Open Access Article
Open Access ArticlePreparation and evaluation of a hierarchical Bi2MoO6/MSB composite for visible-light-driven photocatalytic performance
Lu Cai a,
Yarui Zhoub,
Zhen Wangc,
Jinlong Chend,
Lili Jie,
Jian Guof,
Yaning Wange,
Wendong Songd and
Jianshe Liu
a,
Yarui Zhoub,
Zhen Wangc,
Jinlong Chend,
Lili Jie,
Jian Guof,
Yaning Wange,
Wendong Songd and
Jianshe Liu *a
*a
aCollege of Environmental and Science Technology, Donghua University, Shanghai 201620, China. E-mail: liujianshe@dhu.edu.cn
bCollege of Naval Architecture and Mechanical-Electrical Engineering, Zhejiang Ocean University, Zhoushan 316022, China
cCollege of Marine Science and Technology, Zhejiang Ocean University, Zhoushan 316022, China
dCollege of Petrochemical and Energy Engineering, Zhejiang Ocean University, Zhoushan 316022, China
eInstitute of Innovation & Application, Zhejiang Ocean University, Zhoushan 316022, China
fCollege of Food and Medical, Zhejiang Ocean University, Zhoushan 316022, China
First published on 22nd November 2019
Abstract
In this study, waste mussel shells were used to remove dyes in aqueous solution. Mussel shell was prepared into mussel shell biochar (MSB), which was used as a carrier to support Bi2MoO6. A novel Bi2MoO6/MSB composite photocatalyst was developed by the hydrothermal synthesis method. The as-synthesized sample was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), N2 adsorption–desorption method, Fourier transform infrared spectroscopy (FTIR) and UV-vis diffuse reflectance spectra (DRS). Then, the photocatalytic activity of the prepared samples was determined by testing the photodegradation of Rhodamine B (RhB) under visible-light (λ > 420 nm) irradiation. The pre exfoliated layered MSB was an excellent supporting matrix for the growth of Bi2MoO6 nanoflakes. The obtained hierarchical Bi2MoO6/MSB composites exhibited significantly enhanced performance for photocatalytic degradation of RhB compared with pure Bi2MoO6 under visible light irradiation because of the improved electron–hole pair separation, which boosted the number of exposed catalytic active sites. Moreover, the Bi2MoO6/MSB composite photocatalyst is of good stability and reusability.
1. Introduction
With the high speed of industrialization, dyes have become a major source of water pollution as they are widely used in such fields as the textile, tannery, printing, paper, plastic and cosmetic industries.1,2 Due to the improvement of people's requirements on color, various dyes with the abilities of oxidation resistance, photolysis resistance or biodegradation resistance have sprung up, which are difficult to be treated with traditional water treatment systems. Some dyes are carcinogenic, mutagenic, and teratogenic, and even their products may be carcinogenic or otherwise toxic after being transformed or degraded, such as aromatic amines.3–5 Moreover, dye effluents discharged into water even at low concentrations could threaten the aquatic animals and plants, and destroy the ecological environment. Thus, the efficient treatment of dyestuff wastewater has always been a hot topic. Various methods had been applied to degrade dyes including chemical oxidation, microbiological degradation, electrochemical treatment, adsorption, photocatalytic degradation, etc6–9. In recent years, there has been considerable interest in the utilization of photocatalysis systems for the destruction of dyes, so more and more efficient photocatalysts have been developed. Remarkably, visible-light-driven photocatalysis has received much attention as an economical and practical method. Bismuth-based photocatalysts such as Bi2MoO6, BiVO4, BiOX (X = Br, Cl), Bi2WO6, Bi2Fe4O9 and Bi2O2CO3,10–14 possessing excellent photocatalytic activity under visible-light irradiation, have become representative photocatalytic materials.15–18 Besides, Bi2MoO6 is considered as an ideal photocatalyst from the viewpoint of using visible light.19–21 However, the practical application of individual phase Bi2MoO6 is still limited by its dissatisfactory quantum yield.22 In order to further improve the photocatalytic performance of Bi2MoO6, previous studies have attempted to immobilize the photocatalyst onto various supports such as activated carbon, graphene, carbon nanotubes, zeolite, glass, and silica; however, they exhibited high cost and lack of treatability.23–29Mussel shell, as a by-product of the aquaculture industry, is an abundant residual source of carbonate. As we all know, shell is a kind of well-arranged organic–inorganic composite material formed under the control of organic macromolecules. Shell has a unique multi-scale and multi-level “brick-mud” assembly structure and is a kind of cheap biological material. After modification by simple heat treatment, the organic matter in the mussel shell can be decomposed and released, resulting in the formation of porous biochar skeleton, which is expected to be a low cost and effective carrier material. The biochar had excellent conductivity, which would lower quick recombination of electrons and electron holes during the photocatalysis for enhancing photocatalytic degradation. Efficient attachment of photocatalyst onto the biochar is allowed due to the large specific surface area, rich functional groups and easy surface modification of the biochar.30
Therefore, the present study suggested a biochar-support Bi2MoO6 as an effective photocatalyst to eliminate dyes in water. The effect of biochar-support Bi2MoO6 on photocatalytic activity was investigated by decomposing Rhodamine B (RhB) under visible-light irradiation. The possible photocatalytic mechanism of this composite was also discussed. The as-prepared sample may be expected to be a cheap and efficient photocatalyst, which could realize the value-added use of waste mussel shells.
