Open Access Article
Open Access ArticleHigh pressure extraction of bioactive diterpenes from the macroalgae Bifurcaria bifurcata: an efficient and environmentally friendly approach†
Adriana C. S. Paisa,
Carlos A. Pintob,
Patrícia A. B. Ramosab,
Ricardo J. B. Pinto a,
Daniela Rosac,
Maria F. Duartecd,
M. Helena Abreue,
Silvia M. Rochab,
Jorge A. Saraiva
a,
Daniela Rosac,
Maria F. Duartecd,
M. Helena Abreue,
Silvia M. Rochab,
Jorge A. Saraiva b,
Armando J. D. Silvestre
b,
Armando J. D. Silvestre a and
Sónia A. O. Santos
a and
Sónia A. O. Santos *a
*a
aCICECO-Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, Campus de Santiago, 3810-193 Aveiro, Portugal. E-mail: santos.sonia@ua.pt
bQOPNA/LAQV & REQUIMTE, Department of Chemistry, University of Aveiro, Campus de Santiago, 3810-193 Aveiro, Portugal
cCentro de Biotecnologia Agrícola e Agro-Alimentar do Alentejo (CEBAL), Instituto Politécnico de Beja (IPBeja), Beja, 7801-908, Portugal
dInstituto de Ciências Agrárias e Ambientais Mediterrânicas (ICAAM), Universidade de Évora, Pólo da Mitra, 7002-554 Évora, Portugal
eALGAplus—Prod. e Comerc. De Algas e Seus Derivados, Lda., Ílhavo, 3830-196, Portugal
First published on 2nd December 2019
Abstract
The brown macroalgae Bifurcaria bifurcata have gained special attention due to their ability to biosynthesize linear diterpenes (rarely found in other species). However, the conventional extraction methods normally used to extract these compounds involve organic solvents and often high temperatures, leading to the degradation of thermo-labile compounds. In this context, the main objective of this work was to study and optimize for the first time the extraction of diterpenes from B. bifurcata through an environmentally friendly methodology, namely, high pressure extraction (HPE) using ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water. This was compared with conventional Soxhlet extraction, using dichloromethane. Box–Behnken design was employed to evaluate the linear, quadratic, and interaction effects of 3 independent variables (pressure (X1), ethanol percentage (X2), and time of extraction (X3)) on response variables (extraction yield and diterpenes content (mg g−1 of extract and mg kg−1 of dry weight)) and the optimal extraction conditions (X1: 600 MPa; X2: 80%; X3: 5 min) were estimated by response surface methodology (RSM). B. bifurcata extract obtained under HPE optimal conditions showed a diterpenes content (612.2 mg g−1 of extract) 12.2 fold higher than that obtained by conventional extraction (50.1 mg g−1 of extract). The HPE extract, obtained under optimal conditions, showed antioxidant and antibacterial (against Staphylococcus aureus) activities considerably higher than the Soxhlet extract, and also presented a promising synergic effect with antibiotics, improving the antibiotic efficacy against S. aureus. In conclusion, these results indicate that HPE is a promising methodology, compared to conventional methodologies to obtain linear diterpene rich extracts from B. bifurcata with great potential to be exploited in pharmaceutical or biomedical applications.
water. This was compared with conventional Soxhlet extraction, using dichloromethane. Box–Behnken design was employed to evaluate the linear, quadratic, and interaction effects of 3 independent variables (pressure (X1), ethanol percentage (X2), and time of extraction (X3)) on response variables (extraction yield and diterpenes content (mg g−1 of extract and mg kg−1 of dry weight)) and the optimal extraction conditions (X1: 600 MPa; X2: 80%; X3: 5 min) were estimated by response surface methodology (RSM). B. bifurcata extract obtained under HPE optimal conditions showed a diterpenes content (612.2 mg g−1 of extract) 12.2 fold higher than that obtained by conventional extraction (50.1 mg g−1 of extract). The HPE extract, obtained under optimal conditions, showed antioxidant and antibacterial (against Staphylococcus aureus) activities considerably higher than the Soxhlet extract, and also presented a promising synergic effect with antibiotics, improving the antibiotic efficacy against S. aureus. In conclusion, these results indicate that HPE is a promising methodology, compared to conventional methodologies to obtain linear diterpene rich extracts from B. bifurcata with great potential to be exploited in pharmaceutical or biomedical applications.
1. Introduction
Bioactive natural compounds from marine sources have gained increased interest.1 Bifurcaria bifurcata is a brown macroalga that thrives, year-round, in the intertidal areas (the lower shore, rock-pools and mid-shore) along the coast of the Northern Atlantic, from Morocco (southern limit) to north-western Ireland (northern limit).2–4 The chemical composition of this brown macroalga has gained particular attention, due to the abundance of a variety of acyclic diterpenes.5–12 Compared to their cyclic counterparts, these linear diterpenes are relatively quite rare in nature,2 yet quite interesting since a vast range of promising bioactivities have been recognized for them.2,3,7,8,13–15 Recently, Santos et al.5 demonstrated the antioxidant, anti-inflammatory and antibacterial activities of B. bifurcata lipophilic extracts, mainly composed of linear diterpenes. In addition, a promising synergism was observed when these extracts were used together with antibiotic families of major clinical importance.5In general, this species could be a promising source of bioactive molecules useful for pharmaceutical, biomedical or even cosmetic industries.3,5 Nonetheless, the commonly used conventional extraction methodologies involve the use of large amounts of organic solvents, often toxic to humans and harmful to environment, such as dichloromethane5 or chloroform16 which represents a major limitation to the industrial exploitation of B. bifurcata diterpenes rich extracts. In addition, these extraction methods have demonstrated poor selectivity as at the same time require long operation times frequently at high temperatures, which can induce the degradation of thermo-labile compounds.13,14 Therefore, the evaluation and optimization of sustainable, economically viable and efficient methodologies to extract diterpenes from B. bifurcata macroalgae is an important challenge.
High pressure extraction (HPE) has been one of the emerging technologies that has been successfully exploited in the extraction of bioactive compounds from natural raw materials.17,18 HPE provides a large differential pressure between the interior and exterior of the cells, which causes cell walls and membranes structural damages, increasing their permeability and thus enhancing compounds dissolution in the extraction media.17 Additionally, HPE technology, usually performed at room temperature, presents a higher rate and efficiency of extraction than conventional methods, allowing to use safe solvents even presenting lower selectivity. Furthermore, this extraction methodology can be faster, with higher safety and energetically efficient.17,19 Although a major limitation of HPE has been associated with the high capital and equipment costs, the perspective for an expansion of HPE implementation in the near future is expected to lead to a decrease in costs. Actually, HP technology has been adopted quickly as reflected by the increased number of units installed.20,21
Although some studies have endorsed the use of HPE to extract lipophilic components (namely fatty acids and terpenes) from natural sources,18,19,22–26 no studies have been reported so far concerning the HPE of diterpenes. In addition, HPE has been only exploited in macroalgae to extract higher molecular weight components, namely sulfated polysaccharides from Sargassum muticum.27
In the present work the feasibility of HPE using ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures to extract diterpenes from B. bifurcata macroalga was studied for the first time. Besides, the process parameters of HPE were optimized by both Box–Behnken design and response surface methodology (RSM). As final output of this work, the higher efficiency of HPE to recover diterpenes from B. bifurcata, as well as the higher antioxidant and antibacterial activities and synergism with different antibiotics of the extract obtained, compared with those previously described are demonstrated.
water mixtures to extract diterpenes from B. bifurcata macroalga was studied for the first time. Besides, the process parameters of HPE were optimized by both Box–Behnken design and response surface methodology (RSM). As final output of this work, the higher efficiency of HPE to recover diterpenes from B. bifurcata, as well as the higher antioxidant and antibacterial activities and synergism with different antibiotics of the extract obtained, compared with those previously described are demonstrated.
