Open Access Article
Open Access ArticleFacile synthesis of novel dithioacetal–naphthalene derivatives as potential activators for plant resistance induction†
R. J.
Ji‡
a,
W. M.
Shi‡
a,
D. Y.
Tian
a,
G. P.
Zhang
 *a and
H.
Wang
*b
*a and
H.
Wang
*b
aChemistry and Material Science College, Huaibei Normal University, Huaibei 235000, China. E-mail: hbzgp-1@163.com
bInstitute for Plant Protection and Soil Science, Hubei Academy of Agricultural Sciences, Nanhu Road 6, Wuhan 430064, China. E-mail: wanghua4@163.com
First published on 10th October 2019
Abstract
In this paper, a series of novel dithioacetal–naphthalenes were designed and synthesized for plant immunity. Their antiviral activities were evaluated against tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV). The results indicated that most compounds exhibited better activity against CMV than against TMV. These dithioacetal derivatives also displayed good bacterial activity against rice bacterial leaf blight. Among them, compound S16 exhibited relatively good anti-CMV, anti-TMV, and antibacterial activity. Structure–activity relationships indicated that introducing the naphthalene moiety enhanced their activities for plant resistance induction. Therefore, the basic motif of compound S16 could be the most promising candidate for further structural optimization to develop a potential activator for plant resistance induction.
Introduction
Plant viruses are pathogenic to plants, infecting many plants, especially vegetables, including pepper, tomato, cucumber, and so on.1 Owing to the diversity of virus species, the different transmission mechanisms and the mutability of viruses under field conditions, it is extremely difficult to control viral infection,2 which results in massive economic losses each year.3–5 Although numerous chemical and biological controls have been applied, there have been few efficient compounds that can protect plants completely from virus infection.6 In the field, the reported antiviral agents,7 such as ninominomycin, dufulin, ribavirin, lentinan polysaccharide, emodinmethyl ether, morinanidine hydrochloride, chlorbromo-isocyanurate, DHT, DADHT, and chitooligosaccharide, are often effective only against one virus while disabled against the others. Furthermore, the agents with a preventive effect are less than 60% among the antiviral agents.8 Therefore the development of new antiviral agents with an efficient broad-spectrum is extremely urgent.In fact, plants have self-defend system and can resist the infection of bacteria, mold and viruses.9 Such self-defensive ability is commonly induced by some external or internal elicitors. Based on plant immune resistance, these elicitors are developed into antivirus agents and used for the prevention and treatment of plant virus.10–14 In the development of the chemical antivirus, people mainly consider the virus itself. Many of these reported drugs suffer from low effectiveness and narrow-spectrum antiviral property. In addition, the absolute parasite of the virus on the host is closely related to the plant. In general, two factors of virus inhibition and plant activation must be considered together to develop efficient broad-spectrum antiviral agents.
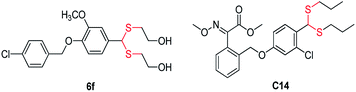
Dithioacetal and its derivatives have extensive biological activities, such as antibacterial,15 antileishmanial,16 antiviral,17 and antifungal.18,19 Song et al. found that dithioacetal derivative 6f (Fig. 1) exerted markedly curative and protective activities against PVY and CMV.20 Further research results by this group showed that dithioacetal C14 (Fig. 1) elicited excellent curative and protective activities against PVY, CMV and TMV.21 Their mechanism associated with the change of SOD, CAT, and POD was also demonstrated. Based on above, introduction of plant immune elicitors, dithiacetal compounds could be developed into novel antiviral agents with effectiveness and spectrum width.
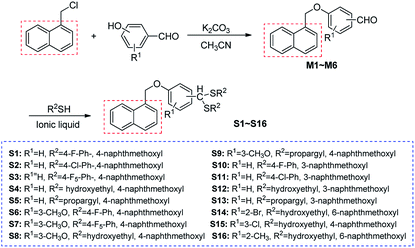
Naproxen acetic acid and naproxen sodium derivatives are important plant regulators for agriculture.22 These regulators can promote plant growth and chlorophyll synthesis. Especially it can promote the formation of adventitious roots and roots with fruit expansion and anti-falling function. We then introduced the plant regulated naphthalene group to dithioacetal skeleton (Fig. 2). Moreover, their bioactivities against cucumber mosaic virus and tobacco mosaic virus were subsequently evaluated and preliminary structure–activity relationship was concluded.
Results and discussion
The condensation reaction of aromatic aldehyde bearing naphthalene ring (M1) with allyl mercaptan was chosen as a model reaction to optimize the reaction conditions and the results are summarized in Table 1. It was observed that the reaction failed to give the product without the catalyst (Table 1, entry 1). In initial studies, different catalysts were screened in dichloromethane as the solvent (1 mL) at 40 °C (Table 1, entry 2 and 3). It was found that the acid ionic liquid (BIL) could effectively catalyzed the condensation reaction. The effects of solvents were then evaluated by using ionic liquid (BIL) as the catalyst (Table 1, entry 3–6). Dichloroethane (Table 1, entry 5) was found to be an optimal solvent, giving S1 in a good yield (up to 97%). Almost no change in reactivity was observed when the loading of BIL–HSO4 was reduced from 10 mol% to 5 mol% (Table 1, entries 5 and 7). However, the product decreased from 97% to 69% when the catalyst load was reduced from 5 mol% to 1 mol% (Table 1, entries 7 and 8).| Entrya | Catalyst (mol%) | Solvent | Temp/°C | Time | Yieldb/% |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1.0 mmol), thiol (1.0 mmol), solvent (1.0 mL). b Isolated yield. | |||||
| 1 | — | DCM | 40 | 6 | 0 |
| 2 | ZrCl4 (10) | DCM | 40 | 6 | 71 |
| 3 | BIL–HSO4 (10) | DCM | 40 | 6 | 81 |
| 4 | BIL–HSO4 (10) | CH3CN | 80 | 6 | 67 |
| 5 | BIL–HSO4 (10) | DCE | 80 | 6 | 97 |
| 6 | BIL–HSO4 (10) | DOX | 110 | 6 | 87 |
| 7 | BIL–HSO4 (5) | DCE | 80 | 12 | 96 |
| 8 | BIL–HSO4 (1) | DCE | 80 | 12 | 69 |
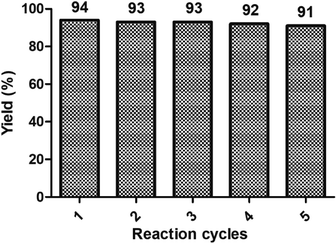
The reusability of the catalyst (BIL–HSO4) was investigated and the results are described in Fig. 3. The reaction mixture was concentrated under reduced pressure to remove the dichloromethane. 20 mL water was added to the mixture. Then the catalyst was recovered by using simple filtration technique. The filtrate containing ionic liquid was dried over vacuum to remove excess water. This catalyst was directly subjected to the condensation reaction using the model reaction with our optimized reaction conditions. It is important to note that the recycled catalysts produced excellent yields of dithioacetal (S1) in 91–94%, respectively (Fig. 3). It was observed that the yields were consistent without significant loss in its catalytic activity.
