Open Access Article
Open Access ArticleZnCl2 loaded TiO2 nanomaterial: an efficient green catalyst to one-pot solvent-free synthesis of propargylamines†
Digambar B.
Bankar
ab,
Ranjit R.
Hawaldar
a,
Sudhir S.
Arbuj
 a,
Mansur H.
Moulavi
a,
Santosh T.
Shinde
d,
Shrikant P.
Takle
a,
Manish D.
Shinde
a,
Dinesh P.
Amalnerkar
c and
Kaluram G.
Kanade
a,
Mansur H.
Moulavi
a,
Santosh T.
Shinde
d,
Shrikant P.
Takle
a,
Manish D.
Shinde
a,
Dinesh P.
Amalnerkar
c and
Kaluram G.
Kanade
 *ac
*ac
aCentre for Materials for Electronics Technology (C-MET), Panchwati, Off Pashan Road, Pune-411008, India. E-mail: kgkanade@yahoo.co.in
bP. G. Department of Chemistry, R. B. Narayanrao Borawake College, Shrirampur, Ahmednagar-413709, India
cP. G. and Research Centre, Yashavantrao Chavan Institute of Science, Satara-415001, India
dP. G. Department of Chemistry, Annasaheb Awate College, Manchar, Pune-410503, India
First published on 14th October 2019
Abstract
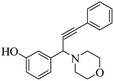
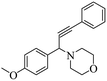
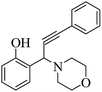
One-pot green synthesis of propargylamines using ZnCl2 loaded TiO2 nanomaterial under solvent-free conditions has been effectively accomplished. The aromatic aldehydes, amines, and phenylacetylene were reacted at 100 °C in the presence of the resultant catalyst to form propargylamines. The nanocrystalline TiO2 was initially synthesized by a sol–gel method from titanium(IV) isopropoxide (TTIP) and further subjected to ZnCl2 loading by a wet impregnation method. X-ray diffraction (XRD) patterns revealed the formation of crystalline anatase phase TiO2. Field emission scanning electron microscopy (FESEM) showed the formation of agglomerated spheroid shaped particles having a size in the range of 25–45 nm. Transmission electron microscopy (TEM) validates cubical faceted and nanospheroid-like morphological features with clear faceted edges for the pure TiO2 sample. Surface loading of ZnCl2 on spheroid TiO2 nanoparticles is evident in the case of the ZnCl2 loaded TiO2 sample. X-ray photoelectron spectroscopy (XPS) confirmed the presence of Ti4+ and Zn2+ species in the ZnCl2 loaded TiO2 catalyst. Energy-dispersive X-ray (EDS) spectroscopy also confirmed the existence of Ti, O, Zn and Cl elements in the nanostructured catalyst. 15% ZnCl2 loaded TiO2 afforded the highest 97% yield for 3-(1-morpholino-3-phenylprop-2-ynyl)phenol, 2-(1-morpholino-3-phenylprop-2-ynyl)phenol and 4-(1,3-diphenylprop-2-ynyl)morpholine under solvent-free and aerobic conditions. The proposed nanostructure-based heterogeneous catalytic reaction protocol is sustainable, environment-friendly and offers economic viability in terms of recyclability of the catalyst.
1. Introduction
Organic reactions involving three or more components are becoming increasingly important day by day since they allow many starting materials to be combined easily in one-pot forming a single compound.1 One-pot, multi-component carbon–carbon and carbon–nitrogen bond forming reactions were successfully accomplished for producing molecular complexity.2 Three-component coupling of aldehyde, amine, and alkyne is one of the most extensively used methods for the preparation of several propargylamines through C–H bond activation.3 Propargylamine derivatives play a significant role in synthetic organic chemistry by acting as a versatile intermediate for the synthesis of many biologically active heterocyclic compounds such as 2-aminoimidazoles,4 pyrrolidines,5 pyrroles,6 phenanthrolines,7 oxazolidinones,8 aminoindolizines,9etc. Moreover, they are the important structural ingredients of therapeutic drug molecules and also of natural products.10 For example, rasagiline containing Azilect as a moiety of propargylamine has been used for the symptomatic therapy in early Parkinson's disease.11–13 Also, propargylamines are essential intermediates for the preparation of PF960IN as a biologically active drug molecule.14The literature survey reveals that the synthesis of propargylamines via classical routes involves the addition of alkynyl-metal reagents to the imines15,16 in the separate step. However, the addition of moisture sensitive Grignard or Li acetylides reagent is tedious. Through direct amination of propargylic electrophiles like propargyl halides, triflates and phosphates,17 the formation of propargylamine derivatives also has been achieved.