2. Materials and methods
2.1 Sample preparation
The company ALGAplus, Produção e Comercialização de Algas e seus derivados, Lda. was responsible for the collection and pre-processing of algal samples. B. bifurcata (batch B1.2643.08F), was harvested in November 2013, at Aguda Beach (41°2′38′′ N, 8°39′10′′ W), region of Oporto, Portugal. Processing consisted in washing the biomass with running tap water and then with distilled water. Samples were preserved at −20 °C and then freeze-dried and milled at the laboratory of University of Aveiro.2.2 Conventional extraction
For comparative purposes, three aliquots (5 g) of lyophilized macroalgae samples were Soxhlet extracted with dichloromethane (160 mL), for 9 h, following a procedure described before.5 The solvent was evaporated to dryness and the extracts were weighed. The results are expressed in percent of dry weight (dw) material (w/w, %).2.3 High pressure extraction
Each aliquot (5 g) of lyophilized macroalgae was dispersed in 50 mL of solvent (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, as described in 2.3.1 and 2.3.2 sub-topics) and placed in a double packaged in low-permeability polyamide-polyethylene bags, which were heat-sealed under vacuum. The bags containing the biomass and solvent mixtures were subject to HPE under different time periods and pressures according to the established extraction conditions (Tables 2 and 3). HPE were performed in a hydrostatic press (Hyperbaric 55, Hyperbaric, Burgos, Spain), which has a pressure vessel of 200 mm inner diameter and 2.0 mm length with a maximum operating pressure of 600 MPa.
water, as described in 2.3.1 and 2.3.2 sub-topics) and placed in a double packaged in low-permeability polyamide-polyethylene bags, which were heat-sealed under vacuum. The bags containing the biomass and solvent mixtures were subject to HPE under different time periods and pressures according to the established extraction conditions (Tables 2 and 3). HPE were performed in a hydrostatic press (Hyperbaric 55, Hyperbaric, Burgos, Spain), which has a pressure vessel of 200 mm inner diameter and 2.0 mm length with a maximum operating pressure of 600 MPa.
After HPE, the aqueous/ethanol extracts and the remaining residue were removed from the bags and filtered through a glass filter funnel (porosity 3). Ethanol was evaporated and the extracts were frozen at −80 °C until freeze-drying. Then, and similarly to the Soxhlet extract, the extraction yield (EY) was determined as weight percentage (w/w, %) of dried extract.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (5 g of dry macroalgae in 50 mL of aqueous/ethanol solution). The extraction time was 15 min.
10 (5 g of dry macroalgae in 50 mL of aqueous/ethanol solution). The extraction time was 15 min.Table 3 presents the Box–Behnken design with three independent variables, designated as X1, X2, X3, at three levels, coded +1, 0, −1 for high, intermediate and low values, respectively: namely extraction pressure (X1: 0.1, 300 and 600 MPa), ethanol percentage (X2: 40, 60 and 80%) and extraction time (X3: 5, 17.5 and 30 min). A solid/liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was used. EY and the diterpenes content (DC) (expressed as milligram per gram of extract and milligram per kilogram of dw macroalgae) were used as response variables to evaluate the influence of the different levels of independent variables combined.
10 was used. EY and the diterpenes content (DC) (expressed as milligram per gram of extract and milligram per kilogram of dw macroalgae) were used as response variables to evaluate the influence of the different levels of independent variables combined.
The response surface design consisted in 15 runs in randomized order, to minimize the effects of unexpected variability in the observed responses,27 with three replicates in the centre point to estimate the pure error sum of squares (Table 3).
A full quadratic model was used to fit the data according to eqn (1):
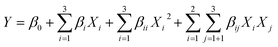 | (1) |
Analysis of variance (ANOVA) for response surface quadratic model validation was performed, and the test for significance of each term to test for goodness of fit was conducted at P < 0.05. The design construction and analysis were achieved through Minitab 18 (Minitab Statistical Software, Pennsylvania State University, State College, PA) software.
2.4 Diterpenes analysis by gas chromatography-mass spectrometry (GC-MS)
Before GC-MS analysis, aliquots of each dried extract (nearly 20 mg each) and an accurate amount of internal standard (hexadecane, 0.8 mg) were dissolved in 1100 μL of dichloromethane. High pressure extracts were, prior to the injection, filtered through a syringe filter (0.2 μm Teflon filter).The extracts were analysed by GC-MS following previously described methodologies5,28,29 on a GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan) equipment, equipped with a DB-1 J&W (Agilent, Santa Clara, CA, United States) capillary column (30 m × 0.32 mm inner diameter, 0.25 μm film thickness). The chromatographic conditions were as follows: initial temperature, 80 °C for 5 min; temperature gradient, 52 °C min−1; final temperature 285 °C for 8 min; injector temperature, 250 °C; transfer-line temperature, 290 °C; split ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.
40.
The identification of compounds was carried out through the comparison of their mass spectra fragmentation profile with library (Wiley 275 and U.S National Institute of Science and Technology (NIST14)), their characteristic retention times obtained under the described experimental conditions5 and by comparing their mass spectra fragmentation profiles with published data9,10,28 or by injection of standards.
For semi-quantitative analysis and to determine the response factor for diterpenes, GC-MS was calibrated with phytol, relative to hexadecane. The respective response factor was calculated as an average of six GC-MS runs. Three aliquots of each extract were injected in duplicate, and the results correspond to the average of the concordant values obtained (less than 5% variation between injections of the same aliquot). The compound contents were expressed as milligram per gram of extract (mg g−1 of extract) and as milligram per kilogram of dw of macroalgae (mg kg−1 dw).
2.5 Scanning electron microscopy (SEM) analysis
SEM micrographs were obtained by a Hitachi SU-70 microscope operating at 4 kV. Dried samples (initial lyophilized macroalgae, and macroalgae after Soxhlet and high-pressure extractions) were placed in an aluminium support with double sided carbon tape and deposited with a carbon coating before SEM analysis.2.6 In chemico and in vitro biological activities evaluation
The dry extracts were previously dissolved in methanol (4 mg mL−1). Sample aliquots (0.5 mL) were mixed with 0.125 mL of DPPH˙ (0.8 mM in methanol) and 1.375 mL of methanol. The ranges of final concentrations were 50–1000 μg mL−1 for Soxhlet extract and 20–100 μg mL−1 for high pressure extracts. Mixtures were homogenized in vortex. After 30 min of incubation in the dark, at room temperature, the absorbance was read at 517 nm, against a blank, using a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan). Duplicate measurements of each extract were carried out and each absorbance was compared to a control without extract.