Under these optimized reaction conditions (Table 1, entry 7), substituted aldehydes bearing naphthalene ring (M1–M6), using acid ionic liquid (BIL),23 reacted with different thiols to generate novel dithioacetals S1–S16 with excellent yields of 83–97%.
First, compounds S1–S16 were measured for their phytotoxic activity against tobacco.24 The data of phytotoxic activity at 500 μg mL−1 indicated that compounds S1–S16 showed no toxicity to the tested plant.
The in vivo anti-TMV activities of target compounds S1–S16 at the concentration of 500 μg mL−1 were evaluated through the half leaf method.25–29 The results were shown in Table 1. Ningnanmycin and dufulin were used as the controls. Most of the title compounds exhibited good antiviral activities against CMV. Compounds S5, S8, S12 and S16 possessed excellent curative and protective activities from 61.5% to 71.3%, which was significantly greater than those of the controls with the vicinity of 50%. Compounds S1, S4, S9 and S13 exhibited good curative and protective activities with 55% or so, which were slightly exceeded than those of controls. Other compounds maintained moderate antiviral activities. The results of the activity against TMV showed that the target compounds displayed common inhibitory effects (Table 2). The curative activities of S1 (45.3%), S5 (45.9%), S11 (50.0%) and S16 (49.6%) against TMV were similar to those of dufulin (46.3%), slightly lower than ningnanmycin (53.1%). The protective activities against TMV of S11 (47.4%) and S16 (49.6%) were similar to those of dufulin (49.4%), much less than ningnanmycin (63.4%). Moreover, the activity of the title compound S8 is excess than those of the accordingly intermediates referring to the literature.20
| Compd | Anti-TMV | Anti-CMV | ||
|---|---|---|---|---|
| Curative activity (%) | Protective activity (%) | Curative activity (%) | Protective activity (%) | |
| a The reaction was conducted in anoxic conditions. b Dufulin was used as the control. c Ningnanmycin was used as the control. | ||||
| S1 | 45.3 ± 2.5% | 37.8 ± 2.2% | 53.2 ± 1.6% | 57.8 ± 2.3% |
| S2 | 31.7 ± 2.4% | 16.2 ± 3.1% | 36.6 ± 2.1% | 31.2 ± 1.9% |
| S3 | 35.8 ± 2.5% | 26.7 ± 2.0% | 47.5 ± 2.2% | 46.9 ± 1.7% |
| S4 | 32.5 ± 1.9% | 28.2 ± 2.1% | 57.4 ± 1.3% | 59.3 ± 2.2% |
| S5 | 45.9 ± 2.4% | 38.6 ± 5.3% | 66.1 ± 2.8% | 68.3 ± 2.4% |
| S6 | 34.1 ± 2.1% | 34.9 ± 1.9% | 44.2 ± 2.3% | 41.6 ± 1.6% |
| S7 | 39.6 ± 3.0% | 37.6 ± 2.7% | 47.1 ± 2.6% | 44.9 ± 2.1% |
| S8 | 40.6 ± 3.4% | 35.3 ± 2.8% | 61.5 ± 3.1% | 64.4 ± 2.0% |
| S9 | 41.2 ± 2.4% | 42.6 ± 3.8% | 56.4 ± 2.3% | 54.8 ± 1.6% |
| S10 | 34.8 ± 1.8% | 29.0 ± 3.3% | 46.1 ± 1.9% | 48.7 ± 3.5% |
| S11 | 50.0 ± 2.7% | 47.4 ± 2.4% | 41.7 ± 2.2% | 43.8 ± 2.6% |
| S12 | 32.4 ± 3.6% | 18.4 ± 3.1% | 62.9 ± 1.5% | 68.3 ± 2.0% |
| S13 | 40.1 ± 2.7% | 36.4 ± 2.3% | 59.6 ± 2.1% | 56.3 ± 1.5% |
| S14 | 39.3 ± 2.5% | 34.5 ± 2.4% | 49.1 ± 2.8% | 46.4 ± 2.2% |
| S15 | 45.1 ± 2.6% | 44.7 ± 2.1% | 65.3 ± 1.9% | 63.1 ± 1.7% |
| S16 | 49.6 ± 2.4% | 48.1 ± 1.7% | 71.5 ± 1.4% | 69.1 ± 2.1% |
| Controlb | 46.3 ± 2.1% | 49.4 ± 2.6% | 51.3 ± 1.8% | 53.1 ± 2.1% |
| Controlc | 53.1 ± 1.7% | 63.4 ± 2.4% | 48.7 ± 2.1% | 49.4 ± 2.6% |
Bioassay results indicated that the introduction of naphthalene moiety to the dithioacetals can effectively improve the antiviral activity. In addition, among these compounds S1–S5, the R2 groups affect the antiviral activity (S1, S5 > S2, S3, S4), with 1-naphthalene-methyl moiety at the para-position of dithioacetals in anti-TMV activity. The effect of the R2 groups in anti-CMV activity is more obvious (S5 > S4 > S1 > S3 > S2). Compound S5 or S4 exhibited high activities when R2 is hydroxyethyl or propargyl moiety. To study the influence of the R1 group, among these compounds S6–S9, its effect on activity is quite small with the CH3O group at the meta-position in anti-TMV activity. Nevertheless, compound S8 with the CH3O group at the R1 position and the hydroxyethyl group showed good activity against CMV, further illustrate that the hydroxyethyl group is favorable for activity (S4, S8 and S12) regardless of naphthalene and R1 position on dithioacetals. To screen out high active compounds, the hydroxyethyl group was choosed to change the R1 group and naphthalene position of the title compounds, the compound S16 exhibited best activity against CMV and TMV. The results indicate that R1 and naphthalene moiety is important for antiviral activities. Meanwhile, the hydroxyethyl group is indispensable for the antiviral activities of these compounds.