In the last few decades, enormous progress has been made for the C–H bond activation of terminal alkynes. A variety of homogeneous transition metallic systems such as Cu(I) salts,18 Ag(I) salts,19 ZnMe2,20 Fe(III) salts,21 Cu(I) salts,22 Zr complex,23 Ni(II) salts,24 InCl3,25 Ir complex26 and AgOAc27 were used for the C–H bond activation of terminal alkynes. Researchers also used Cu–Ru bimetallic system,28 InBr3,29 Co(II)30 and Au(III) salen complexes31 as a homogeneous catalytic system for the activation of C–H bond of the terminal alkynes. However, some of these catalytic systems have certain limitations due to the use of expensive and excess amount of catalyst, toxic solvents, and the requirement of inert atmospheric conditions.
Diverse heterogeneous catalysts such as layered double hydroxide-supported gold (LDH-AuCl4) catalyst,32 polystyrene-supported NHC–Ag(I) catalyst,33 Fe3O4 nanoparticles,34 Ag nanoparticles/Ni metal–organic framework,35 copper(I) anchored onto MCM-41 silica,36 Fe3O4@SBA-15 (ref. 37) etc. were used for the synthesis of propargylamines. Nanoscale metal oxides such as ZnO38 and In2O3 (ref. 39) were also used for the synthesis of propargylamines. Further, copper ferrite nanoparticles,40 Ni–Y zeolites,41 nanosize Co3O4,42 Cu nanoparticles on TiO2,43 Au nanoparticles supported on ionic liquid,44 Au nanoparticles supported on polyacrylamide,45 Zn(II)/HAP/Fe3O4,46 Zn(L-proline)2,47etc. were used as heterogeneous catalysts for the three component synthesis of propargylamines. Besides, a super paramagnetic MCM-41 with dendrites copper complex48 was utilized as a recyclable catalyst for the propargylamine synthesis. Nonetheless, there are disadvantages to some of such catalytic systems viz. requirement of high catalyst amount, use of inert gas, use of solvent, longer reaction time, etc. Hence, there is a need to find a simple and efficient method for the synthesis of propargylamines.
In the context of heterogeneous catalysis, nanocrystalline materials can play significant role due to their large surface to volume ratio, easy separation and desirable physico-chemical characteristics.49 Specifically, nanocrystalline TiO2 is one of the most important materials extending widespread applications in pigments, catalytic systems, inorganic membranes, gas sensors, support ceramics,50etc. Also, non-toxic nature and low cost of TiO2 nano-particulate system made it a better catalyst. The TiO2 supported with transition metals brings the advantage of heterogeneous catalysis in the propargylamine synthesis. Fe doped TiO2 nanoparticles are used as a heterogeneous catalyst for the activation of C–H bond of terminal alkynes under microwave irradiation.51 Recently, Ag/TiO2 and Pt/TiO2 nanocatalyst have been applied as active recyclable catalysts for the synthesis of propargylamines under microwave irradiation at 140 °C.52
Effective use of zinc salts53 for the propargylamine synthesis motivated us to perform one-pot synthesis of propargylamine using ZnCl2 loaded TiO2 nanomaterial.
In this report, we have demonstrated a synthetic strategy in which inexpensive and commercially available ZnCl2 is loaded by wet impregnation technique on sol–gel generated TiO2 nanoparticles. The as-synthesized nanocrystalline ZnCl2 loaded TiO2 has been used as an efficient and green heterogeneous catalyst for the synthesis of propargylamines under solvent-free and aerobic conditions.