The antioxidant activity was expressed as a percentage of DPPH radical reduction, using the following eqn (2):
 | (2) |
The inhibitory concentration of the extract required to decrease the initial DPPH radical concentration by 50% (IC50) was determined from the graph of DPPH reduction percentage in function of extracts concentration. The IC50 values were expressed in μg mL−1.
MBC, defined as the lowest concentration of the extract that results in killing 99.9% of bacterial cells, was determined by subculturing the corresponding MIC onto agar plates. In this assay, MIC and concentrations above were plated on Mueller–Hinton Agar (MHA; Liofilchem, Italy), using the spreading technique. The lowest concentration without visible growth corresponded with the MBC. Experiments were performed three times and each one with duplicates (n = 6).
The synergistic assay was performed following the protocol described above, according to Santos et al.5 B. bifurcata high pressure extract was conjugated with the antibiotics rifampicin (Rif; Duchefa Biochemie, Alfagene), tetracycline (Tetra; Duchefa Biochemie, Alfagene), gentamicin (Gent; Duchefa Biochemie, Alfagene) and ampicillin (Amp; Duchefa Biochemie, Alfagene) in a concentration range from 2 to 256 μg mL−1. Experiments were performed three times and each one with triplicates (n = 9). Factorial inhibitory concentration index (FICI) was calculated to classify interaction between B. bifurcata high pressure extract and antibiotics.33 Each of the combinations was calculated according to the following eqn (3):
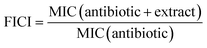 | (3) |
Results were interpreted as follows: FICI ≤ 0.5 synergistic (S), 0.5 < FICI < 1 partially synergistic (PS), FICI = 1 additive (ADD), 1 < FICI ≤ 4 indifferent (I) and FICI > 4 antagonistic (ANT).
3. Results and discussion
3.1 Lipophilic fraction obtained by soxhlet extraction
The lipophilic extract of wild B. bifurcata obtained by Soxhlet extraction presented an EY of 9.4 ± 0.1% (w/w) which was about three-fold higher than the Soxhlet EY obtained previously for B. bifurcata also from Portugal.5 Additionally, the EY observed was considerably higher than those previously reported for other Phaeophyta species.28,34 A detailed study of B. bifurcata Soxhlet extracts composition was performed by GC-MS analysis (Table 1), according to a previously established methodology.5| Compound | mg g−1 of extracta | mg kg−1 dwa | Rt (min) |
|---|---|---|---|
| a Results correspond to the average value estimated from the injection of three aliquots analysed in duplicate (standard deviation < 5). | |||
| Neophytadiene | 0.13 | 15 | 26.7 |
| Phytol | 0.03 | 4 | 32.3 |
| trans-Geranylgeraniol | 0.08 | 10 | 33.9 |
| 6,7,9,10,11,12,14,15-Tetradehydrophytol | 0.07 | 9 | 34.4 |
| 6-Hydroxy-13-oxo-7,7′,10,11-didehydrophytol | 1.12 | 144 | 36.3 |
| Eleganolone | 48.44 | 6180 | 38.0 |
| 1-Acetyl-10,13-dioxo-6,7,11,11′,14,15-tridehydrophytol | 0.27 | 33 | 40.6 |
| Total | 50.14 | 6395 | |
B. bifurcata has already been studied due to its variety of diterpenes.2,6–12 However, only a single detailed study of its lipophilic fraction was performed by GC-MS analysis, in which other compounds were identified and quantified, namely sterols, fatty acids, long-chain aliphatic alcohols, monoglycerides, among others.5
Several diterpenes were identified in B. bifurcata Soxhlet extract, namely neophytadiene, phytol, trans-geranylgeraniol, 6,7,9,10,11,12,14,15-tetrahydrophytol, 6-hydroxy-13-oxo-7,7′,10,11-didehydrophytol, eleganolone, and 1-acetyl-10,13-dioxo-6,7,11,11′,14,15-tridehydrophytol. Some of these linear compounds were previously detected in B. bifurcata collected at different geographical points, such as France,9,11,35 Morocco10 and Spain.36 Phytol and neophytadiene were also reported before as constituents of this macroalga from Portugal.5 trans-Geranylgeraniol was already identified as B. bifurcata constituent from Morocco8,37 and Brittany.9,37
The diterpenes in the studied extracts were identified by comparing the mass spectra fragmentation profile with libraries (Wiley 275 and U.S. National Institute of Science and Technology (NIST14)), their characteristic retention times obtained under the described experimental conditions5 and literature data.9,10,28
Diterpenes accounted for 6395 mg kg−1 dw of B. bifurcata. Eleganolone and 6-hydroxy-13-oxo-7,7′,10,11-didehydrophytol were the major components of this family, accounting for 6180 mg kg−1 dw and 144 mg kg−1 dw, respectively. 1-Acetyl-10,13-dioxo-6,7,11,11′,14,15-tridehydrophytol and neophytadiene were also present in considerable amounts.
These linear diterpenes have been the focus of interest of several studies, since they are relatively rare in nature.2,15 In addition, they have been associated with several biological activities, such as antioxidant, antimicrobial and anti-inflammatory properties.5,38
In order to obtain these extracts without the use of hazardous solvents and using a more sustainable approach we decided to study their extraction with ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water under HPE.
water under HPE.
3.2 Preliminary high-pressure extractions
Preliminary HPE experiments were performed considering two independent variables, namely the extraction pressure (600, 300 and 0.1 MPa) and the ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio (80
water ratio (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 60
20 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). These experiments were carried out to choose the experimental design variables and their ranges of values, and thus the type of experimental design. As shown in Table 2, the differences observed in the EY and DC (mg g−1 of extract and mg kg−1 dw) values of the preliminary experiments indicated that these factors have possible significant effects on the results.
40). These experiments were carried out to choose the experimental design variables and their ranges of values, and thus the type of experimental design. As shown in Table 2, the differences observed in the EY and DC (mg g−1 of extract and mg kg−1 dw) values of the preliminary experiments indicated that these factors have possible significant effects on the results.
| Preliminary experiment no. | Independent variables | Response variablesa | |||
|---|---|---|---|---|---|
| Pressure (MPa) | % Ethanol | EY (w/w, %) | DC (mg g−1 of extract) | DC (mg kg−1 dw) | |
| a Results correspond to the average value estimated from the injection of three aliquots analysed in duplicate (standard deviation < 5%). | |||||
| 1 | 600 | 80 | 6.9 | 413.0 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557 557 |
| 2 | 300 | 60 | 11.9 | 333.5 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527 |
| 3 | 0.1 | 80 | 8.4 | 4299 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 679 |
HPE extracts presented quite different EY values, accounting 6.9% (w/w) at 600 MPa and 80% ethanol and 11.9% (w/w) at 300 MPa and 60% ethanol. These values are also different from that obtained with Soxhlet extraction (9.4% (w/w)). The experiment 3 (Table 2), performed at atmospheric pressure and 80% ethanol, showed an EY of 8.4% (w/w), which is in the range of those obtained at higher pressure and lower than that obtained with Soxhlet extraction.