The antibacterial activities of the title compounds (S1–S16) against rice bacterial leaf blight were evaluated by turbidimeter tests.30 Bismerthiazol was used as the positive control.31 The results showed that the title compounds displayed moderate to good antibacterial activities (Table 3). Among them, compounds S4, S7, S8 exhibited good in vitro antibacterial activity at concentrations of 200 and 100 μg mL−1, which were slightly superior to bismerthiazol (82.3 and 57.8%, respectively). Compounds S2, S6, S15 and S16 displayed moderate antibacterial activity, compared to that of control. Other compounds maintain low antibacterial activities. From the Table 3, SAR is easier to summarize. When R1 = H and 1-naphthalene-methoxy at the 4-position, the order of activities (S1–S5) from high to low activity at 100 μg mL−1 was hydroxyethyl (71.1%), 4-Cl–Ph– (57.0%), 4-F–Ph– (42.4%), 4-F5–Ph– (33.0%), propargyl (19.9%). Different dithioacetal groups obviously affected the activities. When R1 was 3-CH3O and naphthalene-methoxy at 4-position, the order of activities (S6–S9) at 100 μg mL−1 was hydroxyethyl (71.7%), 4-F5–Ph– (70.6%), 4-F–Ph– (63.1%), propargyl (46.8%). Among them, 3-CH3O group could increase the activity. When R1 was H-group and naphthalene-methoxy at 3-position, the order of activities (S10–S13) was hydroxyethyl (51.8%), 4-F–Ph– (30.3%), 4-Cl–Ph– (25.6%), propargyl (22.8%). When R2 was selected for the hydroxyethyl group, the sequence activities of compounds were S15 (63.2%), S16 (57.1%), S14 (39.6%). The above results further suggested that the dithioacetal hydroxyethyl group of the title compounds is critical for the activity and the R1 substituted and 1-naphthalene-methoxy of the derivatives influenced the antibacterial activity.
| Compd | Inhibitiona (%) | Compd | Inhibitiona (%) | ||
|---|---|---|---|---|---|
| 200 μg mL−1 | 100 μg mL−1 | 200 μg mL−1 | 100 μg mL−1 | ||
| a Average of three replicates. b Bismerthiazol was used as the control. | |||||
| S1 | 68.1 ± 1.8 | 42.4 ± 2.2 | S9 | 66.2 ± 3.5 | 46.8 ± 5.8 |
| S2 | 98.9 ± 2.2 | 57.0 ± 4.5 | S10 | 79.2 ± 2.6 | 30.3 ± 5.2 |
| S3 | 74.5 ± 3.8 | 33.0 ± 2.4 | S11 | 49.8 ± 1.3 | 25.6 ± 5.4 |
| S4 | 98.7 ± 3.9 | 71.1 ± 1.9 | S12 | 90.0 ± 3.7 | 51.8 ± 3.8 |
| S5 | 51.8 ± 1.9 | 19.9 ± 1.7 | S13 | 50.0 ± 0.5 | 22.8 ± 6.6 |
| S6 | 84.7 ± 3.0 | 63.1 ± 0.8 | S14 | 69.1 ± 4.6 | 39.6 ± 5.0 |
| S7 | 93.1 ± 3.4 | 70.6 ± 3.7 | S15 | 98.9 ± 3.9 | 63.2 ± 4.5 |
| S8 | 100.0 ± 1.6 | 71.7 ± 3.2 | S16 | 93.3 ± 3.4 | 57.1 ± 1.1 |
| Controlb | 82.3 ± 4.3 | 57.9 ± 3.1 | |||
To investigate the stability property of aromatic dithioacetals, compound S1 was selected to investigate in different conditions. First, compound S1 was stable under the condition of 0.1 M hydrochloric acid solution. Subsequently, compound S1 was also stable in 0.1 M NaOH. The results indicated that aromatic dithioacetals acetals displayed much better stability than acetals.
Conclusions
In summary, a series of novel dithioacetals–naphthalene, total sixteen compounds have been designed and synthesized with moderate yields. Their structures have been fully confirmed. Bioassay results showed that some compounds exhibited good anti-CMV activities, antibacterial activity and moderate anti-TMV activities in vivo. Among them, compound S16 in particularly showed the potent activity against CMV, TMV and good antibacterial activity. Therefore, the basic motif of S16 can be used as lead compound for further development.Experimental
General information
All of the reagents were purchased from commercial suppliers and used without further purification. All of the solvents were used without further purification and drying before use. Thin-layer chromatography with UV detection was conducted on silica gel GF254. The melting points of the products were determined with a WRX-4 microscopic melting point meter (Shanghai Yice Apparatus & Equipment Co., Ltd., China) with an uncorrected thermometer. 1H, and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Ascend-400 spectrometer (Bruker, Germany) in CDCl3 solution with tetramethylsilane as internal standard. High resolution mass spectral (HRMS) data were determined with Thermo Scientific Q Exactive (Thermo).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) affording the aromatic aldehydes (M1–M6).
20) affording the aromatic aldehydes (M1–M6).
4-(Naphthalen-1-ylmethoxy)benzaldehyde (M1). White solid, mp 122–123 °C, yield 95%; 1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 8.07–7.99 (m, 1H), 7.96–7.83 (m, 4H), 7.65–7.43 (m, 4H), 7.16 (d, J = 8.7 Hz, 2H), 5.59 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 190.77 (s), 163.80 (s), 133.83 (s), 132.04 (s), 131.40 (s), 131.27 (s), 130.27 (s), 129.42 (s), 128.84 (s), 126.77 (s), 126.68 (s), 126.09 (s), 125.30 (s), 123.43 (s), 115.21 (s), 68.95 (s).