2. Materials and methods
2.1 Materials
The mussel shells were collected at Shengsi, Zhejiang province, China. Bi(NO3)3·5H2O, Na2MoO6·2H2O, C2H5OH and C2H6O2 were purchased from Sinopharm Chemical Reagent Co., Ltd., in Shanghai, China. RhB(C28H31ClN2O3) was purchased at Beijing Chemical Reagent Co. Ltd., in Beijing, China. All chemicals were analytical grade and used as received without further purification.2.2 Preparation of composite photocatalyst
The mussel shells were washed to remove dirt from its surface, treated with 0.1% HCl for 24 h, then washed with distilled water until neutral and dried at 80 °C. The dried mussel shells loaded in corundum crucible were placed in a tube furnace (Shanghai Jvjing Precision Instrument Manufacturing Co., Ltd., Shanghai, China). It was heated at a rate of 10°C min−1 from room temperature to 900 °C under purified nitrogen (99.99%) flow of 100 mL min−1. Next, the calcination step was held for 3 h at 900 °C. The calcined sample was then cooled to room temperature under nitrogen flow and grounded into powder, which was labelled as MSB.The Bi2MoO6/MSB composite photocatalyst were synthesized through the hydrothermal method. Briefly, Bi (NO3)3·5H2O (0.3630 g) and Na2MoO4·2H2O (0.0907 g) were completely dissolved in two different 7 mL glycol, respectively. 0.1306 g MSB (the mass ratio of Bi2MoO6 to MSB was 1.75) were dissolved in 20 mL ethyl alcohol. After stirring for 30 min, the three solutions were transferred into a 50 mL Teflon-lined stainless autoclave, heated at 160 °C for 12 h in an oven, and then cooled down to room temperature. The resulting mixture was washed with deionized water and ethyl ethanol to remove any ionic residual, and dried in an oven at 60 °C for 6 h in an oven, named as Bi2MoO6/MSB. By adjusting the mass ratio of Bi2MoO6 to BMS, a series of Bi2MoO6/MSB with different mass ratio (0.25, 0.75, 1.25, 1.75 and 2.25) were obtained and named as B/M-0.25, B/M-0.75, B/M-1.25, B/M-1.75, and B/M-2.25, respectively.
2.3 Characterization
FTIR spectra were obtained using a PerkinElmer Fourier transform infrared (Nicoletteis 50, Thermo Fourier, Waltham, MA, USA) spectrometer with KBr as a diluting agent and operated in the frequency range of 4000–500 cm−1. The crystal structures of the as-prepared sample were investigated by XRD analysis at room temperature on an XRD powder diffraction instrument (D/max2500, Shimadzu Co., Ltd., Kyoto, Japan) in the range of 2θ from 20° to 80°. Specific surface area measurements were performed on a micromeritics ASAP 2010 instrument (Micromeritics Instrument Ltd., Atlanta, GA, USA) and analysed by the BET method. The morphology change of the sample was studied by a scanning electron microscopy (SEM, Hitachi-4800, Hitachi Co., Ltd., Tokyo, Japan). The optical properties of the samples were determined by the Shimadzu UV 2600 spectrophotometer (Shimadzu Co., Ltd., Kyoto, Japan) in the wavelength range of 200–800 nm.2.4 Photocatalytic activity
The photocatalytic performance of the sample was evaluated for the degradation of RhB under visible-light irradiation. A 300 W Xe lamp combined with a 400 nm cutoff filter was used as a light source and its distance to the liquid surface of the suspension was about 10 cm. The temperature of the reaction system was kept at 25 °C by cycling water. In the experiment, 0.02 g photocatalyst was added to 50 mL of 6 mg L−1 RhB solution. Prior to irradiation, the suspensions were stirred in the dark for 3 h to establish an adsorption–desorption equilibrium. During irradiation, 2 mL liquid was taken from the suspension at 10, 20, 30, 60, 90 and 120 min followed by the separation of photocatalyst through centrifugation. The absorbance of RhB solution without catalyst was determined at 554 nm using a spectrophotometer (UV 2600, Shimadzu Co., Ltd., Kyoto, Japan). The degradation rate (D%) was calculated by the eqn (1):
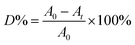 | (1) |
The stability experiment was carried out by the degradation of RhB solution (50 mL, 6 mg L−1) over Bi2MoO6/MSB for four successive cycles. After each cycle, the catalyst was separated and collected by centrifugation, then was washed with deionized water and dried. After that, it was added into the fresh RhB solution (50 mL, 6 mg L−1) to initiate the reaction.
