S. Ye, X. Q. Liang, Y. X. Chen, J. Liu, J. T. Gu, R. Guo and L. Li, Field Crop. Res., 2013, 144, 212–224 CrossRef.
- D. Briassoulis and C. Dejean, J. Polym. Environ., 2010, 18, 384–400 CrossRef CAS.
- A. Wezel, M. Casagrande, F. Celette, J.-F. Vian, A. Ferrer and J. Peigné, Agron. Sustainable Dev., 2014, 34, 1–20 CrossRef.
- A. Schneider Teixeira, L. Deladino and N. Zaritzky, ACS Sustainable Chem. Eng., 2016, 4, 2449–2458 CrossRef CAS.
- A. Rattanamanee, H. Niamsup, L. O. Srisombat, W. Punyodom, R. Watanesk and S. Watanesk, J. Polym. Environ., 2014, 23, 334–340 CrossRef.
- B. R. dos Santos, F. B. Bacalhau, T. dos Santos Pereira, C. F. Souza and R. Faez, Carbohydr. Polym., 2015, 127, 340–346 CrossRef.
- T. Jamnongkan and S. Kaewpirom, J. Polym. Environ., 2010, 18, 413–421 CrossRef CAS.
- J. Ge, R. Wu, X. Shi, H. Yu, M. Wang and W. Li, J. Appl. Polym. Sci., 2002, 86, 2948–2952 CrossRef CAS.
- D. Qiao, H. Liu, L. Yu, X. Bao, G. P. Simon, E. Petinakis and L. Chen, Carbohydr. Polym., 2016, 147, 146–154 CrossRef CAS.
- M. Fernández-Pérez, F. Garrido-Herrera, E. González-Pradas, M. Villafranca-Sánchez and F. Flores-Céspedes, J. Appl. Polym. Sci., 2008, 108, 3796–3803 CrossRef.
- W. Mulder, R. Gosselink, M. Vingerhoeds, P. Harmsen and D. Eastham, Ind. Crops Prod., 2011, 34, 915–920 CrossRef CAS.
- M. Eddaoudi, L. Hailian and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391–1397 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed. Engl., 2004, 43, 2334–2375 CrossRef CAS.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed. Engl., 2009, 48, 7502–7513 CrossRef CAS.
- C. Janiak, Dalton Trans., 2003, 14, 2781–2804 RSC.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626–636 RSC.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS.
- R. M. Abdelhameed, R. E. Abdelhameed and H. A. Kamelc, Mater. Lett., 2019, 237, 72–79 CrossRef CAS.
- M. Anstoetz, N. Sharma, M. Clark and L. H. Yee, J. Mater. Sci., 2016, 51, 9239–9252 CrossRef CAS.
- M. Anstoetz, T. J. Rose, M. W. Clark, L. H. Yee, C. A. Raymond and T. Vancov, PLoS One, 2015, 10, 1–16 CrossRef.
- K. Wu, C. W. Du, F. Ma, Y. Z. Shen, D. Liang and J. M. Zhou, Polymers, 2019, 11, 947 CrossRef PubMed.
- Y. C. Jiang, S. L. Wang, K. H. Lii, N. Nguyen and A. Ducouret, Chem. Mater., 2003, 15, 1633–1638 CrossRef CAS.
- J. R. D. Debord, W. M. Reiff, C. J. Warren, R. C. Haushalter and J. Zubieta, Chem. Mater., 1997, 9, 1994–1998 CrossRef CAS.
- N. Rajic, D. Stojakovic, D. Hanzel and V. Kaucic, J. Serb. Chem. Soc., 2004, 69, 179–185 CrossRef CAS.
- K. Abu-Shandi, H. Winkler, B. Wu and C. Janiak, CrystEngComm, 2003, 5, 180–189 RSC.
- Y. Z. Shen, C. W. Du, J. M. Zhou and F. Ma, Sci. Rep., 2018, 8, 12279 CrossRef.
- J. Z. Xie, Y. C. Yang, B. Gao, Y. S. Wan, C. Y. Li, J. Xu and Q. H. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 15868–15879 CrossRef CAS.
- K. Fujii, M. Aoki and K. Kitayama, Soil Biol. Biochem., 2013, 56, 3–9 CrossRef CAS.
- K. Fujii, C. Hayakawa, P. A. W. VanHees, S. Funakawa and T. Kosaki, Plant Soil, 2010, 334, 475–489 CrossRef CAS.
- D. L. Jones and D. S. Brassington, Eur. J. Soil Sci., 1998, 49, 447–455 CrossRef CAS.
- L. Ström, A. G. Owen, D. L. Godbold and D. L. Jones, Soil Biol. Biochem., 2001, 33, 2125–2133 CrossRef.
- M. Clemente-Leon, E. Coronado, M. C. Gimenez-Lopez, A. Soriano-Portillo, J. C. Waerenborgh, F. S. Delgado and C. Ruiz-perez, Inorg. Chem., 2008, 47, 9111–9120 CrossRef CAS.
- W. Stumm and J. J. Morgan, Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, John Wiley and Sons, 3rd edn, 1995 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06939a |
| This journal is © The Royal Society of Chemistry 2019 |