3-Methoxy-4-(naphthalen-1-ylmethoxy)benzaldehyde (M2). White solid, mp 111–112 °C, yield 94%; 1H NMR (400 MHz, CDCl3) δ 9.84 (s, 1H), 8.04 (d, J = 8.0 Hz, 1H), 7.92–7.81 (m, 2H), 7.62–7.48 (m, 3H), 7.48–7.36 (m, 3H), 7.08 (d, J = 8.2 Hz, 1H), 5.65 (s, 2H), 3.90 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 190.91 (s), 153.73 (s), 150.30 (s), 133.79 (s), 131.28 (s), 131.22 (s), 130.50 (s), 129.14 (s), 128.82 (s), 126.57 (s), 126.54 (s), 126.27 (s), 126.00 (s), 125.35 (s), 123.31 (s), 112.74 (s), 109.60 (s), 69.51 (s), 56.07 (s).
3-(Naphthalen-1-ylmethoxy)benzaldehyde (M3). White solid, mp 95–96 °C, yield 97%; 1H NMR (400 MHz, CDCl3) δ 9.99 (s, 1H), 8.09–8.00 (m, 1H), 7.94–7.83 (m, 2H), 7.62–7.44 (m, 7H), 7.32–7.26 (m, 1H), 5.54 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 192.07 (s), 159.41 (s), 137.91 (s), 133.83 (s), 131.67 (s), 131.51 (s), 130.20 (s), 129.30 (s), 128.78 (s), 126.78 (s), 126.60 (s), 126.03 (s), 125.32 (s), 123.82 (s), 123.60 (s), 122.30 (s), 113.32 (s), 68.94 (s).
3-Chloro-4-(naphthalen-1-ylmethoxy)benzaldehyde (M4). Sandy solid, mp 119–121 °C, yield 96%; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H), 8.07 (d, J = 8.1 Hz, 1H), 8.00–7.83 (m, 3H), 7.77 (dd, J = 8.5, 2.0 Hz, 1H), 7.66 (d, J = 6.8 Hz, 1H), 7.63–7.53 (m, 2H), 7.53–7.46 (m, 1H), 7.23 (d, J = 8.5 Hz, 1H), 5.70 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 189.67 (s), 159.00 (s), 133.77 (s), 131.45 (s), 131.10 (s), 130.70 (s), 130.55 (s), 130.29 (s), 129.36 (s), 128.85 (s), 126.67 (s), 126.22 (s), 126.11 (s), 125.28 (s), 124.47 (s), 123.22 (s), 113.32 (s), 69.73 (s).
2-Bromo-5-(naphthalen-1-ylmethoxy)benzaldehyde (M5). Red solid, mp 129–131 °C, yield 94%; 1H NMR (400 MHz, CDCl3) δ 10.35 (s, 1H), 8.06–7.99 (m, 1H), 7.95–7.86 (m, 2H), 7.65 (d, J = 3.2 Hz, 1H), 7.63–7.52 (m, 4H), 7.49 (dd, J = 8.1, 7.2 Hz, 1H), 7.15 (dd, J = 8.8, 3.2 Hz, 1H), 5.54 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 191.68 (s), 158.46 (s), 134.72 (s), 134.09 (s), 133.82 (s), 131.46 (s), 131.29 (s), 129.41 (s), 128.79 (s), 126.86 (s), 126.64 (s), 126.05 (s), 125.26 (s), 123.83 (s), 123.50 (s), 118.30 (s), 113.94 (s), 69.20 (s).
5-Methyl-2-(naphthalen-1-ylmethoxy)benzaldehyde (M6). White solid, mp 114–116 °C, yield 93%; 1H NMR (400 MHz, CDCl3) δ 10.43 (s, 1H), 8.06–8.02 (m, 1H), 7.96–7.82 (m, 2H), 7.67 (d, J = 2.1 Hz, 1H), 7.63–7.42 (m, 4H), 7.38 (dd, J = 8.5, 2.3 Hz, 1H), 7.10 (d, J = 8.5 Hz, 1H), 5.60 (s, 2H), 2.33 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 189.88 (s), 159.26 (s), 136.52 (s), 133.81 (s), 131.60 (s), 131.40 (s), 130.62 (s), 129.30 (s), 128.84 (s), 128.47 (s), 126.67 (s), 126.53 (s), 126.06 (s), 125.26 (s), 125.12 (s), 123.36 (s), 113.24 (s), 69.38 (s), 20.30 (s).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) or recrystallization with alcohol.
2, v/v) or recrystallization with alcohol.
Bis(4-fluorophenyl)-4-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S1). White solid, mp 103–105 °C, yield 96%; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.4 Hz, 1H), 7.95–7.86 (m, 2H), 7.61–7.47 (m, 4H), 7.37–7.29 (m, 4H), 7.24 (d, J = 8.6 Hz, 2H), 7.01–6.91 (m, 6H), 5.49 (s, 2H), 5.26 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 164.08 (s), 161.61 (s), 158.62 (s), 135.71 (d, J = 8.4 Hz), 133.80 (s), 132.05 (s), 131.72 (s), 131.49 (s), 129.30 (s), 129.11 (s), 128.75 (s), 126.57 (d, J = 12.3 Hz), 125.97 (s), 125.30 (s), 123.64 (s), 116.07 (s), 115.85 (s), 114.87 (s), 68.72 (s), 61.55 (s); IR (KBr, cm−1) ν 1604.8 (s), 1584.1 (s), 1509.1 (s), 1487.6 (s), 1244.6 (s), 1222.7 (s); HRMS (ES) m/z for C30H22F2OS2 [M + Na]+ cacld 523.0972, found 523.0978.
Bis(4-chlorophenyl)-4-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S2). White solid, mp 113–114 °C, yield 92%; 1H NMR (400 MHz, CDCl3) δ 8.09–8.02 (m, 1H), 7.96–7.85 (m, 2H), 7.62–7.47 (m, 4H), 7.34–7.20 (m, 10H), 6.98 (d, J = 8.7 Hz, 2H), 5.49 (s, 2H), 5.36 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 158.75 (s), 134.21 (s), 134.07 (s), 133.80 (s), 132.72 (s), 132.02 (s), 131.50 (s), 131.28 (s), 129.15 (s), 129.04 (s), 128.75 (s), 126.66 (s), 126.52 (s), 125.98 (s), 125.31 (s), 123.64 (s), 114.99 (s), 68.75 (s), 60.21 (s); IR (KBr, cm−1) ν 1505.4 (s), 1471.6 (s), 1233.5 (s), 1091.4 (s), 1005.4 (s); HRMS (ES) m/z for C30H22Cl2OS2 [M + Na]+ cacld 555.0381, found 555.0384.