All the preliminary extractions showed total amounts of diterpenes (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557 and 43
557 and 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 mg kg−1 dw) higher than that obtained with Soxhlet extraction (6394 mg kg−1 dw). The experiments with 80% ethanol (1 and 3) showed the highest DC values, suggesting that the ethanol percentage could have a high effect on the diterpenes yield. Comparing the experiments at different pressures (1 and 3), with the same ethanol percentage, the DC at atmospheric pressure was slightly higher than that obtained at 600 MPa. However, the standard deviation associated to experiment 3 was considerably high and therefore the differences may not be statistically significant.
527 mg kg−1 dw) higher than that obtained with Soxhlet extraction (6394 mg kg−1 dw). The experiments with 80% ethanol (1 and 3) showed the highest DC values, suggesting that the ethanol percentage could have a high effect on the diterpenes yield. Comparing the experiments at different pressures (1 and 3), with the same ethanol percentage, the DC at atmospheric pressure was slightly higher than that obtained at 600 MPa. However, the standard deviation associated to experiment 3 was considerably high and therefore the differences may not be statistically significant.
When a lower ethanol percentage (60%) was used with a pressure of 300 MPa, higher EY and lower DC were achieved, which could mean that under these HPE conditions, the extraction of other compounds, such as polysaccharides, can be favored in the detriment of diterpenes extraction.
With the preliminary experiments, it was verified that HPE could be more selective to the diterpenic compounds, than the conventional extraction methodology. Therefore, pressure and ethanol percentage were chosen as variables to optimize. In addition, and taking into account the high number of studies showing a high effect of extraction time on the EY of target compounds,27,39,40 time was selected as the third variable for the experimental design. EY and DC (expressed as mg g−1 of extract and mg kg−1 dw) were selected as responses to optimize.
A pressure range between 0.1 and 600 MPa was selected, due to the highest DC observed for the preliminary experiments 1 (600 MPa) and 3 (0.1 MPa). Actually, 600 MPa correspond to the maximum value of pressure enabled by the equipment, so the full possible range was considered in order to enhance the maximum rupture of macroalga cell walls.27
In the same way, DC values were different at diverse mixture concentration. Therefore, the effect of this factor was evaluated in an extended range, namely between 40% and 80% of ethanol.
Finally, extraction time was selected to be 5–30 min, which has been in the range of most of the optimal extraction times reported in several studies.27,39,40
3.3 Analysis of the designed HPE experiments
HPE of diterpenes from B. bifurcata was optimized by response surface methodology using a Box–Behnken design. EY and DC expressed in mg g−1 of extract and in mg kg−1 dw of B. bifurcata extracts are shown in Table 3. The predicted values, within the limits of the experimental factors, are also listed in Table 3.| Run no. | Coded levels of independent variables | Responses variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 (pressure, MPa) | X2 (% ethanol) | X3 (time, min) | EY (w/w, %) | DCa (mg g−1 of extract) | DC (mg kg−1 dw) | ||||
| Observed | Predicted | Observed | Predicted | Observed | Predicted | ||||
| a Diterpenes content. | |||||||||
| 1 | 0 (300) | +1 (80) | −1 (5) | 3.5 | 3.6 | 475.7 | 436.7 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 651 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517 517 |
| 2 | +1 (600) | +1 (80) | 0 (17.5) | 4.1 | 5.2 | 430.3 | 409.1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
| 3 | 0 (300) | −1 (40) | −1 (5) | 9.2 | 10.2 | 49.0 | 16.1 | 4484 | 4988 |
| 4 | −1 (0.1) | 0 (60) | −1 (5) | 9.1 | 9.2 | 15.0 | 26.8 | 1370 | 1378 |
| 5 | 0 (300) | 0 (60) | 0 (17.5) | 7.7 | 7.6 | 125.3 | 110.7 | 9620 | 8336 |
| 6 | +1 (600) | 0 (60) | +1 (30) | 12.6 | 12.5 | 27.8 | 16.0 | 3496 | 3488 |
| 7 | 0 (300) | +1 (80) | +1 (30) | 9.6 | 8.6 | 136.4 | 169.3 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 134 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630 |
| 8 | −1 (0.1) | −1 (40) | 0 (17.5) | 9.9 | 8.7 | 15.6 | 36.8 | 1540 | 1028 |
| 9 | +1 (600) | 0 (60) | −1 (5) | 9.7 | 8.4 | 188.9 | 249.1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 906 |
| 10 | 0 (300) | 0 (60) | 0 (17.5) | 8.3 | 7.6 | 85.0 | 110.7 | 7048 | 8336 |
| 11 | +1 (600) | −1 (40) | 0 (17.5) | 10.9 | 11.0 | 33.2 | 6.0 | 3608 | 3482 |
| 12 | −1 (0.1) | 0 (60) | +1 (30) | 9.1 | 10.3 | 90.2 | 30.0 | 8213 | 8592 |
| 13 | 0 (300) | −1 (40) | +1 (30) | 10.5 | 10.4 | 14.6 | 53.7 | 1541 | 1675 |
| 14 | −1 (0.1) | +1 (80) | 0 (17.5) | 6.2 | 6.0 | 142.7 | 170.0 | 8888 | 9013 |
| 15 | 0 (300) | 0 (60) | 0 (17.5) | 6.8 | 7.6 | 121.8 | 110.7 | 8340 | 8336 |
From a qualitative point of view and similarly to the observed in preliminary experiments, the lipophilic composition of HPE extracts was very similar to the Soxhlet extract.
The results of 15 experiments including three replicates at the centre point were analysed, using the response surface methodology. Linear and quadratic effects of the three variables studied as well as their interactions were evaluated for regression coefficients.
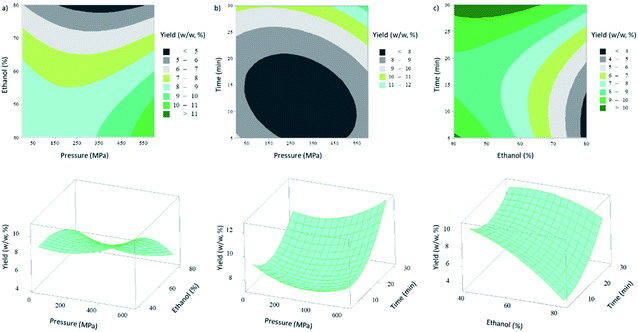
The main effects of independent variables on the measured responses can be observed through the interpretation of the 2D contour and 3D surface response plots (Fig. 1). The increase of extraction time and the decrease of ethanol percentage led to an increase of the extraction yield, which is also verified in the positive and negative values of the β-coefficient value (for coded variables) of the linear term (Table 1S†), respectively. This negative effect of the ethanol percentage could be related to the co-extraction of other components, namely polysaccharides, which are quite abundant in macroalgae, and their extraction may occur with high water contents on the extraction solvent mixture.