3. Results and discussion
3.1 Materials characterization
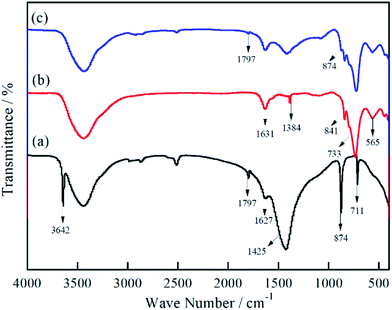
Fig. 1 illustrates the FTIR spectra of MSB, Bi2MoO6, and Bi2MoO6/MSB. As can be seen from Fig. 1(a), the MSB spectra has a strong and wide peak at 1425 cm−1, a narrow peak at 874 cm−1 and a weak and narrow peak at 711 cm−1, corresponding to the asymmetric stretching vibration peak, in-plane bending vibration peak and out-of-plane bending vibration peak of CO32− group, respectively.31 The absorption peak at 1797 cm−1 is the vibration peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the CO32− group.32 The above peaks are characteristic peaks of carbonate minerals, which coincide with the characteristic peaks of CaCO3. In addition, the absorption peak at 3642 cm−1 is the stretching vibration peak of –OH, which indicates the existence of Ca(OH)2 in MSB.33
O in the CO32− group.32 The above peaks are characteristic peaks of carbonate minerals, which coincide with the characteristic peaks of CaCO3. In addition, the absorption peak at 3642 cm−1 is the stretching vibration peak of –OH, which indicates the existence of Ca(OH)2 in MSB.33
 | ||
| Fig. 1 FTIR spectra of MSB, pure Bi2MoO6 and Bi2MoO6/MSB composite catalyst ((a) MSB; (b) Bi2MoO6; (c) Bi2MoO6/MSB). | ||
Fig. 1(b) shows the infrared spectrum of pure Bi2MoO6, the band at 1384 cm−1 is the vibration of the Bi–O bond, and the absorption bands at 841 cm−1, 733 cm−1 and 565 cm−1 correspond to the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, MoO6 asymmetric stretching and MoO6 flexural vibration.34,35 As shown in Fig. 1(c), all characteristic absorption peaks of Bi2MoO6 can be observed in the Bi2MoO6/MSB curve, and CaCO3 characteristic absorption peaks are found at 1797 and 874 cm−1, indicating the existence of Bi2MoO6 and MSB.
O stretching vibration, MoO6 asymmetric stretching and MoO6 flexural vibration.34,35 As shown in Fig. 1(c), all characteristic absorption peaks of Bi2MoO6 can be observed in the Bi2MoO6/MSB curve, and CaCO3 characteristic absorption peaks are found at 1797 and 874 cm−1, indicating the existence of Bi2MoO6 and MSB.
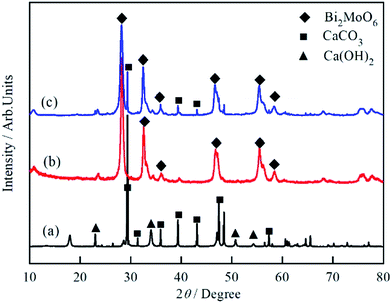
The XRD patterns of MSB, Bi2MoO6 and Bi2MoO6/MSB composites are shown in Fig. 2. The profile in Fig. 2(a) confirms that pure MSB consists of the calcite type of CaCO3 (JCPDS 83-1762) and hexagonal phase of Ca(OH)2 (JCPDS 04-0733). The existence of Ca(OH)2 is mainly because high temperature decomposes CaCO3 into CaO, which reacts with H2O in the air to form Ca(OH)2. Fig. 2(b) confirms that the as-prepared sample is phase-pure orthorhombic Bi2MoO6, which is consistent with the reported result (JCPDS76-2388). As shown in Fig. 2(c), the main diffraction peaks of Bi2MoO6/MSB at about 23.3°, 28.1°, 32.3°, 35.8°, 46.7°, 55.3° and 58.2° could be perfectly indexed to the (111), (131), (200), (151), (062), (331) and (191) crystal planes of Bi2MoO6, which consistent with pure Bi2MoO6. At the same time, the diffraction peaks of MSB located at 2θ = 29.4°, 39.5°, and 43.1° could be obviously observed, corresponding to the (104), (202) and (024) crystal planes of calcite type CaCO3 (JCPDS 83-1762). The results showed that the characteristic peaks of MSB and Bi2MoO6 were observed, which indicates that Bi2MoO6 was successfully combined with MSB.
 | ||
| Fig. 2 XRD patterns of MSB, pure Bi2MoO6 and Bi2MoO6/MSB composite catalyst ((a) MSB; (b) Bi2MoO6; (c) Bi2MoO6/MSB). | ||
The morphology of the sample was characterized by SEM. As shown in Fig. 3, MSB is a nano-flake structure with different size holes, which is a porous calcium compound skeleton formed by organic matter escaping from shells after high-temperature treatment. Pure Bi2MoO6 is a hollow spherical structure composing of nanosheets, with a diameter of about 1.5–2 μm. Compared with pure Bi2MoO6, the nanomorphology of Bi2MoO6 in the composite catalyst is changed. In the composite photocatalyst, the hollow spherical structure of Bi2MoO6 is missing, and the Bi2MoO6 nanosheets attached to MSB surface form an obvious hierarchical structure. Moreover, after hydrothermal treatment, the particles of the MSB in the composite photocatalyst become smaller and the hierarchical structure becomes more obvious.