Bis(pentafluorophenyl)-4-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S3). White solid, mp 137–139 °C, yield 83%; 1H NMR (400 MHz, CDCl3) δ 8.05–7.98 (m, 1H), 7.95–7.83 (m, 2H), 7.62–7.51 (m, 3H), 7.50–7.44 (m, 1H), 7.41 (d, J = 8.7 Hz, 2H), 6.98 (d, J = 8.7 Hz, 2H), 5.66 (s, 1H), 5.48 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 159.67 (s), 133.79 (s), 131.73 (s), 131.43 (s), 129.20 (s), 128.76 (s), 129.11 (s), 128.46 (s), 126.64 (s), 126.55 (s), 125.99 (s), 125.27 (s), 123.52 (s), 115.24 (s), 68.76 (s), 57.63 (s); IR (KBr, cm−1) ν 1513.8 (s), 1487.9 (s), 1230.5 (s), 1098.2 (s); HRMS (ES) m/z for C30H14F10OS2 [M + Na]+ cacld 667.0219, found 667.0216.
Bis(2-hydroxyethyl)-4-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S4). White solid, mp 105–107 °C, yield 94%; 1H NMR (400 MHz, CDCl3) δ 8.08–8.01 (m, 1H), 7.93–7.84 (m, 2H), 7.61–7.45 (m, 4H), 7.44–7.37 (m, 2H), 7.07–6.99 (m, 2H), 5.49 (s, 2H), 5.07 (s, 1H), 3.74 (t, J = 5.5 Hz, 4H), 2.86 (dt, J = 14.0, 5.8 Hz, 2H), 2.73 (dt, J = 14.0, 5.9 Hz, 2H), 2.17 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 158.77 (s), 133.81 (s), 132.34 (s), 132.07 (s), 131.52 (s), 129.12 (s), 128.94 (s), 128.72 (s), 126.65 (s), 126.50 (s), 125.95 (s), 125.30 (s), 123.65 (s), 115.13 (s), 68.80 (s), 61.31 (s), 52.68 (s), 35.66 (s); IR (KBr, cm−1) ν 3477.8 (s), 3414.4 (s), 1617.8 (s), 1510.5 (s), 1238.2 (s); HRMS (ES) m/z for C22H24O3S2 [M + Na]+ cacld 423.1059, found 423.1064.
Bis(propenyl)-4-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S5). White solid, mp 114–116 °C, yield 97%; 1H NMR (400 MHz, CDCl3) δ 8.07–8.01 (m, 1H), 7.92–7.83 (m, 2H), 7.59 (d, J = 6.8 Hz, 1H), 7.57–7.49 (m, 2H), 7.49–7.43 (m, 1H), 7.38 (t, J = 5.8 Hz, 2H), 7.01 (d, J = 8.7 Hz, 2H), 5.80 (ddt, J = 17.1, 10.0, 7.2 Hz, 2H), 5.48 (s, 2H), 5.18–5.06 (m, 4H), 4.77 (s, 1H), 3.27 (dd, J = 13.7, 7.2 Hz, 2H), 3.06 (dd, J = 13.7, 7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 158.54 (s), 133.89 (s), 133.82 (s), 132.33 (s), 132.20 (s), 131.55 (s), 129.30 (s), 129.08 (s), 128.72 (s), 126.65 (s), 126.48 (s), 125.94 (s), 125.32 (s), 123.70 (s), 117.52 (s), 114.95 (s), 68.77 (s), 49.99 (s), 35.28 (s); IR (KBr, cm−1) ν 3351.2 (s), 2123.6 (s), 1537.4 (s), 1432.1 (s), 1218.6 (s); HRMS (ES) m/z for C24H24OS2 [M + Na]+ cacld 437.1216, found 437.1219.
Bis(4-fluorophenyl)-4-(naphthalen-1-yl-methoxy)-3-methoxylphenyldithioacetal (S6). White solid, mp 117–119 °C, yield 92%; 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 8.0 Hz, 1H), 7.92–7.79 (m, 2H), 7.60–7.40 (m, 4H), 7.36–7.27 (m, 4H), 6.99–6.89 (m, 4H), 6.87 (d, J = 2.0 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.68 (dd, J = 8.2, 2.1 Hz, 1H), 5.55 (s, 2H), 5.19 (s, 1H), 3.80 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 164.12 (s), 161.65 (s), 149.92 (s), 148.06 (s), 135.80 (d, J = 8.5 Hz), 133.75 (s), 132.54 (s), 132.21 (s), 131.37 (s), 129.16 (d, J = 3.4 Hz), 128.77 (d, J = 13.2 Hz), 126.31 (d, J = 13.6 Hz), 125.86 (s), 125.30 (s), 123.57 (s), 120.29 (s), 116.06 (s), 115.84 (s), 114.15 (s), 111.46 (s), 69.76 (s), 61.82 (s), 56.02 (s); IR (KBr, cm−1) ν 1588.4 (s), 1510.1 (s), 1486.9 (s), 1466.3 (s), 1265.5 (s), 1227.3 (s), 1136.0 (s); HRMS (ES) m/z for C31H24F2O2S2 [M + H]+ cacld 531.1259, found 531.1257.
Bis(pentafluorophenyl)-4-(naphthalen-1-ylmethoxy)-3-methoxylphenyldithioacetal (S7). White solid, mp 118–120 °C, yield 85%; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.9 Hz, 1H), 7.91–7.82 (m, 2H), 7.58–7.50 (m, 3H), 7.46–7.42 (m, 1H), 7.11 (s, 1H), 6.83 (s, 2H), 5.64 (s, 1H), 5.54 (s, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.23 (s), 149.20 (s), 133.74 (s), 131.84 (s), 131.29 (s), 129.06 (s), 128.92 (s), 128.72 (s), 126.42 (s), 126.21 (s), 125.89 (s), 125.27 (s), 123.43 (s), 120.47 (s), 113.97 (s), 110.90 (s), 69.66 (s), 57.91 (s), 56.10 (s); IR (KBr, cm−1) ν 1514.3 (s), 1489.8 (s), 1236.1 (s), 1088.2 (s); HRMS (ES) m/z for C31H16F10O2S2 [M + Na]+ cacld 697.0324, found 697.0321.