Extraction time also had a significant effect on the EY. As example, the yield increased from 3.5% (w/w) at 300 MPa, 80% ethanol and 5 min to 9.6% (w/w) at 300 MPa, 80% ethanol and 30 min, which is a variation of 64%. As HPE is known to be a faster methodology than other extraction methods,19 the extraction time only has to be long enough to ensure the contact between the compounds and the solvent.40 Notwithstanding, a higher amount of extractives is expected when increasing the extraction time.
No significant effect was observed for pressure (P > 0.05) (Fig. 1a and b). However, the maximum EY (12.6% (w/w)) was obtained at 600 MPa, corresponding to an increase of 28% compared to the yield obtained with the same conditions at atmospheric pressure (9.1% (w/w)).
According to the model, the EY was maximized at 600 MPa, 40% ethanol and an extraction time of 30 min, corresponding to a predicted value of 13.4% (w/w). A R2 of 0.894 was obtained, which means that 10.6% of total variations are not explained by the model. However, the P-value for the lack-of-fit was 0.17, which shows that the model developed can represent well the results observed.27,41
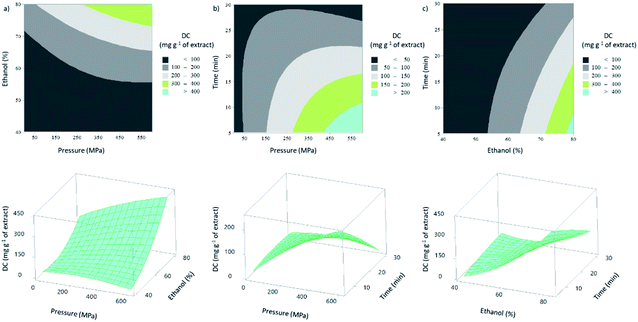
Higher ethanol percentages and/or higher extraction pressures led to higher DC (mg g−1 of extract), as can be seen in contour and surface response plots (Fig. 2), through the positive β-coefficients values (for coded variables) of the linear terms (X1 and X2).
In general, an increase in extraction pressure resulted in an increase on DC. When the pressure changed from 0.1 MPa to 600 MPa, maintaining the remaining extraction conditions (X2: 80% and X3: 17.5 min), the DC increased from 142.7 to 430.3 mg g−1 of extract (∼67%). This positive effect of pressure was expected, since at higher pressures the cell structures and membranes are destroyed, which increases mass transfer of solvents into raw materials, as well as of the soluble constituents into the solvents.19 Concerning the effect of extraction time, the highest DC was obtained for the lower extraction time (5 min), which is also reflected in the negative value of the β-coefficient value (for coded variables) of the linear term. At 600 MPa and 60% of ethanol, the extractions carried out during 5 and 30 minutes, resulted in DC of 188.9 and 27.8 mg g−1 of extract, respectively. This means that the longer the extraction time, the lower the DC.
A hypothesis to explain the negative effect of extraction time is based on a higher abundance of co-extracted compounds, such as, polysaccharides, and a possible adsorption of diterpenes in the macromolecules. In fact, brown macroalgae are known for their high content in polysaccharides.42,43 Before GC-MS analysis, several steps are performed, such as filtration, where losses can result in the reduction of co-extracted compounds. Upon elimination, the polysaccharides may consequently retain part of the diterpenes, which results in a decrease in this response.
The maximum of DC (475.7 mg g−1 of extract) was achieved at 300 MPa, the value of extraction pressure, with the lowest extraction time (5 min) and with the highest percentage of ethanol (80%). However, at 300 MPa an extraction time of 30 min and with a percentage of ethanol of 40%, the minimum amount of diterpenes per g of extract (14.6) was extracted. This means that the ethanol percentage and the extraction time were the most significant effects, which is in accordance to the F values of their linear effects (44.60 and 8.19, respectively) (Table 2S†).
According to the model, the maximum predicted DC (593.5 mg g−1 of extract) could be obtained with the following HPE conditions: extraction pressure (X1), 600 MPa; ethanol percentage (X2), 80%; and extraction time (X3), 5 min.
The R2 value (Table 2S†) of this model was 0.943, which represents a good correlation between the observed and predicted values, where more than 94% of responses variability are explained by the model. Furthermore, the P-value for the lack-of-fit non-significant (0.09) suggests once more that the developed model can represent the observed results.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
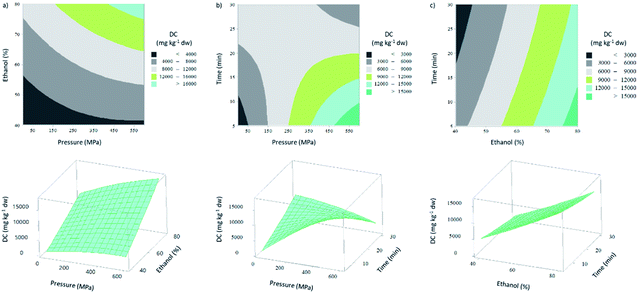
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) time interaction term (X2X3), all the linear and interaction effects showed to have significant effect (P-value < 0.05) (Table 3S†). The 2D contour and 3D surface response plots in Fig. 3 show that DC (mg kg−1 dw) increased with increased ethanol percentage and extraction pressure. In fact, the positive β-coefficients values (for coded variables) of the corresponding linear terms (Table 3S†) are in agreement with this effect. However, and similarly with the observed for the DC in a basis of extract, the effect of extraction time was opposite, obtaining higher diterpenes amount per kg of dw when the extraction has a short length. As expected, the linear effect of each variable in this response was quite similar to those of the previous response.
time interaction term (X2X3), all the linear and interaction effects showed to have significant effect (P-value < 0.05) (Table 3S†). The 2D contour and 3D surface response plots in Fig. 3 show that DC (mg kg−1 dw) increased with increased ethanol percentage and extraction pressure. In fact, the positive β-coefficients values (for coded variables) of the corresponding linear terms (Table 3S†) are in agreement with this effect. However, and similarly with the observed for the DC in a basis of extract, the effect of extraction time was opposite, obtaining higher diterpenes amount per kg of dw when the extraction has a short length. As expected, the linear effect of each variable in this response was quite similar to those of the previous response.
In this measured response, the effect of extraction pressure was more evident when compared with the other models. The two HPE performed with 60% of ethanol, for 5 min, at different values of pressure (0.1 and 600 MPa), resulted in a minimum (1370 mg kg−1 dw) and maximum (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 mg kg−1 dw) DC, respectively. Thus, the highest extraction pressure ensured a higher DC. In fact, when the compression level applied exceeds the deformation limit of the cells, can lead to formation of cracks,19 resulting in more solvent inside the cells and consequently more compounds permeate the damaged cell membrane.40
285 mg kg−1 dw) DC, respectively. Thus, the highest extraction pressure ensured a higher DC. In fact, when the compression level applied exceeds the deformation limit of the cells, can lead to formation of cracks,19 resulting in more solvent inside the cells and consequently more compounds permeate the damaged cell membrane.40
Notwithstanding, the most important effect to achieve higher amounts of diterpenes was the ethanol percentage, which had a F value of 269.12 (linear effect), represented in Table 3S.† DC (mg kg−1 dw) increased 79%, when the ethanol percentage was changed from 40% (3608 mg kg−1 dw) to 80% (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 mg kg−1 dw).