2. Experimental
2.1. General
All the chemicals were AR grade and purchased from a local dealer. The chemicals were used as received without further purification. The progress of reaction was monitored by thin layer chromatography (TLC) technique. The crystal structure of the synthesized catalyst was detected by XRD using a Rigaku MiniFlex-600 diffractometer (Cu Kα radiation, λ = 1.5406 Å). The surface morphology and particle size were examined using field emission scanning electron microscope (Hitachi S-4800). Elemental compositions were determined by energy-dispersive X-ray spectroscopy (EDS) analysis. For fine-scale nanostructural evaluation, field emission transmission electron microscopic (FETEM) images of the typical sample were obtained using the JEOL JEM-2200 FS instrument. The same instrument was also used for obtaining the Energy Dispersive Spectroscopy (EDS) and allied elemental mapping information using scanning transmission electron microscopy (STEM) in bright field mode. For the confirmation of chemical composition, X-ray photoelectron spectroscopic (XPS) scans were recorded using Al Kα (Monochromatic) anode with 6 mA beam current and 12 kV X-ray source. The as-synthesized propargylamine derivatives were spectrally characterized by Nuclear Magnetic Resonance (NMR) spectroscopy and High-Resolution Mass Spectrometry (HRMS) techniques. 1H and 13C NMR spectra were recorded using CDCl3 and DMSO-d6 solvents on 500 and 125 MHz Bruker NMR spectrometer, respectively. The HRMS was taken in methanol solvent using high-resolution mass spectrometer (Bruker Germany, Model: Impact HD, UHR Impact II ESI-Q-TOF). All yields refer to the isolated products after purification by column chromatography with ethyl acetate and n-hexane as an eluent.2.2. Synthesis of TiO2 nanoparticles
Synthesis of TiO2 nanoparticles was performed by sol–gel method using titanium(IV) isopropoxide as a titanium precursor. Initially, 80 ml of glacial acetic acid was added drop-wise to a 40 ml of titanium(IV) isopropoxide (TTIP) in a 500 ml round bottom flask immersed in an ice bath with constant stirring. Later, 100 ml isopropyl alcohol was gradually added with vigorous stirring for a period of 30 min at room temperature. In the resulting solution, 10 g polyvinyl alcohol (PVA) in 50 ml isopropyl alcohol was added, stirred for 10 min and subsequently, 10 ml of 1 M HCl was added and the resultant solution was subjected to constant stirring at room temperature for 4 h. For natural gel formation, the obtained mixture was transferred into 500 ml beaker and kept for 5 days. Finally, the gel was dried at 110 °C for 12 h, transferred into a mortar, powdered with pestle and further calcined in a muffle furnace at 400 °C for 4 h.2.3. Synthesis of ZnCl2 loaded TiO2 nanomaterial (ZnCl2–TiO2 catalyst)
Loading of 15 wt% ZnCl2 over TiO2 nanoparticles was performed by wet impregnation technique. A mixture of ZnCl2 (0.3 g) and TiO2 nanoparticles (2.0 g) was grinded for 10 min and subsequently transferred into the 250 ml round bottom flask containing 50 ml ethanol. Further, it was stirred at room temperature for 1 h and then refluxed for 4 h with persistent stirring. The excess ethanol was concentrated under vacuum distillation and obtained solid material was dried at 200 °C for 6 h. The same procedure was repeated for the loading of 5 wt%, 10 wt% and 20 wt% ZnCl2 over TiO2 nanoparticles.2.4. General procedure for the synthesis of propargylamine derivatives
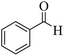
In a typical procedure, aromatic aldehyde (1 mmol), secondary amine (1.2 mmol) and phenylacetylene (1.5 mmol) were taken in a clean and oven-dried round bottom flask. The nanocrystalline ZnCl2–TiO2 catalyst (0.2 mmol) was added into the round bottom flask and the resulting reaction mixture was placed in pre-heated oil bath at 100 °C and stirred at the same temperature for 6 h. Progress of the reaction was checked by thin layer chromatography (TLC). After cooling, ethyl acetate was added to the reaction mass and stirred at room temperature for 15 min. Ethyl acetate layer was separated by filtration and again remaining reaction residue was washed with ethyl acetate. Combined ethyl acetate washings were mixed with water. The organic layer was separated by extraction, dried over anhydrous Na2SO4 and filtered. The organic solvent was concentrated under reduced pressure and obtained crude residue was purified by column chromatography to yield the desired propargylamine derivative. The pure organic compounds were characterized by 1H NMR, 13C NMR, and HRMS spectral techniques (please see ESI†).3. Results and discussion
3.1. Catalyst characterization
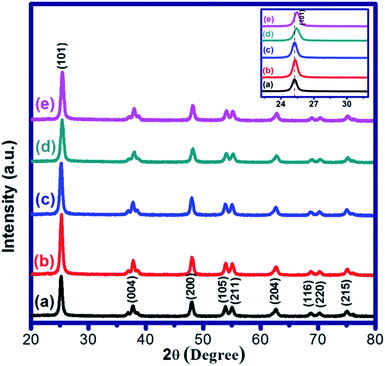 | ||
| Fig. 1 XRD patterns of (a) pure TiO2, (b) 5% ZnCl2–TiO2, (c) 10% ZnCl2–TiO2, (d) 15% ZnCl2–TiO2 and (e) 20% ZnCl2–TiO2. Inset is enlarged view of (101) plane. | ||
The crystallite size of all as-synthesized samples was calculated by Scherrer's equation using broadening of (101) anatase peak of TiO2. The crystallite size of unloaded TiO2 is 16 nm, whereas 5%, 10%, 15% and 20% ZnCl2 loaded TiO2 showed crystallite size values of 16, 15, 13 and 14 nm respectively. It may be, however, noted that there is no prominent change in the overall crystallite size of all the samples under investigation. The miniscule decrease in crystallite size of ZnCl2–TiO2 in comparison to pure TiO2 as well as the marginal shift in 2θ value of (101) plane with increasing loading percentage of ZnCl2 on TiO2 nanoparticles can be ascribed to the effective surface coating of ZnCl2 over TiO2 and/or might be due to partial incorporation of Zn species in the crystalline lattice of TiO2.