Bis(2-hydroxyethyl)-4-(naphthalen-1-ylmethoxy)-3-methoxylphenyldithioacetal (S8). White solid, mp 113–114 °C, yield 93%; 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 8.1 Hz, 1H), 7.91–7.87 (m, 1H), 7.83 (d, J = 8.3 Hz, 1H), 7.59 (d, J = 6.8 Hz, 1H), 7.53 (td, J = 7.7, 1.4 Hz, 2H), 7.47–7.42 (m, 1H), 7.07 (s, 1H), 6.91 (s, 2H), 5.56 (s, 2H), 5.03 (s, 1H), 3.89 (s, 3H), 3.74 (t, J = 5.8 Hz, 4H), 2.84 (dt, J = 14.0, 5.8 Hz, 2H), 2.72 (dt, J = 14.0, 5.9 Hz, 2H), 2.18 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 150.33 (s), 148.34 (s), 133.76 (s), 133.18 (s), 132.24 (s), 131.39 (s), 128.83 (s), 128.67 (s), 126.36 (s), 126.28 (s), 125.84 (s), 125.32 (s), 123.58 (s), 120.08 (s), 114.18 (s), 111.34 (s), 69.84 (s), 61.34 (s), 56.15 (s), 53.09 (s), 35.72 (s); IR (KBr, cm−1) ν 3414.5 (s), 32.46.4 (s), 1508.7 (s), 1466.4 (s), 1259.1 (s), 1212.0 (s), 1141.1 (s), 1004.7 (s); HRMS (ES) m/z for C23H26O4S2 [M + Na]+ cacld 453.1165, found 453.1161.
Bis(propenyl)-4-(naphthalen-1-ylmethoxy)-3-methoxyl-phenyldithioacetal (S9). Yellow solid, mp 90–92 °C, yield 95%; 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 8.1 Hz, 1H), 7.92–7.79 (m, 2H), 7.63–7.40 (m, 4H), 7.06 (d, J = 1.8 Hz, 1H), 6.94–6.83 (m, 2H), 5.79 (ddt, J = 17.1, 10.0, 7.2 Hz, 2H), 5.56 (s, 2H), 5.17–5.04 (m, 4H), 4.73 (s, 1H), 3.88 (s, 3H), 3.27 (dd, J = 13.7, 7.1 Hz, 2H), 3.06 (dd, J = 13.7, 7.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 150.17 (s), 148.06 (s), 133.87 (s), 133.76 (s), 133.18 (s), 132.37 (s), 131.40 (s), 128.78 (s), 128.67 (s), 126.34 (s), 126.26 (s), 125.82 (s), 125.35 (s), 123.62 (s), 120.40 (s), 117.54 (s), 114.15 (s), 111.72 (s), 69.84 (s), 56.09 (s), 50.41 (s), 35.34 (s); IR (KBr, cm−1) ν 3331.2 (s), 2118.4 (s), 1557.4 (s), 1462.1 (s), 1208.9 (s); HRMS (ES) m/z for C25H26O2S2 [M + Na]+ cacld 445.1266, found 445.1269.
Bis(4-fluorophenyl)-3-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S10). White solid, mp 105–106 °C, yield 92%; 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 7.8 Hz, 1H), 7.94–7.84 (m, 2H), 7.61–7.51 (m, 3H), 7.49–7.45 (m, 1H), 7.35–7.28 (m, 4H), 7.18 (t, J = 7.9 Hz, 1H), 7.00–6.86 (m, 7H), 5.43 (s, 2H), 5.21 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 164.15 (s), 161.68 (s), 158.82 (s), 140.85 (s), 135.81 (d, J = 8.2 Hz), 133.82 (s), 132.10 (s), 131.52 (s), 129.55 (s), 129.08 (s), 128.71 (s), 126.57 (d, J = 18.1 Hz), 125.95 (s), 125.28 (s), 123.66 (s), 120.61 (s), 116.05 (s), 115.84 (s), 115.01 (s), 114.26 (s), 68.71 (s), 62.01 (s); IR (KBr, cm−1) ν 1643.2 (s), 1593.7 (s), 1488.9 (s), 1212.1 (s); HRMS (ES) m/z for C30H22F2OS2 [M + Na]+ cacld 523.0972, found 523.0977.
Bis(4-chlorophenyl)-3-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S11). White solid, mp 119–121 °C, yield 91%; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.1 Hz, 1H), 7.97–7.84 (m, 2H), 7.66–7.52 (m, 3H), 7.51–7.44 (m, 1H), 7.36–7.18 (m, 9H), 7.06–7.01 (m, 1H), 7.00–6.90 (m, 2H), 5.45 (s, 2H), 5.32 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 158.91 (s), 140.47 (s), 134.63 (s), 134.22 (s), 133.81 (s), 132.51 (s), 132.05 (s), 131.51 (s), 129.68 (s), 129.08 (s), 129.04 (s), 128.72 (s), 126.66 (s), 126.51 (s), 125.95 (s), 125.29 (s), 123.65 (s), 120.60 (s), 115.19 (s), 114.26 (s), 68.72 (s), 60.76 (s); IR (KBr, cm−1) ν 1544.6 (s), 1491.6 (s), 1230.5 (s), 1081.4 (s); HRMS (ES) m/z for C30H22Cl2OS2 [M + Na]+ cacld 555.0381, found 555.0376.
Bis(2-hydroxyethyl)-3-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S12). White solid, mp 101–103 °C, yield 94%; 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 7.9 Hz, 1H), 7.97–7.81 (m, 2H), 7.67–7.42 (m, 4H), 7.34–7.26 (m, 1H), 7.21–7.14 (m, 1H), 7.07 (d, J = 7.7 Hz, 1H), 6.98 (dd, J = 8.0, 2.1 Hz, 1H), 5.51 (s, 2H), 5.04 (s, 1H), 3.73 (s, 4H), 2.84 (dt, J = 14.0, 5.8 Hz, 2H), 2.72 (dt, J = 14.0, 5.9 Hz, 2H), 2.10 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 159.11 (s), 141.65 (s), 133.81 (s), 132.10 (s), 131.54 (s), 129.86 (s), 129.08 (s), 128.70 (s), 126.72 (s), 126.50 (s), 125.95 (s), 125.27 (s), 123.70 (s), 120.40 (s), 114.94 (s), 114.24 (s), 68.76 (s), 61.30 (s), 53.17 (s), 35.70 (s); IR (KBr, cm−1) ν 3415.1 (s), 3282.6 (s), 1514.8 (s), 1464.7 (s), 1011.2 (s); HRMS (ES) m/z for C22H24O3S2 [M + Na]+ cacld 423.1059, found 423.1062.