470 mg kg−1 dw).
Pressure![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) time interaction also had a significant effect (F value of 124.54) (Table 3S†) on the DC in a dw basis. According to the model, the maximum predicted DC (25
time interaction also had a significant effect (F value of 124.54) (Table 3S†) on the DC in a dw basis. According to the model, the maximum predicted DC (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625.2 mg kg−1 dw) is achieved at X1: 600 MPa; X2: 80%; and X3: 5 min. At these extraction conditions, there is a large pressure differential between the intra- and extracellular medium, which will lead to a rapid permeation of the compounds obtaining the equilibrium in a shorter time.40
625.2 mg kg−1 dw) is achieved at X1: 600 MPa; X2: 80%; and X3: 5 min. At these extraction conditions, there is a large pressure differential between the intra- and extracellular medium, which will lead to a rapid permeation of the compounds obtaining the equilibrium in a shorter time.40
The R2 and Radj2 of the predicted model were 0.990 and 0.973, respectively, which did not differ significantly. In agreement with these statistical parameters, the P-value of lack-of-fit was 0.84 (P-value > 0.05) (Table 3S†), which is another evidence that the model equation for DC (mg kg−1 dw) was adequate to predict the respective values under any sets of combination within the range of experimental values. Additionally, only 27% of samples showed a variation between values higher than 10% (Table 3).
3.4 Optimization of high-pressure extraction conditions
The optimization of HPE of diterpenes from B. bifurcata was performed in order to maximize the DC (expressed as mg g−1 of extract and mg kg−1 dw) and not EY, because an increase in the EY might mean an increase of co-extracted polysaccharides. The optimized extraction pressure, ethanol percentage and extraction time were 600 MPa, 80% and 5 min, respectively. According to the models, the predicted results in extract and dw basis under these conditions were 593.5 mg g−1 of extract and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 mg kg−1 dw, respectively. The experimental results achieved at this point were 612.2 ± 10.6 mg g−1 of extract and 38
625 mg kg−1 dw, respectively. The experimental results achieved at this point were 612.2 ± 10.6 mg g−1 of extract and 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 ± 633 mg kg−1 dw (Table 4). The difference between the predicted and experimental values was satisfactory, being the variation lower than 5% for DC (mg g−1 of extract), which validates the respective model.
954 ± 633 mg kg−1 dw (Table 4). The difference between the predicted and experimental values was satisfactory, being the variation lower than 5% for DC (mg g−1 of extract), which validates the respective model.
| Optimal conditions | Responses variables | ||
|---|---|---|---|
| EY (w/w, %) | DC (mg g−1 of extract) | DC (mg kg−1 dw) | |
| a HPE – high pressure extraction; dw – dry weight. | |||
| Soxhlet extraction | 9.4 ± 0.1 | 50.1 ± 13.2 | 6394 ± 767 |
| HPE | 6.9 ± 0.6 | 612.2 ± 10.6 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 ± 633 954 ± 633 |
3.5 Comparative perspective between HPE at optimal conditions and conventional extraction
Concerning the amount of diterpenes in the extract, HPE allowed obtaining 612.2 ± 10.6 mg g−1 of extract, which is 12.2-fold higher than conventional extraction (50.1 mg g−1 of extract). In the same way, the DC in a dw basis in HPE accounted for 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 ± 633 mg kg−1 dw, which is considerably higher (6.1-fold) than that obtained with Soxhlet extraction (6395 mg kg−1 dw).
954 ± 633 mg kg−1 dw, which is considerably higher (6.1-fold) than that obtained with Soxhlet extraction (6395 mg kg−1 dw).
Macroalgae before (Fig. 4a) and after conventional (Fig. 4b) and HPE at optimal conditions (Fig. 4c) were analysed by SEM. The higher magnification images (×2.5k) showed the biggest differences at the cell surface level, between the three samples. The image of the initial macroalga shows a regular surface, whereas the image correspondent to macroalga after Soxhlet extraction present already some damages. Nonetheless, the most evident surface damages are present in the macroalga after HPE, where gaps can be observed in the cell structure.
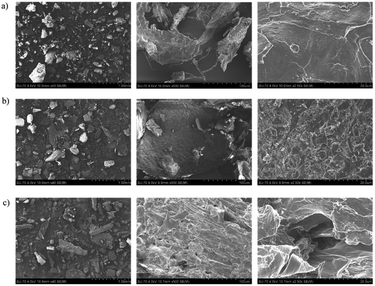 | ||
| Fig. 4 SEM micrographs of macroalgae (a) before extraction, (b) after Soxhlet extraction and (c) after HPE for three different magnifications (×40, ×500 and ×2.50k). | ||
The SEM images of these samples corroborated the results reported above, notably the higher amount of diterpenes extracted in HPE, since greater damage in the cell structure leads to a reduction of mass transfer resistance and, consequently, to a higher amount of compounds extracted.
Previous antioxidant activity results of solid–liquid and Soxhlet B. bifurcata extract against DPPH˙, reported for a wild sample collected from Peniche Coast Portugal (345 μg mL−1 (246.10–482.80))3 and for a sample from an integrated multi-trophic aquaculture from Portugal (366 ± 10 μg mL−1),5 respectively, showed quite similar IC50 values. In the present work, the Soxhlet extract of B. bifurcata collected in the Portuguese north coast, presented an IC50 value of about 777 ± 16 μg mL−1, demonstrating a lower antioxidant activity than those reported before.