 | ||
| Fig. 4 TEM-EDS spectrum exhibiting quantitative elemental composition data for nanostructured 15% ZnCl2–TiO2. | ||
The EDS spectrum discloses that the 15% ZnCl2–TiO2 catalyst contains 33.36 wt% of oxygen, 6.08 wt% of chlorine, 43.29 wt% of titanium and 17.27 wt% of Zn, even though the XRD pattern did not show any Zn peaks. Thus, EDS spectrum revealed that the sample is composed of Ti, O, Zn, and Cl which is in good agreement with results obtained by XPS (Fig. 6).
The EDS-elemental mapping images of 15% ZnCl2–TiO2 sample in scanning transmission electron microscopy (STEM) mode are shown in Fig. 5.
 | ||
| Fig. 5 FETEM-STEM-EDS elemental mapping images of 15% ZnCl2–TiO2 catalyst: (a) Electron image (b) Ti, (c) O, (d) Zn and (e) Cl. | ||
The elemental mapping images due to Ti and O (Fig. 5b and c, respectively) overlap well with corresponding electron image (Fig. 5a). The intensity of the colours assigned to Ti and O is also very high. The elemental mapping images for Zn and Cl (Fig. 5d and e, respectively) also overlap with the corresponding electron image, however, the intensity of the colours ascribable to these two elements is low which is quite obvious as ZnCl2 is used only for the purpose of surface loading. FETEM images correspond well with FESEM images and endorse the surface loading of ZnCl2 over TiO2 nanoparticles.
 | ||
| Fig. 6 High resolution XPS scans of the respective elements corresponding to 15% ZnCl2–TiO2 sample. The inset of (b) displays deconvoluted spectrum. | ||
The Ti binding energy peaks observed at 458.72 and 464.35 eV are related to Ti 2p3/2 and Ti 2p1/2, in close resemblance with that of Ti4+ in TiO2.57 The two O 1s peaks (inset of Fig. 6b) can be assigned to lattice oxygen (O–Ti) at 529.80 eV and surface-bound hydroxyl group (Ti–OH) at 531.78 eV. The binding energy peaks at 1022.20 eV and 1045.20 eV can be associated with Zn 2p3/2 and Zn 2p1/2 energy states, respectively (Fig. 6c). The peak at 1022.20 eV could be assigned to Zn2+ species in ZnO (1021.9 eV),58 ZnCl2 (1022.5 eV)59 or Zn(OH)2 (1022.6).60 Subsequently, we studied the chloride XPS peak (Fig. 6d) to detect the presence of Zn2+ species among the ZnO, ZnCl2 or Zn(OH)2. The binding energy at 199.59 eV signifies the Cl 2p3/2 energy state of metal chloride. It apparently confirms the presence of ZnCl2 (ref. 61) confined to surface in the ZnCl2–TiO2 catalyst (since XPS is surface sensitive technique).
The overall appearance of peaks in XPS scans demonstrates the successful loading of ZnCl2 on the surface of TiO2 nanoparticles.