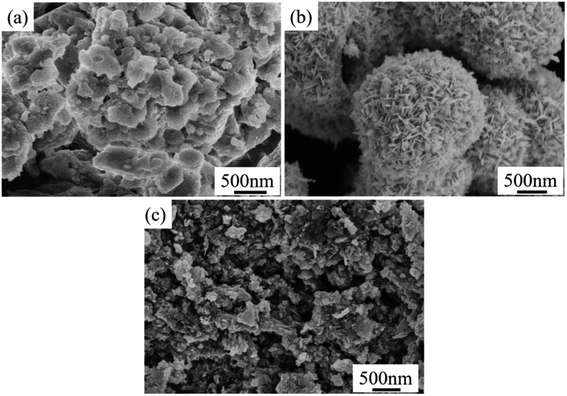 | ||
| Fig. 3 SEM of Bi2MoO6/MSB composite catalyst, pure Bi2MoO6 and MSB ((a) MSB; (b) Bi2MoO6; (c) Bi2MoO6/MSB). | ||
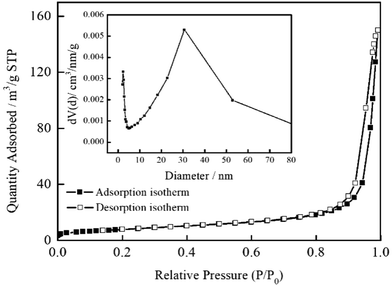
The specific surface areas, pore volumes and average pore diameters of MSB, Bi2MoO6, and Bi2MoO6–MSB are shown in Table 1. Compared with pure Bi2MoO6, the surface area, pore volume and average pore diameter of Bi2MoO6/MSB composite decrease, because the addition of MSB changes the self-assembly structure of Bi2MoO6 nanosheets, which is deposited on the surface and pores of MSB. Moreover, the specific surface area of the Bi2MoO6/MSB composite gradually decreased with the increase of MSB addition. According to Fig. 4, the nitrogen adsorption isotherm of Bi2MoO6/MSB(B/M-1.75) accords with type IV adsorption isotherm with a hysteresis loop at pressure (P/P0 > 0.8), indicating that there may be a certain number of mesoporous in composite photocatalyst.36 The pore size distribution of Bi2MoO6/MSB also reflects the above results, mainly in the range of 2–20 nm.
| Samples | BET (m2 g−1) | Total pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| MSB | 2.47 | 0.004 | 6.53 |
| Bi2MoO6 | 43.15 | 0.183 | 16.98 |
| Bi2MoO6/MSB(B/M-2.25) | 32.36 | 0.110 | 8.18 |
| Bi2MoO6/MSB(B/M-1.75) | 28.64 | 0.069 | 9.63 |
| Bi2MoO6/MSB(B/M-1.25) | 27.63 | 0.076 | 8.79 |
| Bi2MoO6/MSB(B/M-0.75) | 20.59 | 0.042 | 8.15 |
| Bi2MoO6/MSB(B/M-0.25) | 18.48 | 0.036 | 8.97 |
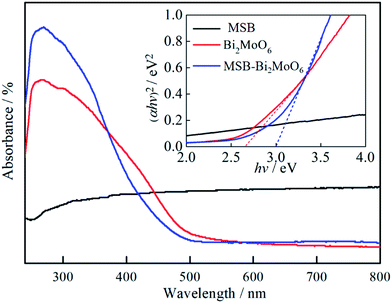
The difference in optical absorption could highly affect the photocatalytic performance of the sample. Fig. 5 exhibits the UV-vis diffuse reflectance spectra of MSB, Bi2MoO6 and Bi2MoO6/MSB are various due to their different photoabsorption properties from UV light region to visible light region. Pure Bi2MoO6 and Bi2MoO6/MSB all show significantly absorbance in the visible-light region (λ > 400 nm). The obtained diffuse reflectance spectra are further used to determine their optical band gap energies with the formula (αhν)2 = A(hν − Eg), where α, ν, A and Eg are the absorption coefficient, the incident light frequency, a constant and the band gap energy. The band gap energies of Bi2MoO6 and Bi2MoO6/MSB could be evaluated from a plot of (αhν)2 vs. hν as presented in Fig. 5 (inset). The estimated band gap energies for the Bi2MoO6 and Bi2MoO6/MSB were about 2.65 and 3.00 eV, respectively.
3.2 Photocatalytic activity
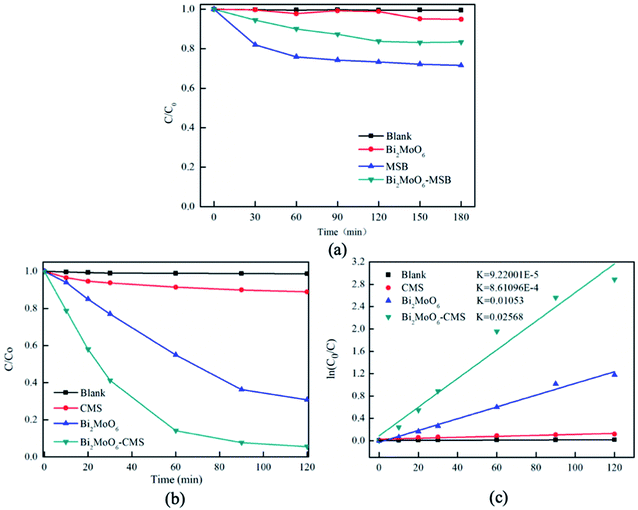
The photocatalytic activity of the as-prepared catalysts was evaluated for the photocatalytic degradation of RhB under visible light irradiation. The dark adsorption data was shown in Fig. 6(a), the adsorption equilibrium between the photocatalyst and the dye solution has established. As is illustrated in Fig. 6(b), when the RhB suspension was irradiated with visible light for 120 min in the absence of any photocatalyst, no obvious change can be observed in the RhB solution; therefore, the photodegradation of RhB can be neglected in this study. Under the same conditions, 11.0% RhB is degraded by MSB after 120 min visible light illumination, which indicates that MSB has the photocatalytic activity against RhB.The degradation efficiency of pure Bi2MoO6 was 69.2%. Furthermore, it is worth noting that the combination of a certain amount of MSB with Bi2MoO6 (mass ratio = 1.75) result in a sharp increase of RhB degradation from 69.2% to 94.4%. The improvement of photocatalytic activity of Bi2MoO6/MSB can be attributed to the following reasons. Bi2MoO6 was deposited on the surface or in the pore of MSB, which could cover or block the pores of MSB, leading to a decrease in the specific surface area of composite materials. However, Bi2MoO6 is dispersed on MSB carrier, so as to expose more photocatalytic activity sites, which leads to the improvement of photocatalytic activity of composite materials. Besides, as is known, mussel shell has abundant transition metal elements, so MSB internally contains transition metal oxides,37 acting as photogenerated holes and electrons traps, which hinder the recombination of hole–electron pairs, and significantly improve the photocatalytic activity of Bi2MoO6/MSB. The forbidden bandwidth of Bi2MoO6/MSB is larger than that of the pure Bi2MoO6, indicating it needs more energy to activate, however, it has higher efficiency of light utilization, shows significantly absorbance in the visible-light region, and exhibits an enhanced photocatalytic activity.