On the contrary, the antioxidant activity of B. bifurcata HPE extract obtained at the optimal conditions accounted for 28 ± 2 μg mL−1, which is a noticeably improved result compared to the Soxhlet extract obtained from the same macroalgae sample as well as from those previously reported in the literature. In addition, this IC50 value is in the same range of those reported in the literature for extracts rich in antioxidant compounds (e.g. phenolic compounds),44 which could emerge as a consequence of using H2O and ethanol as solvents, due to their higher polarity. Thus, these compounds not extracted with DCM can also contribute to an improvement of antioxidant activity. Although, these antioxidant activity results clearly demonstrate the potential of HPE in this context. This difference may be related with the higher abundance of diterpenes observed in HPE extract. In fact, diterpenes have been well recognized by their bioactivities including antioxidant.5
| S. aureus ATCC® 43300 | ||
|---|---|---|
| MIC (μg mL−1) | MBC (μg mL−1) | |
| a HPE – high pressure extraction; MIC – minimal inhibitory concentration; MBC – minimal bactericidal concentration. | ||
| Soxhlet extract | 2048 | >2048 |
| HPE extract | 1024 | 2048 |
The antibiotics used represent drug families of major clinical importance, such as aminoglycosides (Gent: gentamicin), tetracyclines (Tetra: tetracycline), macrocyclics (Rif: rifampicin), and β-lactams antibiotics, such as aminopenicillins (Amp: ampicillin). The results are expressed in MIC and FICI (Table 6). The antibacterial activity observed for the B. bifurcata Soxhlet extract (MIC = 2048 μg mL−1) matched that obtained by Santos et al.5
| MIC (μg mL−1) | FICI | |
|---|---|---|
| a HPE ext – high pressure extract; Rif – rifampicin; Gent – gentamicin; Tetra – tetracycline; Amp – ampicillin; MIC – minimal inhibitory concentration; FICI – factorial inhibitory concentration index; synergistic (S) if FICI ≤ 0.5; partially synergistic (PS), if 0.5 < FICI < 1; additive (ADD), if FICI = 1; indifferent (IND), if 1 < FICI ≤ 4 and antagonistic (ANT), if FICI > 4. | ||
| Rif | 16 | <0.125 (S) |
| Rif + HPE ext | <2 | |
| Gent | >256 | <0.125 (S) |
| Gent + HPE ext | 32 | |
| Tetra | >256 | <0.125 (S) |
| Tetra + HPE ext | <2 | |
| Amp | 128 | 0.125 (S) |
| Amp + HPE ext | 16 | |
Whereas the activity of B. bifurcata HPE extract (MIC = 1024 μg mL−1) was 2-fold higher than that of Soxhlet extract. Furthermore, MBC determination showed that B. bifurcata HPE extract has bactericidal effect against S. aureus ATCC® 43300 (MBC = 2048 μg mL−1), which indicates that HPE method is more efficient than Soxhlet in what concerns antibacterial potential of the ensuing diterpene rich extracts. The higher content in diterpenes may explain the antibacterial activity obtained.
The combination of the HPE extract with distinct antibiotics resulted in a considerable decrease of antibiotic MICs values against the S. aureus ATCC® 43300. Outstanding decreases were observed with gentamicin and tetracycline, which MIC values decreased from >256 μg mL−1 to 32 and <2 μg mL−1, respectively. Regarding FICI values, performed according to eqn (3), B. bifurcata HPE extract with antibiotics resulted in a synergistic effect against S. aureus ATCC® 43300. HPE extract, obtained at optimal conditions, shows thus high potential to be further studied as a possible strategy to eradicate S. aureus.5
The possible use of natural compounds as coadjuvants in conventional antibiotherapy has already been described.45,46 Since their structures are quite different from those of antibiotics, the mechanism of action and/or target may be different and, therefore, other pathways/targets might be involved in bactericidal effect. This fact leads to better outcomes such as enhanced efficacy, decreased dosage and delayed development of drug resistance.45 Given the favourable results obtained, it would be of interest to better understand the mechanism against bacterial cells as well as to assess effectiveness over time of the combinations used.
4. Conclusions
In this study it was demonstrated the feasibility of HPE to enhance extraction of linear diterpenes from B. bifurcata. In addition, HPE showed to be more selective and efficient than conventional Soxhlet extraction with dichloromethane. The effect of pressure, ethanol percentage and time of extraction were evaluated and the HPE conditions were optimized using RSM. HPE optimal conditions achieved were: extraction pressure – 600 MPa; ethanol percentage – 80%; and extraction time – 5 min. At these conditions, the B. bifurcata extract presented a DC of 612.2 ± 10.6 mg g−1 of extract, which is 12.2-fold higher than Soxhlet extraction (50.1 mg g−1 of extract). In the same way, the DC in a dw basis was considerably higher in HPE, accounting for 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 ± 633 (mg kg−1 dw), which is 6.1-fold than that obtained with Soxhlet extraction (6394 mg kg−1 dw). HPE at optimal conditions also resulted in an extract with considerably higher antioxidant and antibacterial activities and a synergism with distinct antibiotics was also observed. Therefore, HPE shows to be a promising technique to obtain B. bifurcata extracts with potential to be used in pharmaceutical or biomedical applications, particularly in the management of antibiotic-resistant pathogenic bacteria.
954 ± 633 (mg kg−1 dw), which is 6.1-fold than that obtained with Soxhlet extraction (6394 mg kg−1 dw). HPE at optimal conditions also resulted in an extract with considerably higher antioxidant and antibacterial activities and a synergism with distinct antibiotics was also observed. Therefore, HPE shows to be a promising technique to obtain B. bifurcata extracts with potential to be used in pharmaceutical or biomedical applications, particularly in the management of antibiotic-resistant pathogenic bacteria.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks are due to the University of Aveiro and FCT/MCT for the financial support for the CICECO (FCT UID/CTM/50011/2019) and QOPNA (FCT UID/QUI/00062/2019) research Units through national founds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement. This work was also funded through FCT under the Project UID/AGR/00115/2019 to ICAAM. S. A. O. Santos thanks the AgroForWealth: Biorefining of agricultural and forest by-products and wastes: integrated strategic for valorisation of resources towards society wealth and sustainability project (CENTRO-01-0145-FEDER-000001), funded by Centro2020, through FEDER and PT2020 for the contract. P. A. B. Ramos acknowledges the “MultiBiorefinery” project (POCI-01-0145-FEDER-016403) for her post-doctoral grant.Notes and references
- E. Hussain, L. J. Wang, B. Jiang, S. Riaz, G. Y. Butt and D. Y. Shi, RSC Adv., 2016, 6, 12592–12610 RSC.
- J. Muñoz, G. Culioli and M. Köck, Phytochem. Rev., 2013, 12, 407–424 CrossRef.
- C. Alves, S. Pinteus, T. Simões, A. Horta, J. Silva, C. Tecelão and R. Pedrosa, Int. J. Food Sci. Technol., 2016, 51, 1638–1646 CrossRef CAS.
- L. Pereira, As Algas Marinhas e Respectivas Utilidades, Universidade de Coimbra, 2008, vol. 913, pp. 1–19 Search PubMed.
- S. Santos, S. Trindade, C. Oliveira, P. Parreira, D. Rosa, M. Duarte, I. Ferreira, M. Cruz, A. Rego, M. Abreu, S. Rocha and A. Silvestre, Mar. Drugs, 2017, 15, 340 CrossRef PubMed.
- K. Le Lann, J. Rumin, S. Cérantola, G. Culioli and V. Stiger-Pouvreau, J. Appl. Phycol., 2014, 26, 1207–1214 CrossRef CAS.
- R. Valls, L. Piovetti, B. Banaigs, A. Archavlis and M. Pellegrini, Phytochemistry, 1995, 39, 145–149 CrossRef CAS PubMed.
- R. Valls, B. Banaigs, L. Piovetti, A. Archavlis and J. Artaud, Phytochemistry, 1993, 34, 1585–1588 CrossRef CAS.
- G. Culioli, M. Daoudi, V. Mesguiche, R. Valls and L. Piovetti, Phytochemistry, 1999, 52, 1447–1454 CrossRef CAS.
- G. Culioli, M. Daoudi, A. Ortalo-Magné, R. Valls and L. Piovetti, Phytochemistry, 2001, 57, 529–535 CrossRef CAS PubMed.