Bis(propenyl)-3-(naphthalen-1-ylmethoxy)-phenyl-dithioacetal (S13). White solid, mp 91–93 °C, yield 96%; 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 7.8 Hz, 1H), 7.95–7.81 (m, 2H), 7.65–7.44 (m, 4H), 7.32–7.26 (m, 1H), 7.17 (d, J = 1.8 Hz, 1H), 7.05 (d, J = 7.6 Hz, 1H), 6.97 (dd, J = 7.9, 2.1 Hz, 1H), 5.80 (ddt, J = 17.1, 10.0, 7.2 Hz, 2H), 5.51 (s, 2H), 5.21–5.00 (m, 4H), 4.76 (s, 1H), 3.28 (dd, J = 13.7, 7.2 Hz, 2H), 3.07 (dd, J = 13.7, 7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 159.05 (s), 141.61 (s), 133.81 (s), 133.78 (s), 132.21 (s), 131.58 (s), 129.64 (s), 129.07 (s), 128.69 (s), 126.75 (s), 126.47 (s), 125.92 (s), 125.30 (s), 123.78 (s), 120.84 (s), 117.64 (s), 114.62 (s), 114.50 (s), 68.71 (s), 50.48 (s), 35.31 (s); IR (KBr, cm−1) ν 3321.2 (s), 2123.4 (s), 1551.4 (s), 1458.1 (s), 1208.9 (s); HRMS (ES) m/z for C24H24OS2 [M + Na]+ cacld 415.1161, found 415.1166.
Bis(2-hydroxyethyl)-5-(naphthalen-1-ylmethoxy)-2-bromo-phenyldithioacetal (S14). Red solid, mp 123–124 °C, yield 93%; 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 7.9 Hz, 1H), 7.88 (dd, J = 13.8, 8.2 Hz, 2H), 7.68–7.38 (m, 6H), 6.85 (dd, J = 8.8, 2.7 Hz, 1H), 5.51 (s, 3H), 3.76 (t, J = 5.1 Hz, 4H), 2.90–2.79 (m, 2H), 2.79–2.60 (m, 2H), 2.16 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 158.67 (s), 140.15 (s), 133.81 (s), 133.54 (s), 131.65 (s), 131.49 (s), 129.26 (s), 128.75 (s), 126.91 (s), 126.60 (s), 126.02 (s), 125.25 (s), 123.65 (s), 117.12 (s), 115.85 (s), 114.05 (s), 69.06 (s), 61.09 (s), 51.53 (s), 36.01 (s); IR (KBr, cm−1) ν 3415.1 (s), 3291.4 (s), 1517.8 (s), 1467.1 (s), 1016.2 (s); HRMS (ES) m/z for C22H23BrO3S2 [M + Na]+ cacld 501.0164, found 501.0161.
Bis(2-hydroxyethyl)-4-(naphthalen-1-ylmethoxy)-3-chloro-phenyldithioacetal (S15). White solid, mp 102–104 °C, yield 94%; 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 8.2 Hz, 1H), 7.94–7.82 (m, 2H), 7.65 (d, J = 6.9 Hz, 1H), 7.61–7.43 (m, 4H), 7.31 (dd, J = 8.5, 2.3 Hz, 1H), 7.05 (d, J = 8.5 Hz, 1H), 5.59 (s, 2H), 5.04 (s, 1H), 3.76 (q, J = 5.6 Hz, 4H), 2.85 (dt, J = 14.0, 5.8 Hz, 2H), 2.71 (dt, J = 14.0, 5.9 Hz, 2H), 2.15 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 154.10 (s), 133.74 (s), 133.63 (s), 131.55 (s), 131.23 (s), 129.71 (s), 129.05 (s), 128.74 (s), 126.98 (s), 126.49 (s), 126.16 (s), 125.96 (s), 125.31 (s), 123.69 (s), 123.44 (s), 114.08 (s), 69.68 (s), 61.47 (s), 52.25 (s), 35.59 (s); IR (KBr, cm−1) ν 3418.8 (s), 3271.4 (s), 1537.8 (s), 1467.1 (s), 1016.2 (s); HRMS (ES) m/z for C22H23ClO3S2 [M + Na]+ cacld 457.0669, found 457.0663.
Bis(2-hydroxyethyl)-2-(naphthalen-1-ylmethoxy)-5-methyl-phenyldithioacetal (S16). White solid, mp 139–140 °C, yield 91%; 1H NMR (400 MHz, CDCl3) δ 8.11–8.03 (m, 1H), 7.97–7.82 (m, 2H), 7.63–7.40 (m, 5H), 7.12–7.04 (m, 1H), 7.00 (d, J = 8.3 Hz, 1H), 5.52 (s, 2H), 5.43 (s, 1H), 3.48 (q, J = 5.9 Hz, 4H), 2.67 (dd, J = 12.8, 7.0 Hz, 2H), 2.57 (dd, J = 12.9, 7.1 Hz, 2H), 2.32 (s, 3H), 1.90 (d, J = 6.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 152.77 (s), 133.83 (s), 132.16 (s), 131.57 (s), 131.06 (s), 129.63 (s), 129.28 (s), 129.14 (s), 128.85 (s), 128.78 (s), 126.91 (s), 126.52 (s), 126.03 (s), 125.37 (s), 123.69 (s), 112.24 (s), 69.38 (s), 60.93 (s), 45.21 (s), 36.05 (s), 20.64 (s); IR (KBr, cm−1) ν 3421.2 (s), 3271.4 (s), 1547.6 (s), 1477.3 (s), 1018.2 (s); HRMS (ES) m/z for C23H26O3S2 [M + Na]+ cacld 437.1216, found 437.1219.