The degradation process follows pseudo-first-order kinetics, as clearly shown in Fig. 6(c). The degradation rate of RhB over Bi2MoO6/MSB is much faster than other samples, and the apparent rate constant k of the composite is 0.02568 min−1, which is 2.44 times of that over pure Bi2MoO6. Moreover, compared with some previous reports (Table 2), the K value of present Bi2MoO6/MSB is somehow higher than other semiconductor composites in previous reports, such as Bi2MoO6 with K = 0.022 min−1, Bi2MoO6/graphene with K = 0.0136 min−1, Bi2MoO6/CNTs/g-C3N4 with K = 0.0078 min−1.38–40 Although the K value of SnO2/Bi2MoO6 is slightly higher than Bi2MoO6/MSB, the catalyst cost in this study is lower.41 All the results showed that Bi2MoO6/MSB composite photocatalyst was a kind of high efficient photocatalyst with high degradation activity against RhB.
| Photocatalyst | Light source | Catalyst conc. (mg mL−1) | Dye | Dye conc. | Irradiation time (min) | K (min−1) | Reference |
|---|---|---|---|---|---|---|---|
| Bi2MoO6/MSB | 300 W Xe lamp | 0.4 | RhB | 6 mg L−1 | 120 | 0.02568 | This work |
| Bi2MoO6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K Xe lamp 000 K Xe lamp |
1 | RhB | 5 mg L−1 | 300 | 0.022 | 37 |
| Bi2MoO6/graphene | 500 W Xe lamp | 0.5 | MB | 1 × 10−5 M | 120 | 0.0136 | 38 |
| Bi2MoO6/CNTs/g-C3N4 | 500 W Xe lamp | 1 | 2,4-DBP | 20 mg L−1 | 120 | 0.0078 | 39 |
| SnO2/Bi2MoO6 | 400 W halogen lamp | 1 | RhB | 10 mg L−1 | 150 | 0.02831 | 40 |
To further study the photocatalytic process of RhB, the UV-vis spectra of the RhB solution as a function of visible light irradiation time in the presence of Bi2MoO6/MSB is shown in Fig. 7. The main peak wavelength of the degrade solution gradually shifts from 554 nm to 497 nm as the irradiation time increases. Studies has shown that the blue-shift of the absorption band is caused by de-ethylation of RhB because of the attack by one of the active oxygen species on the N-ethyl group. When the de-ethylated process is fully completed, the absorption band shifts to 497 nm and RhB is turned to rhodamine. Rhodamine is then gradually decomposed due to the further destruction of the conjugated structure.42–45
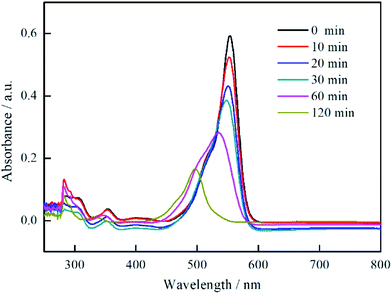 | ||
| Fig. 7 UV absorption spectrum curves of RhB Solution with Bi2MoO6/MSB as photocatalyst under visible light irradiation at different time. | ||
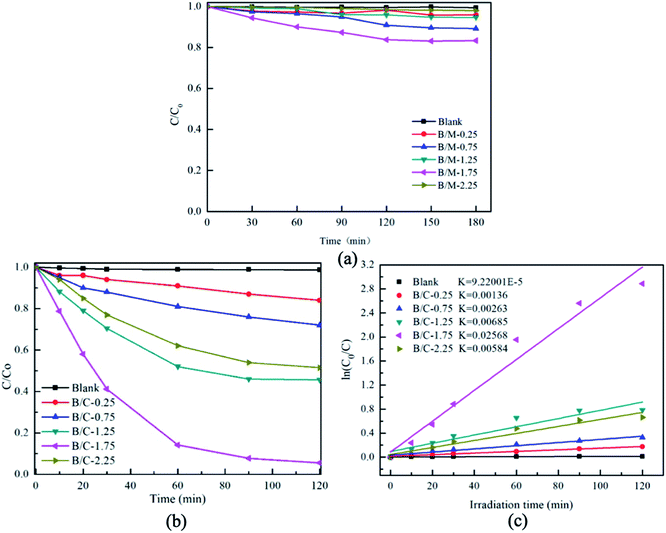
Fig. 8 shows the results of photocatalytic efficiency of degradation of RhB solution by Bi2MoO6/MSB with different mass ratio. Fig. 8(a) exhibit the dark adsorption data of different photocatalyst, the adsorption equilibrium between the photocatalyst and the dye solution was reached. As shown in Fig. 8(b), it is obvious that the degradation efficiency increases gradually as the content of Bi2MoO6 increase in the composite photocatalysts from B/M-0.25 to B/M-1.75 and the degradation efficiency of B/M-1.75 reaches the highest (94.4%). However, the degradation efficiency of B/M-2.25 decreases to 48.5% in comparison with the B/M-1.75. This is because too much Bi2MoO6 is prone to agglomerate, resulting in the effective adsorption sites and photolytic active sites of photocatalysts are reduced.46 Fig. 8(c) is the degradation kinetics of RhB degradation by Bi2MoO6/MSB in different mass ratios. As can be seen from Fig. 8(c), ln(C0/C) and t showed a good linearity relationship. Compared with other samples, B/M-1.75 exhibits the excellent visible light catalytic activity with the k value of 0.02568 min−1.