- G. Culioli, A. Ortalo-Magné, M. Richou, R. Valls and L. Piovetti, Biochem. Syst. Ecol., 2002, 30, 61–64 CrossRef CAS.
- Q. Göthel, J. Muñoz and M. Köck, Phytochem. Lett., 2012, 5, 693–695 CrossRef.
- G. Culioli, A. Ortalo-Magné, M. Daoudi, H. Thomas-Guyon, R. Valls and L. Piovetti, Phytochemistry, 2004, 65, 2063–2069 CrossRef CAS PubMed.
- S. Pinteus, J. Silva, C. Alves, A. Horta, N. Fino, A. I. Rodrigues, S. Mendes and R. Pedrosa, Food Chem., 2017, 218, 591–599 CrossRef CAS PubMed.
- E. M. Balboa, E. Conde, A. Moure, E. Falqué and H. Domínguez, Food Chem., 2013, 138, 1764–1785 CrossRef CAS PubMed.
- V. Smyrniotopoulos, C. Merten, M. Kaiser and D. Tasdemir, Mar. Drugs, 2017, 15, 1–10 CrossRef PubMed.
- E. M. C. Alexandre, L. M. G. Castro, S. A. Moreira, M. Pintado and J. A. Saraiva, Food Eng. Rev., 2017, 9, 1–23 CrossRef.
- E. M. C. Alexandre, S. Silva, S. A. O. Santos, A. J. D. Silvestre, M. F. Duarte, J. A. Saraiva and M. Pintado, Food Res. Int., 2019, 115, 167–176 CrossRef CAS PubMed.
- H. W. Huang, C. P. Hsu, B. B. Yang and C. Y. Wang, Trends Food Sci. Technol., 2013, 33, 54–62 CrossRef CAS.
- H. Mújica-Paz, A. Valdez-Fragoso, C. T. Samson, J. Welti-Chanes and A. Torres, Food Bioprocess Technol., 2011, 4, 969–985 CrossRef.
- V. M. Balasubramaniam, S. I. Martínez-Monteagudo and R. Gupta, Annu. Rev. Food Sci. Technol., 2015, 6, 435–462 CrossRef CAS PubMed.
- V. Briones-Labarca, M. Plaza-Morales, C. Giovagnoli-Vicuña and F. Jamett, LWT--Food Sci. Technol., 2015, 60, 525–534 CrossRef CAS.
- X. Jun, High Pressure Res, 2006, 26, 33–41 CrossRef CAS.
- J. Xi, Chem. Eng. Technol., 2006, 29, 736–739 CrossRef CAS.
- G. Ruiz-Montañez, J. A. Ragazzo-Sánchez, M. Calderón-Santoyo, G. Velázquez-De La Cruz, J. A. Ramírez De León and A. Navarro-Ocaña, Food Chem., 2014, 159, 267–272 CrossRef PubMed.
- X. Jun, Crit. Rev. Food Sci. Nutr., 2013, 53, 837–852 CrossRef CAS PubMed.
- D. Rodrigues, A. C. Freitas, R. Queirós, T. A. P. Rocha-Santos, J. A. Saraiva, A. M. P. Gomes and A. C. Duarte, J. Food Process. Preserv., 2017, 41, 1–12 Search PubMed.
- S. A. O. Santos, C. S. D. Oliveira, S. S. Trindade, M. H. Abreu, S. S. M. Rocha and A. J. D. Silvestre, J. Appl. Phycol., 2016, 28, 3151–3158 CrossRef CAS.
- R. Touati, S. A. O. Santos, S. M. Rocha, K. Belhamel and A. J. D. Silvestre, Ind. Crops Prod., 2015, 69, 238–243 CrossRef CAS.
- J. B. Patel, F. R. Cockerill and P. A. Bradford, Performance standards for antimocrobial susceptibility testing: twenty-fifth informational supplement, Clinical and Laboratory Standards Institute, Wayne, PA, USA, 2015, vol. 35 Search PubMed.
- I. Wiegand, K. Hilpert and R. E. W. Hancock, Nat. Protoc., 2008, 3, 163–175 CrossRef CAS PubMed.
- T. L. Riss, A. L. Niles and L. Minor, Assay Guid. Man., 2004, 1–23 Search PubMed.
- V. Pereira, C. Dias, M. C. Vasconcelos, E. Rosa and M. J. Saavedra, Ind. Crops Prod., 2014, 52, 1–7 CrossRef CAS.
- P. Kumari, M. Kumar, V. Gupta, C. R. K. Reddy and B. Jha, Food Chem., 2010, 120, 749–757 CrossRef CAS.
- A. Ortalo-Magné, G. Culioli, R. Valls, B. Pucci and L. Piovetti, Phytochemistry, 2005, 66, 2316–2323 CrossRef PubMed.
- M. Daoudi, S. Bakkas, G. Culioli, A. Ortalo-Magné, L. Piovetti and M. D. Guiry, Biochem. Syst. Ecol., 2001, 29, 973–978 CrossRef CAS PubMed.
- G. Culioli, V. Mesguiche, L. Piovetti and R. Valls, Biochem. Syst. Ecol., 1999, 27, 665–668 CrossRef CAS.
- J. Hu, B. Yang, X. Lin, X.-F. Zhou, X.-W. Yang and Y. Liu, Handbook of Marine Macroalgae, John Wiley & Sons, Ltd, Chichester, UK, 2011 Search PubMed.
- E. M. C. Alexandre, P. Araújo, M. F. Duarte, V. de Freitas, M. Pintado and J. A. Saraiva, Food Bioprocess Technol., 2017, 10, 886–900 CrossRef CAS.
- E. M. C. Alexandre, P. Araújo, M. F. Duarte, V. de Freitas, M. Pintado and J. A. Saraiva, J. Food Sci. Technol., 2017, 54, 2519–2531 CrossRef CAS PubMed.
- E. Puértolas, G. Saldaña, I. Álvarez and J. Raso, Food Chem., 2011, 126, 1482–1487 CrossRef.
- S. L. Holdt and S. Kraan, J. Appl. Phycol., 2011, 23, 543–597 CrossRef CAS.
- T. N. Zvyagintseva, N. M. Shevchenko, A. O. Chizhov, T. N. Krupnova, E. V. Sundukova and V. V. Isakov, J. Exp. Mar. Biol. Ecol., 2003, 294, 1–13 CrossRef CAS.
- R. Touati, S. A. O. Santos, S. M. Rocha, K. Belhamel and A. J. D. Silvestre, Ind. Crops Prod., 2015, 76, 936–945 CrossRef CAS.
- M. Ayaz, F. Ullah, A. Sadiq, F. Ullah, M. Ovais, J. Ahmed and H. P. Devkota, Chem.-Biol. Interact., 2019, 308, 294–303 CrossRef CAS PubMed.
- M. Simoes, S. Rocha, M. Coimbra and M. Vieira, Med. Chem., 2008, 4, 616–623 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06547d |
| This journal is © The Royal Society of Chemistry 2019 |