3.2. Catalytic activity of ZnCl2 loaded TiO2 nanomaterial
After successful synthesis and characterization of ZnCl2–TiO2 catalysts, it is employed as heterogeneous catalyst in multicomponent synthesis of propargylamine. To optimize the reaction conditions, several parameters such as catalyst, temperature and time were studied and corresponding results are summarized in Table 1. To achieve sustainable green chemistry approach, prior reaction was set under solvent-free and aerobic conditions. In the initial optimizations, 3-hydroxybenzaldehyde, morpholine, and phenylacetylene were used as substrates for the model reaction (Scheme 1).| Entry | Catalyst | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 3-hydroxybenzaldehyde (1 mmol), morpholine (1.2 mmol), phenylacetylene (1.5 mmol), catalyst (0.2 mmol), temp., time, solvent-free condition. b Isolated yield. | ||||
| 1 | ZnCl2 | 30 °C | 6.0 | — |
| 2 | ZnCl2 | 70 °C | 6.0 | 53 |
| 3 | ZnCl2 | 100 °C | 6.0 | 76 |
| 4 | Pure TiO2 | 100 °C | 6.0 | 38 |
| 5 | 5% ZnCl2–TiO2 | 100 °C | 6.0 | 76 |
| 6 | 10% ZnCl2–TiO2 | 100 °C | 3.0 | 57 |
| 7 | 10% ZnCl2–TiO2 | 100 °C | 6.0 | 86 |
| 8 | 15% ZnCl2–TiO2 | 30 °C | 6.0 | — |
| 9 | 15% ZnCl2–TiO2 | 70 °C | 6.0 | 74 |
| 10 | 15% ZnCl2–TiO2 | 100 °C | 3.0 | 68 |
| 11 | 15% ZnCl2–TiO2 | 100 °C | 6.0 | 97 |
| 12 | 20% ZnCl2–TiO2 | 100 °C | 6.0 | 97 |
When the reaction was performed at 30 °C in the presence of commercial ZnCl2, no product formation was observed even after 6 h (Table 1, entry 1). However, when the reaction was carried out at 70 °C, low yield of product was obtained in 6 h (Table 1, entry 2). In addition, an increase in yield (76%) was observed as the temperature was raised to 100 °C (Table 1, entry 3). This indicates that the temperature plays an important role in this reaction. The lower yield using commercial ZnCl2 can be due to its hygroscopic nature. We also performed the reaction using pure TiO2, but observed only 38% yield (Table 1, entry 4).
Subsequently, the catalytic activities of 5%, 10%, 15% and 20% ZnCl2 loaded TiO2 nanoparticles (nanocrystalline ZnCl2–TiO2 catalysts) were examined. For 5% and 10% ZnCl2 supported TiO2 catalysts, yields of 76% and 86%, respectively, were obtained (Table 1, entries 5 and 7). This means that as the loading of ZnCl2 on TiO2 increases, an increase in product yield is observed under a constant mmol of catalyst (0.2 mmol). Therefore, we studied the reaction with 15% ZnCl2 loaded TiO2. No product formation was detected at 30 °C using 15% ZnCl2 loaded TiO2 catalyst (Table 1, entry 8), 74% yield obtained at 70 °C in 6 h (Table 1, entry 9), 68% yield obtained at 100 °C in 3 h (Table 1, entry 10). However, when the reaction was performed at 100 °C for 6 h, 97% yield of the desired product was observed (Table 1, entry 11). 15% ZnCl2 loading over TiO2 was necessary as the optimum amount of ZnCl2 loading and increasing the loading of ZnCl2 (20%) did not improve the yield (Table 1, entry 12).
It is clearly noticed that the nanostructured ZnCl2–TiO2 system exhibits excellent catalytic activity after loading ZnCl2 over TiO2 nanoparticles. The excellent catalytic activity of the nanostructured ZnCl2–TiO2 can be attributed to the greater degree of crystallinity at nanoscale.
It is very interesting to see the effect of solvents on catalytic activity of 15% ZnCl2–TiO2 catalyst. Therefore, we investigated the role of various solvents such as toluene, CH3CN, DMF, THF, and H2O.
From Table 2, is very clear that catalyst exhibits poor activity in polar solvents (CH3CN, DMF, H2O and THF). Non-polar solvent (toluene) shows good catalytic activity, while under solvent-free conditions, catalyst shows excellent catalytic activity. The reason for lowering the yield of propargylamine and hence catalytic activity lies in solvation of reactants and catalyst. In solvent, reactants are dispersed so it delays the contact with the catalyst. Under solvent-free conditions, reactants and catalyst can quickly come in intimate contact with each other which, in turn, can accelerate the reaction on the surface of the catalyst. Thus, best results could be observed for 15% ZnCl2 loaded TiO2 nanoparticle catalyst with 97% yield under solvent-free conditions.
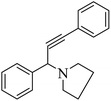
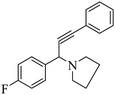
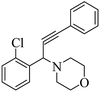
After optimization of the reaction parameters, we have extended the scope of reaction to the different aromatic aldehydes, cyclic secondary amines (pyrrolidine and morpholine), and phenylacetylene using 15% ZnCl2–TiO2 (Scheme 2 and Table 3, entries 1–12).