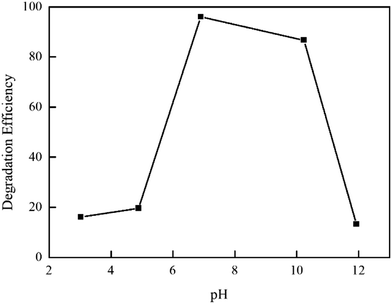
As is well known, the solution pH value plays an important role in the photocatalytic degradation of organic pollutants. The effect of pH on the photodegradation of RhB was studied in the pH range of 3–12. As shown in Fig. 9, with the increase of pH, the degradation rate of RhB increase firstly and then decrease. It can be observed that the optimum pH is 7 in the highest degradation rate (96%). Under the acidic conditions, the H+ in solution will react with carbonyl, hydroxyl and other oxygen-containing functional groups on MSB surface, resulting in reducing the number of active contact sites and weakening adsorption and catalytic capacity. When the pH value is more than 7, the OH− in solution will compete with MSB surfactant functional group for adsorption, causing increasing repulsive force between the composite catalyst and organic dye molecules; therefore, it is not conductive to the adsorption of the catalyst on RhB, leading to a decrease of degradation ability.
Furthermore, the stability and reusability of the composite photocatalyst were also tested. As shown in Fig. 10, after four successive cycles of RhB degradation experiments, the photocatalytic degradation efficiency exhibits only a slight decrease from initial 94.4% for the first run to 82.7% for the fourth run. The XRD pattern of the used Bi2MoO6/MSB composite further confirm that there no obvious changes in the crystalline phase after recycling reactions, implying Bi2MoO6/MSB composite has better stability and reusability. Thus, Bi2MoO6/MSB composite is a good candidate for photocatalytic degradation of persistent organic pollutants in solution under visible light irradiation.
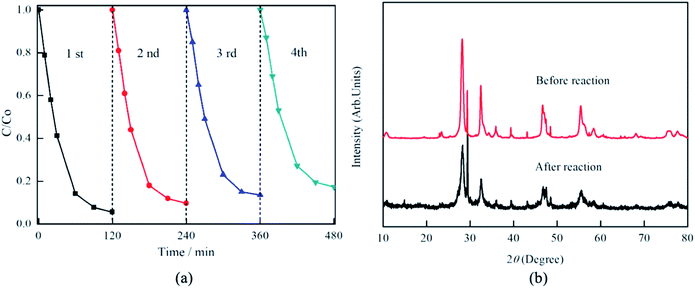 | ||
| Fig. 10 (a) Photocatalytic degradation of RhB by Bi2MoO6/MSB composite photocatalysts in different cycling runs, (b) XRD patterns of Bi2MoO6/MSB before and after cycling photocatalytic test. | ||
3.3 Photocatalytic mechanism
As is well known, photoinduced holes (h+), superoxide radicals (·O2−) and hydroxyl radicals (·OH) are extremely active in photocatalytic reaction, which results in the degradation of organic pollutants.47–50 In order to clarify the photocatalytic mechanism, it is vital to identify the main oxidants in the photodegradation process. In this study, sodium oxalate, p-benzoquinone, and isopropyl alcohol are used as h+,·O2− and ·OH scavenger, respectively and their effects on the photocatalytic degradation of RhB are shown in Fig. 11. As is illustrated in Fig. 11, the degradation efficiency of RhB decreases from 94.4% to 31.2%, 22.6% and 55.7% by adding photoinduced holes, superoxide radicals, and hydroxyl radicals scavenger, respectively. Therefore, it can be indicated that h+ and ·O2− are major active species for the degradation of RhB, and ·OH plays a secondary role.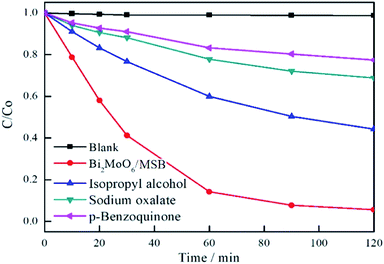 | ||
| Fig. 11 The effects of photogenerated carriers trapping on photocatalytic degradation activity of Bi2MoO6/MSB. | ||
Based on the above photocatalytic experiment results and the characterization of Bi2MoO6/MSB composite, the photocatalytic degradation of RhB by Bi2MoO6/MSB composite photocatalyst under visible light irradiation mainly includes the following process:
| Bi2MoO6 + hν → Bi2MoO6 (e− + h+) |
| Bi2MoO6 (e−) + MSB → Bi2MoO6 + MSB (e−) |
| MSB (e−) + O2 → MSB+ ·O2− |
| ·O2− + H2O → HO2·+ OH− |
| HO2·+ H2O → H2O2 + ·OH− |
| H2O2 → 2·OH− |
| ·OH− + RhB → degraded or mineralized products |
| Bi2MoO6 (h+) + RhB → degraded or mineralized products |
The results showed that the introduction of MSB did not widen the optical response range of Bi2MoO6, and the specific surface area did not increase. Therefore, the improvement of the photocatalytic activity of Bi2MoO6/MSB can be attributed to the following aspects: (1) MSB has photocatalytic degradation activity on RhB solution. The electrons of Bi2MoO6 are active under visible light irradiation, which makes the electrons jump between valence bands. The generation of the heterojunction structure of MSB and Bi2MoO6 can improve the photo-generated carrier transfer speed and reduce the composite rate of electron–hole pairs, so as to improve the photocatalytic activity of the composite catalyst. (2) Bi2MoO6 nanosheet dispersed on the surface or in the pore of MSB, exposing more photocatalytic active sites, which enhance the photocatalytic activity of composite materials. (3) MSB has special pores and surface structure, which can promote the effective adsorption of RhB by composite photocatalyst and further improve the photocatalytic performance of composite photocatalyst. (4) MSB has a variety of trace metal elements, which can be used as active sites in photocatalytic reactions to promote the catalytic degradation of Bi2MoO6/MSB composite photocatalyst.