Biological assays
Conflicts of interest
The authors confirm that this article content has no conflict of interest.Acknowledgements
The authors gratefully acknowledge financial support by the National Key Research and Development Program of China (No. 2017YFD0200506), the National Natural Science Foundation of China (No. 21807037), the National Special Fund For Agro-Scientific Research in the Public Interest of China (No. 201503112-8), the Provincial Major Project of Education Department in Anhui (No. KJ2018A0386), the Provincial Major Project of Excellent Youth Talent Support Program in Anhui (No. gxyqZD2018092), National innovation training program for college students (20171402085). We also sincerely thank all co-workers who have contributed to the work.Notes and references
- (a) J. A. Tomlinson, Ann. Appl. Biol., 1987, 110, 661 CrossRef; (b) A. N. Creager, K. B. Scholthof, V. Citovsky and H. B. Scholthof, Plant Cell, 1999, 11, 301 CrossRef CAS PubMed; (c) M. Jacquemond, Adv. Virus Res., 2012, 84, 439 Search PubMed.
- (a) S. Boquel, J. Zhang, C. Goyer, M. A. Giguère, C. Clark and Y. Pelletier, Pest Manage. Sci., 2015, 71, 1106 CrossRef CAS; (b) A. V. Karasev and S. M. Gray, Annu. Rev. Phytopathol., 2013, 51, 571 CrossRef CAS.
- K. B. G. Scholthof, S. Adkins, H. Czosnek, P. Palukaitis, E. Jacquot, T. Hohn, B. Hohn, K. Saunders, T. Candresse, P. Ahlquist, C. Hemenway and G. D. Foster, Mol. Plant Pathol., 2011, 12, 938 CrossRef CAS.
- H. Xiao, P. Li, D. Y. Hu and B. A. Song, Bioorg. Med. Chem. Lett., 2014, 24, 3452 CrossRef CAS.
- M. H. Chen, P. Li, D. Y. Hu and B. A. Song, Bioorg. Med. Chem. Lett., 2016, 26, 168 CrossRef CAS.
- J. Wu and B. A. Song, Sci. Sin.: Chim., 2016, 46, 1165 Search PubMed.
- B. A. Song, S. Yang, L. H. Jin and P. S. Bhadury, Environment friendly antiviral agents for plants, Springer, Berlin, 2009 Search PubMed.
- Z. Z. Wang, D. D. Xie, X. H. Gan, D. Y. Hu and B. A. Song, Bioorg. Med. Chem. Lett., 2017, 27, 4096 CrossRef CAS.
- J. D. Jones and J. L. Dangl, Nature, 2006, 444, 323 CrossRef CAS.
- F. P. Silverman, P. D. Petracek, Z. Ju, C. M. Fledderman, D. F. Heiman and P. Warrior, J. Agric. Food Chem., 2005, 53, 9764 CrossRef CAS.
- H. Kauss, K. Krause and W. Jeblick, Biochem. Biophys. Res. Commun., 1992, 189, 304 CrossRef CAS.
- Z. J. Fan, Z. G. Shi, H. K. Zhang, X. F. Liu, L. L. Bao, L. Ma, X. Zuo, Q. X. Zheng and N. Mi, J. Agric. Food Chem., 2009, 57, 4279 CrossRef CAS.
- F. P. Silverman, P. D. Petracek, Z. Ju, C. M. Fledderman, D. F. Heiman and P. Warrior, J. Agric. Food Chem., 2005, 53, 9769 CrossRef CAS.
- F. P. Silverman, P. D. Petracek, D. F. Heiman, C. M. Fledderman and P. Warrior, J. Agric. Food Chem., 2005, 53, 9775 CrossRef CAS.
- M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. D. De Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271 CrossRef CAS.
- L. Sakthikumar, J. Kavitha and R. Mahalakshmi, Der Pharma Chem., 2014, 6, 294 Search PubMed.
- H. Koga, Y. Tsuji, K. Inoue, K. Kanai, T. Majima, T. Kasai, K. Uchida and H. Yamaguchi, J. Infect. Chemother., 2006, 12, 163 CrossRef CAS.
- H. Koga, Y. Nanjoh, K. Makimura and R. Tsuboi, Med. Mycol., 2009, 47, 640 CrossRef CAS PubMed.
- S. Pandey, S. Suryawanshi, S. Gupta and V. Srivastava, Eur. J. Med. Chem., 2005, 40, 751 CrossRef CAS.
- J. Zhang, L. Zhao, C. Zhu, Z. X. Wu, G. P. Zhang, X. H. Gan, D. Y. Liu, J. K. Pan, D. Y. Hu and B. A. Song, J. Agric. Food Chem., 2017, 65, 4582 CrossRef CAS PubMed.
- J. Chen, J. Shi, L. Yu, D. Y. Liu, X. H. Gan, B. A. Song and D. Y. Hu, J. Agric. Food Chem., 2018, 66, 5335 CrossRef CAS.
- H. C. Zhang and X. L. Li, Instruction manual for plant growth regulators, China Agriculture Press, 2011 Search PubMed.
- K. K. Pasunooti, H. Chai, C. N. Jensen, B. K. Gorityala, S. Wang and X. W. Liu, Tetrahedron Lett., 2011, 52, 80 CrossRef CAS.
- Z. W. Wang, A. Feng, M. Cui, Y. Liu, L. Wang and Q. M. Wang, PLoS One, 2012, 7, e52933 CrossRef CAS.
- S. G. Bhansali, et al. , Int. J. PharmTech Res., 2013, 5, 1224 CAS.
- G. V. Gooding Jr and T. T. Hebert, Phytopathology, 1967, 57, 1285 CAS.
- H. Scott, Virology, 1963, 20, 103 CrossRef CAS.
- D. G. Zhou, D. D. Xie, F. C. He, B. A. Song and D. Y. Hu, Bioorg. Med. Chem. Lett., 2018, 28, 2091 CrossRef CAS.
- Z. X. Wu, J. Zhang, J. X. Chen, J. K. Pan, L. Zhao, D. Y. Liu, A. W. Zhang, J. Chen, D. Y. Hu and B. A. Song, Pest Manage. Sci., 2017, 73, 2079 CrossRef CAS PubMed.
- (a) Z. H. Ma, M. G. Zhou and Z. Y. Ye, Acta Phytopathol. Sin., 1997, 27, 237 Search PubMed; (b) G. B. Shen and M. G. Zhou, Plant Prot., 2002, 28, 9 Search PubMed.
- P. Li, D. Y. Hu, D. D. Xie, J. X. Chen, L. H. Jin and B. A. Song, J. Agric. Food Chem., 2018, 66, 3093 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06843k |
| ‡ These authors contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2019 |