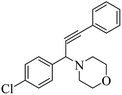
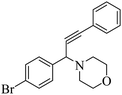
The results in Table 3 indicate that the aromatic aldehydes with electron-donating and halogen substituents showed high reactivity and produced the corresponding propargylamines in better yields. Aryl aldehydes with a hydroxyl group at meta or ortho positions (Table 3, entries 1, 4 and 5), afforded excellent yields as compared to aldehydes having methoxy group at para position (Table 3, entries 2 and 3). The reactivity of 2-chloro and 4-chlorobenzaldehydes (Table 3, entries 9–11) was found to be slightly more than 4-flourobenzaldehyde (Table 3, entry 8) and 4-bromobenzaldehyde (Table 3, entry 12). Benzaldehyde afforded the coupling products in excellent yield (Table 3, entries 6 and 7). We observed slightly higher yields of the products using morpholine as compared to pyrrolidine. Thus, 15% ZnCl2–TiO2 catalyst worked as an efficient catalyst for the synthesis of propargylamines under solvent-free conditions. It may be noted that, the as-synthesized propargylamines are all chiral compounds and spectral data of all products confirmed that they are single compounds and not mixture of isomers.
The reusability of the ZnCl2–TiO2 catalyst was also examined for the reaction between 3-hydroxybenzaldehyde, morpholine, and phenylacetylene under the optimized reaction conditions (Table 3, entry 1). After the first run, the solid catalyst was separated from the reaction mixture by filtration, washed with the ethyl acetate and dried at 120 °C for 4 h. The dried catalyst was then subjected to the next run of the reaction under optimized conditions and afforded 94% yield. Similar procedure was followed for next runs. The relevant results are presented in Table 4. As can be seen, the catalyst could be recycled for five successive runs without significant loss of catalytic activity.
The recycled ZnCl2–TiO2 catalyst was further characterized by XRD which confirms retention of anatase phase of TiO2 (Fig. S1, ESI†). It is, thus, noted that the crystal structure of the recovered catalyst has not changed. The sharp peaks signify that the nature of recovered catalyst is crystalline.
The transition metals are generally used to activate the C–H bond of terminal alkynes in carbon–carbon coupling reactions. The transition metal supported on solid support such as TiO2 nanoparticles might bring the advantage of heterogeneous catalysis in C–C bond forming reactions.51 In the proposed reaction, the ZnCl2 loaded TiO2 nanomaterial (ZnCl2–TiO2) can also lead to activation of C–H bond of terminal alkyne. A tentative mechanism for the one-pot propargylamine synthesis under solvent-free condition using ZnCl2 loaded TiO2 nanomaterial is pictorially demonstrated in Scheme 3. The reaction initially involves activation of C–H bond of phenylacetylene (3) by ZnCl2 from the nanostructured ZnCl2–TiO2 catalyst. The generated phenylacetylide–ZnCl2–TiO2 intermediate (a) is then reacted with iminium ion (b) (which is produced in situ from aldehyde 1 and amine 2) in order to offer the desired product of propargylamine 4. Here, ZnCl2 in the ZnCl2–TiO2 nanomaterial is the main species to activate the C–H bond of terminal alkynes and anatase TiO2 provides solid support for ZnCl2. Due to their synergistic effect, nanostructured ZnCl2–TiO2 can work as an efficient heterogeneous catalyst for the synthesis of propargylamines.
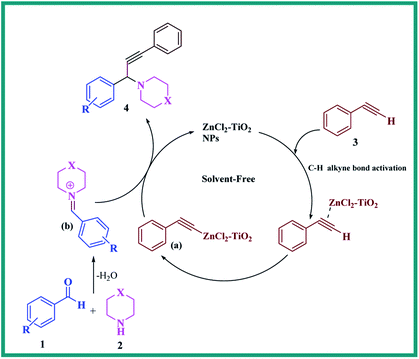 | ||
| Scheme 3 A tentative mechanism for the synthesis of propargylamine catalysed by ZnCl2–TiO2 nanoparticles (NPs). | ||
4. Conclusion
We have reported a simple and efficient route for the synthesis of propargylamine using small amount of nanocrystalline ZnCl2–TiO2 as a heterogeneous catalyst. The role of catalyst is significantly observed for alkyne C–H bond activation. The reported technique is solvent-free and required mild reaction conditions. The synthesized 15% ZnCl2 loaded TiO2 nanomaterial was found to be an excellent catalyst for the synthesis of diverse propargylamine derivatives. We believe that our present protocol is eco-friendly for green approach and can become an alternative to conventional homogeneous catalysis for the synthesis of propargylamine.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. B. Bankar is obliged to Dr B. B. Kale, Director of C-MET, Pune, India for providing research facility. He is also indebted to the Principal and Head of the Chemistry Department of R. B. Narayanrao Borawake College, Shrirampur, India for support to this research work. Dr K. G. Kanade is thankful to RUSA, India for financial and the Rayat Shikshan Sanstha, Satara, for administrative support. Authors are grateful to the Director of NCL, Pune for providing XPS facility.References
- M. Jeganathan, A. Dhakshinamoorthy and K. Pitchumani, ACS Sustainable Chem. Eng., 2014, 2, 781–787 CrossRef CAS.
- K. Gong, H. Wang, S. Wang and X. Ren, Tetrahedron, 2015, 71, 4830–4834 CrossRef CAS.
- A. Teimouri, A. N. Chermahini and M. Narimani, Bull. Korean Chem. Soc., 2012, 33, 1556–1560 CrossRef CAS.
- D. S. Ermolat'ev, J. B. Bariwal, H. P. Steenackers, S. C. De Keersmaecker and E. V. Van der Eycken, Angew. Chem., Int. Ed., 2010, 49, 9465–9468 CrossRef.
- D. F. Harvey and D. M. Sigano, J. Org. Chem., 1996, 61, 2268–2272 CrossRef CAS.
- Y. Yamamoto, H. Hayashi, T. Saigoku and H. Nishiyama, J. Am. Chem. Soc., 2005, 127, 10804–10805 CrossRef CAS.
- D. Shibata, E. Okada, J. Molette and M. Medebielle, Tetrahedron Lett., 2008, 49, 7161–7164 CrossRef CAS.
- W. J. Yoo and C. J. Li, Adv. Synth. Catal., 2008, 350, 1503–1506 CrossRef CAS.
- B. Yan and Y. Liu, Org. Lett., 2007, 9, 4323–4326 CrossRef CAS.
- M. L. Kantam, V. Balasubrahmanyam, K. S. Kumar and G. Venkanna, Tetrahedron Lett., 2007, 48, 7332–7334 CrossRef CAS.
- C. Binda, F. Hubálek, M. Li, Y. Herzig, J. Sterling, D. E. Edmondson and A. Mattevi, J. Med. Chem., 2004, 47, 1767–1774 CrossRef CAS.
- M. Naoi, W. Maruyama, H. Yi, Y. Akao, Y. Yamaoka and M. Shamoto-Nagai, J. Neural Transm., 2007, 121–131 CAS.
- T. K. Saha and R. Das, ChemistrySelect, 2018, 3, 147–169 CrossRef CAS.
- B. Agrahari, S. Layek, R. Ganguly and D. D. Pathak, New J. Chem., 2018, 42, 13754–13762 RSC.
- (a) V. V. Kouznetsov and L. Y. V. Mendez, Synthesis, 2008, 2008(04), 491–506 CrossRef; (b) G. Blay, A. Monleon and J. Pedro, Curr. Org. Chem., 2009, 13, 1498–1539 CrossRef CAS.
- E. Ramu, R. Varala, N. Sreelatha and S. R. Adapa, Tetrahedron Lett., 2007, 48, 7184–7190 CrossRef CAS.
- Y. Imada, M. Yuasa, I. Nakamura and S.-I. Murahashi, J. Org. Chem., 1994, 59, 2282–2284 CrossRef CAS.
- A. Bisai and V. K. Singh, Org. Lett., 2006, 8, 2405–2408 CrossRef CAS.
- C. Wei, Z. Li and C.-J. Li, Org. Lett., 2003, 5, 4473–4475 CrossRef CAS.
- L. Zani, S. Alesi, P. G. Cozzi and C. Bolm, J. Org. Chem., 2006, 71, 1558–1562 CrossRef CAS.
- P. Li, Y. Zhang and L. Wang, Chem.–Eur. J., 2009, 15, 2045–2049 CrossRef CAS.
- (a) J. Lim, K. Park, A. Byeun and S. Lee, Tetrahedron Lett., 2014, 55, 4875–4878 CrossRef CAS; (b) C. Zhao and D. Seidel, J. Am. Chem. Soc., 2015, 137, 4650–4653 CrossRef CAS.
- L. C. Akullian, M. L. Snapper and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4244–4247 CrossRef CAS.
- S. Samai, G. C. Nandi and M. Singh, Tetrahedron Lett., 2010, 51, 5555–5558 CrossRef CAS.
- Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364–4367 CrossRef CAS.
- S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commun., 2004, 1638–1639 RSC.
- X. Chen, T. Chen, Y. Zhou, C.-T. Au, L.-B. Han and S.-F. Yin, Org. Biomol. Chem., 2014, 12, 247–250 RSC.
- C.-J. Li and C. Wei, Chem. Commun., 2002, 268–269 RSC.
- J. Yadav, B. S. Reddy, A. H. Gopal and K. Patil, Tetrahedron Lett., 2009, 50, 3493–3496 CrossRef CAS.
- W.-W. Chen, H.-P. Bi and C.-J. Li, Synlett, 2010, 2010, 475–479 CrossRef.