4. Conclusions
In summary, the Bi2MoO6/MSB composite have been successfully prepared by the hydrothermal synthesis method. The results show that Bi2MoO6/MSB composite consists of ortho-phase Bi2MoO6 and calcite CaCO3, and no other phase appears. With the addition of MSB, the space structure of Bi2MoO6 is transformed from a hollow spherical structure into a lamellar structure assembled by nano-particle, and the specific surface area is reduced. The Bi2MoO6/MSB composite photocatalyst absorb in both ultraviolet and visible light area. The Bi2MoO6/MSB composite photocatalyst exhibited remarkable photocatalytic activity in degrading RhB than the pure Bi2MoO6. At 120 min of visible light irradiation, the degradation rate was 94.4%, which was 1.36 times as high as pure Bi2MoO6. Moreover, the Bi2MoO6/MSB composite photocatalyst has good stability and reusability. The improvement of photocatalytic performance of Bi2MoO6/MSB composite photocatalyst is related to the heterojunction generation, MSB structure, and trace metal elements in MSB. The addition of MSB can not only improve the photocatalytic activity of composite photocatalyst but also effectively reduce the cost. Therefore, Bi2MoO6/MSB composite photocatalyst is expected to be used in many fields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the State Scholarship Fund of China, Key Research and Development Projects of Zhejiang Province of China (No. 2018C02043), Demonstration Project of Marine Economic Innovation and Development of Zhoushan City of China, and Demonstration Project of Marine Economic Innovation and Development of Yantai City of China (No. YHCX-SW-L-201705).References
- R. Sandhiya, K. Begum and D. Charumathi, Int. J. Pharm. Pharm. Sci., 2016, 8, 258–266 CrossRef CAS.
- N. Barka, A. Assabbane, A. Nounah, L. Laanab and Y. A. Ichou, Desalination, 2009, 235, 264–275 CrossRef CAS.
- A. Afkhami and R. Moosavi, J. Hazard. Mater., 2010, 174, 398–403 CrossRef CAS.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Bioresour. Technol., 2001, 77, 247–255 CrossRef CAS.
- C. Namasivayam and D. Sangeetha, J. Hazard. Mater., 2006, 135, 449–452 CrossRef CAS PubMed.
- N. A. Abdelwahab and M. Emh, Int. J. Biol. Macromol., 2018, 108, 1035–1044 CrossRef CAS PubMed.
- Y. Zhou, H. Zhang, L. Cai, J. Guo, Y. Wang, L. Ji and W. Song, Materials, 2018, 11, 1709 CrossRef.
- K. Dutta, S. Mukhopadhyay, S. Bhattacharjee and B. Chaudhuri, J. Hazard. Mater., 2001, 84, 57–71 CrossRef CAS.
- V. K Gupta, D. Pathania, S. Agarwal and P. Singh, J. Hazard. Mater., 2012, 243, 179–186 CrossRef.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163–2169 CrossRef CAS.
- X. Zhang, Z. Ai, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CrossRef CAS.
- Q. J. Ruan and W. D. Zhang, J. Phys. Chem. C, 2009, 113, 4168–4173 CrossRef CAS.
- Y. Liu, Z. Wang, B. Huang, K. Yang, X. Zhang, X. Qin and Y. Dai, Appl. Surf. Sci., 2010, 257, 172–175 CrossRef CAS.
- L. Guo, K. L. Zhang, H. Shen, C. Wang, Q. Zhao, D. Wang and Y. Liang, Chem. Eng. J., 2019, 370, 1522–1533 CrossRef CAS.
- M. Zhang, C. Shao, P. Zhang, C. Su, X. Zhang, P. Liang and Y. Liu, J. Hazard. Mater., 2012, 225, 155–163 CrossRef.
- Y. Chen, G. Tian, Y. Shi, Y. Xiao and H. Fu, Appl. Catal., B, 2015, 164, 40–47 CrossRef CAS.
- W. Wei, Y. Dai and B. Huang, J. Phys. Chem. C, 2009, 113, 5658–5663 CrossRef CAS.