- V. K.-Y. Lo, Y. Liu, M.-K. Wong and C.-M. Che, Org. Lett., 2006, 8, 1529–1532 CrossRef CAS.
- M. L. Kantam, B. V. Prakash, C. R. V. Reddy and B. Sreedhar, Synlett, 2005, 2005, 2329–2332 CrossRef.
- P. Li, L. Wang, Y. Zhang and M. Wang, Tetrahedron Lett., 2008, 49, 6650–6654 CrossRef CAS.
- S. Kaur, M. Kumar and V. Bhalla, Chem. Commun., 2015, 51, 16327–16330 RSC.
- S. Wang, X. He, L. Song and Z. Wang, Synlett, 2009, 2009, 447–450 CrossRef.
- H. Naeimi and M. Moradian, Appl. Organomet. Chem., 2013, 27, 300–306 CrossRef CAS.
- D. Bhuyan, M. Saikia and L. Saikia, Catal. Commun., 2015, 58, 158–163 CrossRef CAS.
- K. Satyanarayana, P. A. Ramaiah, Y. Murty, M. R. Chandra and S. Pammi, Catal. Commun., 2012, 25, 50–53 CrossRef CAS.
- M. Rahman, A. K. Bagdi, A. Majee and A. Hajra, Tetrahedron Lett., 2011, 52, 4437–4439 CrossRef CAS.
- M. L. Kantam, J. Yadav, S. Laha and S. Jha, Synlett, 2009, 2009, 1791–1794 CrossRef.
- K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 2010, 411–415 CrossRef.
- K. D. Bhatte, D. N. Sawant, K. M. Deshmukh and B. M. Bhanage, Catal. Commun., 2011, 16, 114–119 CrossRef CAS.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 2012, 3093–3104 CrossRef CAS.
- S. K. Movahed, N. F. Lehi and M. Dabiri, RSC Adv., 2014, 4, 42155–42158 RSC.
- M. Gholinejad, F. Hamed and C. Najera, Synlett, 2016, 27, 1193–1201 CrossRef CAS.
- Z. Zarei and B. Akhlaghinia, RSC Adv., 2016, 6, 106473–106484 RSC.
- S. Layek, B. Agrahari, S. Kumari and D. D. Pathak, Catal. Lett., 2018, 148, 2675–2682 CrossRef CAS.
- N. Gharibpour, M. Abdollahi Alibeik and A. Moaddeli, ChemistrySelect, 2017, 2, 3137–3146 CrossRef CAS.
- A. Moshfegh, J. Phys. D: Appl. Phys., 2009, 42, 233001 CrossRef.
- W. Buraso, V. Lachom, P. Siriya and P. Laokul, Mater. Res. Express, 2018, 5, 115003 CrossRef.
- D. A. Kotadia and S. S. Soni, Appl. Catal., A, 2014, 488, 231–238 CrossRef CAS.
- Y. M. Mohamed, H. A. El Nazer and E. J. Solum, Chem. Pap., 2019, 73, 435–445 CrossRef CAS.
- S. Mishra, A. K. Bagdi, M. Ghosh, S. Sinha and A. Hajra, RSC Adv., 2014, 4, 6672–6676 RSC.
- U. Sakee and R. Wanchanthuek, Mater. Res. Express, 2017, 4, 115504 CrossRef.
- M. Ahamed, M. M. Khan, M. J. Akhtar, H. A. Alhadlaq and A. Alshamsan, Sci. Rep., 2016, 6, 30196 CrossRef CAS.
- S. Honglong, Z. Guling, Z. Bin, L. Minting and W. Wenzhong, Microsc. Res. Tech., 2013, 76, 641–647 CrossRef.
- S. K. M. Saad, A. A. Umar, H. Q. Nguyen, C. F. Dee, M. M. Salleh and M. Oyama, RSC Adv., 2014, 4, 57054–57063 RSC.
- J. Liqiang, W. Dejun, W. Baiqi, L. Shudan, X. Baifu, F. Honggang and S. Jiazhong, J. Mol. Catal. A: Chem., 2006, 244, 193–200 CrossRef.
- A. Abidli, S. Hamoudi and K. Belkacemi, Dalton Trans., 2015, 44, 9823–9838 RSC.
- L. Dake, D. Baer and J. Zachara, Surf. Interface Anal., 1989, 14, 71–75 CrossRef CAS.
- Y. Yu, J. Wang, W. Li, W. Zheng and Y. Cao, CrystEngComm, 2015, 17, 5074–5080 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06693d |
| This journal is © The Royal Society of Chemistry 2019 |