- G. Tian, Y. Chen, R. Zhai, J. Zhou, W. Zhou, R. Wang and H. Fu, J. Mater. Chem. A, 2013, 1, 6961–6968 RSC.
- G. Tian, Y. Chen, W. Zhou, K. Pan, Y. Dong, C. Tian and H. Fu, J. Mater. Chem., 2011, 21, 887–892 RSC.
- C. Kongmark, R. Coulter, S. Cristol, A. Rubbens, C. Pirovano, A. Löfberg and R. N. Vannier, Cryst. Growth Des., 2012, 12, 5994–6003 CrossRef CAS.
- L. Guo, Q. Zhao, H. Shen, X. Han, K. Zhang, D. Wang and B. Xu, Catal. Sci. Technol., 2019, 9, 3193–3202 RSC.
- Z. Lin, W. Wang and L. Zhang, J. Mol. Catal. A: Chem., 2007, 268, 195–200 CrossRef.
- M. Cao, P. Wang, Y. Ao, C. Wang and J. Hou, Chem. Eng. J., 2015, 264, 113–124 CrossRef CAS.
- Y. J. Jang, Y. H. Jang and D. H. Kim, Sci. Adv. Mater., 2015, 7, 956–963 CrossRef CAS.
- S. Ragupathy, K. Raghu and P. Prabu, Spectrochim. Acta, Part A, 2015, 138, 314–320 CrossRef CAS PubMed.
- M. J. Sampaio, C. G. Silva, A. M. Silva, L. M. Pastrana-Martinez, C. Han, S. Morales-Torres and J. L. Faria, Appl. Catal., B, 2015, 170, 74–82 CrossRef.
- A. Y. Shan, T. I. M. Ghazi and S. A. Rashid, Appl. Catal., A, 2010, 389, 1–8 CrossRef CAS.
- V. Vaiano, O. Sacco, D. Sannino and P. Ciambelli, Appl. Catal., B, 2015, 170, 153–161 CrossRef.
- J. J. Wang, Y. H. Jing, T. Ouyang and C. T. Chang, J. Nanosci. Nanotechnol., 2015, 15, 6141–6149 CrossRef CAS.
- J. R. Kim and E. Kan, J. Environ. Manage., 2016, 180, 94–101 CrossRef CAS PubMed.
- B. Plav, S. Kobe and B. Orel, Kovine, Zlitine, Tehnol., 1999, 33, 517–521 Search PubMed.
- N. Huang and J. Wang, J. Anal. Appl. Pyrolysis, 2009, 84, 124–130 CrossRef CAS.
- I. G. Lodeiro, D. E. Macphee, A. Palomo and A. Fernández-Jiménez, Cem. Concr. Res., 2009, 39, 147–153 CrossRef.
- A. Phuruangrat, P. Jitrou, P. Dumrongrojthanath, N. Ekthammathat, B. Kuntalue, S. Thongtem and T. Thongtem, J. Nanomater., 2013, 1–8 Search PubMed.
- L. Zhang, T. Xu, X. Zhao and Y. Zhu, Appl. Catal., B, 2010, 98, 138–146 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- B. J. Becker, F. J. Fodrie, P. A. McMillan and L. A. Levin, Limnol. Oceanogr., 2005, 50, 48–61 CrossRef CAS.
- A. Martínez-de la Cruz, S. O. Alfaro, E. L. Cuéllar and U. O. Méndez, Catal. Today, 2007, 129, 194–199 CrossRef.
- F. Zhou, R. Shi and Y. Zhu, J. Mol. Catal. A: Chem., 2011, 340, 77–82 CrossRef CAS.
- D. Ma, J. Wu, M. Gao, Y. Xin and C. Chai, Chem. Eng. J., 2017, 316, 461–470 CrossRef CAS.
- B. Liu, X. Liu, M. Ni, C. Feng, X. Lei, C. Li and L. Pan, Appl. Surf. Sci., 2018, 453, 280–287 CrossRef CAS.
- T. Jia, W. Wang, F. Long, Z. Fu, H. Wang and Q. Zhang, J. Phys. Chem. C, 2009, 113, 9071–9077 CrossRef CAS.
- Q. He, Y. Ni and S. Ye, RSC Adv., 2017, 7, 27089–27099 RSC.
- J. Bi, L. Wu, J. Li, Z. Li, X. Wang and X. Fu, Acta Mater., 2007, 55, 4699–4705 CrossRef CAS.
- W. Zhao, C. Chen, X. Li, J. Zhao, H. Hidaka and N. Serpone, J. Phys. Chem. B, 2002, 106, 5022–5028 CrossRef CAS.
- L. Cai, J. Gong, J. Liu, H. Zhang, W. Song and L. Ji, Materials, 2018, 11, 267 CrossRef PubMed.
- Z. He, L. Xie, J. Tu, S. Song, W. Liu, Z. Liu and J. Chen, J. Phys. Chem. C, 2009, 114, 526–532 CrossRef.
- Y. Ou, J. D. Lin, H. M. Zou and D. W. Liao, J. Mol. Catal. A: Chem., 2005, 241, 59–64 CrossRef CAS.
- M. Ge, Y. Li, L. Liu, Z. Zhou and W. Chen, J. Phys. Chem. C, 2011, 115, 5220–5225 CrossRef CAS.
- F. Fu, H. Shen, X. Sun, W. Xue, A. Shoneye, J. Ma and J. Tang, Appl. Catal., B, 2019, 247, 150–162 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |