Open Access Article
Open Access ArticleWater-soluble lanthanide coordination polymers particles with white-light emission and color tuning†
Kaiqi
Fan
 a,
Xiaobo
Wang
b,
Yongpeng
Ma
a,
Yu
Li
a,
Guanglu
Han
*a,
Zhigang
Yin
*a and
Jian
Song
*c
a,
Xiaobo
Wang
b,
Yongpeng
Ma
a,
Yu
Li
a,
Guanglu
Han
*a,
Zhigang
Yin
*a and
Jian
Song
*c
aSchool of Material and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450002, P. R. China
bJournal Editorial Department, Zhengzhou University of Light Industry, Zhengzhou 450002, P. R. China
cSchool of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: songjian@tju.edu.cn
First published on 9th October 2019
Abstract
Water-soluble polymer particles (PPs) with strong fluorescence emission were prepared from hyperbranched poly(ethylenimine) (PEI) and terpyridine-bearing aldehyde (TPy) via Schiff base reaction and self-assembly in aqueous phase. TPy/PEI PPs were then used to develop a series of luminescent lanthanide coordination polymers particles (Ln-CPPs). The optical properties of these Ln-CPPs are readily modulated over a wide spectrum in water systems. Finally, water-soluble white-emitting Ln-CPPs were achieved by controlling the lanthanide ion stoichiometry. This Ln-CPPs design approach offers a robust pathway for white-luminescent materials in water systems.
In recent decades, dynamic metal coordination polymers (M-CPs) have attracted great interest in catalysis, drug delivery, chemical sensors and bioanalysis applications.1–7 M-CPs are constructed from metal ions and organic ligands with a variety of structures and interesting properties for many potential applications. M-CPs acting as chemical sensors are mainly explored by making use of their luminescence properties.8–10 The luminescent M-CPs can emit a stable and intense luminescent emission, so some substances can be detected by observing changes in luminescence intensity. It is well known that lanthanide ions have high color purity and long lifetime excitation lifetime, and the emission covers the entire visible range of 400 to 700 nm. In particular, Eu(III) and Tb(III) ions can emit intense red and green light, respectively. Lanthanide coordination polymers (Ln-CPs) are promising luminescent materials because lanthanide ions have similar chemical properties and two or more lanthanide ions can be randomly distributed in coordination polymers with metal sites, which can modulate the color and brightness of the emission.11 For the above reasons, Ln-CPs have attracted the attention of many scientists and have been effectively used to design multiple color and white light emitting materials. For example, He and co-workers12 developed a new fluorophore that exhibits white light by combining an Eu(III) moiety (red emission) with an organic ligand (blue and green emission). Ma et al.13 reported a white-light-emitting La(III)/Tb(III)/Eu(III) coordination polymers based on combination of blue-emitting ligand/La(III), green-emitting Tb(III) and red-emitting Eu(III) units. Song et al.14 developed a white-light-emitting compound by doping a Eu(III) ion into the Gd(III) framework.
Meanwhile, the selection of suitable ligands plays a crucial role in the synthesis of Ln-CPs with good luminescent properties, since organic ligands can be used not only as building blocks for the construction of new backbones of Ln-CPs, but also as effective sensitizer for Ln(III) ions.15,16 However, ligands are generally poorly water soluble, which limits the practical sensing application in environmental and biological systems.17 An efficient strategy to promote dispersion in water is to prepare Lanthanide coordination polymer particles (Ln-CPPs) by miniemulsion method, reprecipitation method, and so on.18 Nevertheless, several drawbacks still exist for their preparation and application, such as sophisticated multistep synthetic pathways, use of environmentally unfriendly organic solvents, and the possibility of fluorescence self-quenching in aqueous solution. Therefore, the systematic investigation of water-soluble Ln-CPPs with white-light emission is quite rare. More research studies are urgently needed to accelerate the development of white-light luminescent Ln-CPPs in the water system.
Based on the above considerations, we rationally designed water-soluble polymer particles with blue emission and selected Tb(III)/Eu(III) to construct white-light-emitting Ln-CPPs (Fig. 1). The water-soluble polymer particles were constructed from hyperbranched poly(ethylenimine) (PEI) and terpyridine-bearing aldehyde (TPy) via Schiff base reaction and self-assembly. Structural characterization and luminescence properties in the water system of Ln-CPPs are studied in detail. An important clue could be obtained from the result that Ln-CPPs constructed by terpyridine ligands can maintain their structural and luminescent properties in the water system. This research also provides a basis for realizing the controllability of water-soluble white-light-emitting Ln-CPPs in the future.
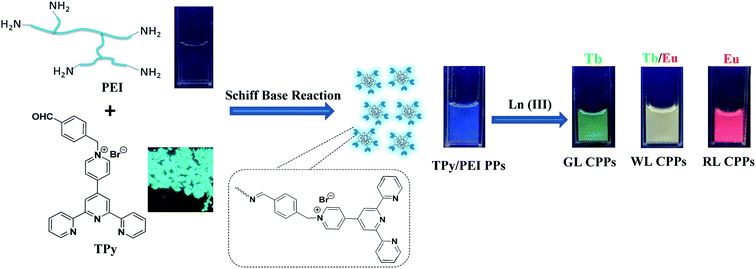 | ||
| Fig. 1 Schematic preparation of TPy/PEI PPs and Ln(III) coordination-based luminescent polymer particles (Ln-CPPs) under UV light (λex = 365 nm). | ||
Synthesis of the TPy/PEI PPs is based on facile Schiff base reaction, which refers to the reaction between primary amine on PEI and aldehyde group on TPy, resulting in a product containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. Moreover, the diluted TPy/PEI PPs solution emits blue fluorescence under a 365 nm UV lamp. Fig. 2A displays the fluorescence excitation and emission spectra of the TPy/PEI PPs solution, and the maximum excitation and emission wavelengths are 330 and 448 nm, respectively. The UV-vis absorption spectra of TPy/PEI PPs, PEI and TPy were shown in Fig. S4.† Compared with TPy, the absorption peak at 250 nm in TPy/PEI PPs solution is weakened, which may be due to the decolorization effect caused by the formation of copolymer by TPy and PEI. In addition, the absorption peak at 335 nm in TPy/PEI PPs solution is attributed to n → π* transitions of C
N bonds. Moreover, the diluted TPy/PEI PPs solution emits blue fluorescence under a 365 nm UV lamp. Fig. 2A displays the fluorescence excitation and emission spectra of the TPy/PEI PPs solution, and the maximum excitation and emission wavelengths are 330 and 448 nm, respectively. The UV-vis absorption spectra of TPy/PEI PPs, PEI and TPy were shown in Fig. S4.† Compared with TPy, the absorption peak at 250 nm in TPy/PEI PPs solution is weakened, which may be due to the decolorization effect caused by the formation of copolymer by TPy and PEI. In addition, the absorption peak at 335 nm in TPy/PEI PPs solution is attributed to n → π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds.18,19 These phenomena indicated TPy/PEI PPs were a newly generated subject.
N bonds.18,19 These phenomena indicated TPy/PEI PPs were a newly generated subject.
The morphologies of TPy/PEI PPs were characterized by transmission electron microscopy (TEM), Fig. S5A† is a TEM image and reveals that the TPy/PEI PPs are monodisperse spherical shape with the size distribution in the range of 26–50 nm. Formation of water-soluble nanoparticles is due to the following factors. In TPy/PEI copolymer, ample amine groups and pyridinium groups are hydrophilic, whereas Schiff base bonds are hydrophobic. As a result, the hyperbranched structure of TPy/PEI copolymer tends to fold and collapse, shrinking and self-assembling into uniform polymer nanoparticles in aqueous medium.18 Many hydrophilic groups on the surface of TPy/PEI PPs make the excellent water dispersity possible. To further explore the chemical composition of TPy/PEI PPs, we performed FT-IR spectra of PEI, TPy, and TPy/PEI PPs (Fig. 2B). Several featured vibration bands at 3284 and 1590 cm−1 in PEI are associated with the stretching vibration of N–H bond, and their intensity is decreased in TPy/PEI PPs, which indicates that some amine groups have reacted with TPy. In addition, another remarkable new peak at 1630 cm−1 was observed in TPy/PEI PPs, which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.20–25 Meanwhile, a new peak at 8.37 ppm was observed in the 1H NMR spectra of TPy/PEI PPs (Fig. S6†), which can be assigned to N
N bond.20–25 Meanwhile, a new peak at 8.37 ppm was observed in the 1H NMR spectra of TPy/PEI PPs (Fig. S6†), which can be assigned to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH protons.26 The monitoring of the aldehyde conversion into imine units can be carried out by measuring the C
CH protons.26 The monitoring of the aldehyde conversion into imine units can be carried out by measuring the C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O/C
O/C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N integral ratio, and the conversion rate of the aldehyde into imine units is 69%. The estimation of the conversion rate from the 1H-NMR spectrum agrees well with the calculation from the weighting measurements with a conversion rate of 73%. These analysis results well demonstrated the formation of Schiff base bonds between TPy and PEI.
N integral ratio, and the conversion rate of the aldehyde into imine units is 69%. The estimation of the conversion rate from the 1H-NMR spectrum agrees well with the calculation from the weighting measurements with a conversion rate of 73%. These analysis results well demonstrated the formation of Schiff base bonds between TPy and PEI.
TPy/PEI PPs possess intrinsic fluorescence, good water solubility, and functional terpyridine structure unit, allowing us to incorporate the Ln(III)-TPy coordination complexes into polymer networks. With the incremental addition of Tb(NO3)3 to the TPy/PEI PPs solution (2% v/v), the TPy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln ratio is 2
Ln ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which produces green-luminescent Ln-CPPs, GL CPPs (τ = 0.35 ms, Φ = 4.3%, CIE coordinates (0.27, 0.36), Fig. S7, S8 and Table S1†). In the corresponding emission spectrum, a decrease in the luminescence intensity of the ligand centred emission band at 448 nm with the concomitant emergence of sharp bands at 489 nm, 544 nm, 583 nm, and 622 nm was observed (Fig. S7†). A decrease in the luminescence intensity of the central emission band of the ligand was observed. These emission bands were assigned to 5D4–7F6, 5D4–7F5, 5D4–7F4, and 5D4–7F3 based transitions, respectively, for Tb(III).27–30 A similar procedure was observed upon addition of Eu(NO3)3 to the TPy/PEI PPs solution with the occurrence of five characteristic Eu(III)-based emission bands having maxima at 579 nm (5D0–7F0), 592 nm (5D0–7F1), 617 nm (5D0–7F2), 649 nm (5D0–7F3), and 687 nm (5D0–7F4), resulting in a clear red-luminescent Ln-CPPs, RL CPPs (τ = 0.81 ms, Φ = 11.3%, CIE coordinates (0.52, 0.29)). These emission spectra demonstrate that Ln3+ (Eu3+ or Tb3+) ions were successfully doped to the TPy/PEI PPs. More importantly, strong fluorescence could still be detected even after these Ln-CPPs were stored for over a week, implying that the coordination between the TPy/PEI PPs and Ln3+ ions is very stable. The interactions between the TPy/PEI PPs and Ln3+ ions were further monitored by FT-IR spectroscopy (Fig. S9†). Strong absorbent bands at 3256, 1549 and 1398 cm−1 in TPy/PEI PPs are attributed to the stretching vibrations of N–H bond.18,19 After the formation of RL CPPs, GL CPPs or WL CPPs using Ln3+ ions, a dramatically red shift appeared, which indicated the coordination of the TPy/PEI PPs to Ln3+ ions. The medium-to-weak bands at 760 cm−1 for RL CPPs, 769 cm−1 for GL CPPs and 765 cm−1 for WL CPPs are observed as additional evidence of the Ln–N formation.31
1, which produces green-luminescent Ln-CPPs, GL CPPs (τ = 0.35 ms, Φ = 4.3%, CIE coordinates (0.27, 0.36), Fig. S7, S8 and Table S1†). In the corresponding emission spectrum, a decrease in the luminescence intensity of the ligand centred emission band at 448 nm with the concomitant emergence of sharp bands at 489 nm, 544 nm, 583 nm, and 622 nm was observed (Fig. S7†). A decrease in the luminescence intensity of the central emission band of the ligand was observed. These emission bands were assigned to 5D4–7F6, 5D4–7F5, 5D4–7F4, and 5D4–7F3 based transitions, respectively, for Tb(III).27–30 A similar procedure was observed upon addition of Eu(NO3)3 to the TPy/PEI PPs solution with the occurrence of five characteristic Eu(III)-based emission bands having maxima at 579 nm (5D0–7F0), 592 nm (5D0–7F1), 617 nm (5D0–7F2), 649 nm (5D0–7F3), and 687 nm (5D0–7F4), resulting in a clear red-luminescent Ln-CPPs, RL CPPs (τ = 0.81 ms, Φ = 11.3%, CIE coordinates (0.52, 0.29)). These emission spectra demonstrate that Ln3+ (Eu3+ or Tb3+) ions were successfully doped to the TPy/PEI PPs. More importantly, strong fluorescence could still be detected even after these Ln-CPPs were stored for over a week, implying that the coordination between the TPy/PEI PPs and Ln3+ ions is very stable. The interactions between the TPy/PEI PPs and Ln3+ ions were further monitored by FT-IR spectroscopy (Fig. S9†). Strong absorbent bands at 3256, 1549 and 1398 cm−1 in TPy/PEI PPs are attributed to the stretching vibrations of N–H bond.18,19 After the formation of RL CPPs, GL CPPs or WL CPPs using Ln3+ ions, a dramatically red shift appeared, which indicated the coordination of the TPy/PEI PPs to Ln3+ ions. The medium-to-weak bands at 760 cm−1 for RL CPPs, 769 cm−1 for GL CPPs and 765 cm−1 for WL CPPs are observed as additional evidence of the Ln–N formation.31
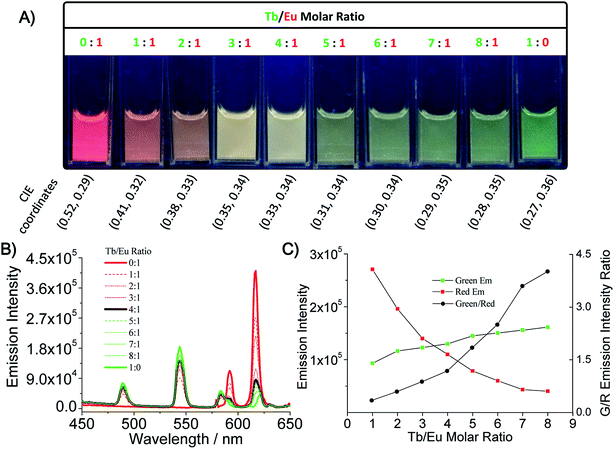
Next we investigated how to modulate the emission of polymer particles by adjusting the stoichiometry of the two lanthanide chromophores. Titration of the Tb/Eu molar ratio resulted in a series of Ln-CPPs with a broad spectrum of emission under UV irradiation (Fig. 3A). By testing the emission spectrum (Fig. 3B and C), it was found that the intensity of the green band at 544 nm increased gradually at the expense of the intensity of the red band at 616 nm as a function of Tb/Eu molar ratio. Interestingly, an intense white-luminescent Ln-CPPs, WL CPPs (CIE coordinates (0.33, 0.34)), were observed when the Eu/Tb molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The smart illumination control strategy here provides a simple design approach for broad-spectrum color adjustment of luminescent polymer materials.
4. The smart illumination control strategy here provides a simple design approach for broad-spectrum color adjustment of luminescent polymer materials.
In conclusion, we created polymer particles with blue emission from PEI and TPy via Schiff base reaction and self-assembly under mild conditions. The structural characterization and the fundamental properties of the TPy/PEI PPs have been studied. Because of the specific structure, the TPy/PEI PPs exhibit excellent water solubility. Furthermore, we have used the TPy/PEI PPs to develop a series of luminescent Ln-CPPs with Eu(III), Tb(III), and mixed Eu(III)/Tb(III) in aqueous medium. The individual Ln-CPPs exhibited bright red (Eu-CPPs) and green (Tb-CPPs) fluorescence upon exposure to UV light (λex = 365 nm). Careful tuning of the stoichiometric ratio of Eu(III) and Tb(III) helped in achieving water-soluble white-emitting Ln-CPPs, which could offer a suitable pathway for preparing white-luminescent materials in water systems. Due to their stability in water, in our next work efforts will be focused on exploring their potential applications in biological and environmental areas as luminescence sensing and quantitative detection materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21671178) and Scientific Research Foundation for the Doctoral Program (No. 2014BSJJ061).Notes and references
- A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540 RSC.
- X. Yu, L. Chen, M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346 RSC.
- G. L. Fiore, S. J. Rowan and C. Weder, Chem. Soc. Rev., 2013, 42, 7278 RSC.
- C. Neaime, C. Daiguebonne, G. Calvez, S. Freslon, K. Bernot, F. Grasset, S. Cordier and O. Guillou, Chem. - Eur. J., 2015, 21, 17466 CrossRef CAS.
- K. Fan, Z. Su, S. Wang, S. Zhao, G. Han, S. Yu, B. Zhang, Z. Yin and J. Song, J. Zhengzhou Univ. Light Ind., Nat. Sci. Ed., 2018, 33, 56 Search PubMed.
- K. Fan, H. Kong, X. Wang, X. Yang and J. Song, RSC Adv., 2016, 6, 80934 RSC.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC.
- K. Fan, J. Yang, X. Wang and J. Song, Soft Matter, 2014, 10, 8370 RSC.
- B. Y. Guan, A. Kushima, L. Yu, S. Li, J. Li and X. W. Lou, Adv. Mater., 2017, 29, 1605902 CrossRef.
- K. Fan, X. Wang, Z. Yin, C. Jia, B. Zhang, L. Zhou and J. Song, J. Mater. Chem. C, 2018, 6, 10192 RSC.
- J. Jia, J. Xu, S. Wang, P. Wang, L. Gao, M. Yu, Y. Fan and L. Wang, CrystEngComm, 2015, 17, 6030 RSC.
- G. He, D. Guo, C. He, X. Zhang, X. Zhao and C. Duan, Angew. Chem., Int. Ed., 2009, 48, 6132 CrossRef CAS.
- M.-L. Ma, C. Ji and S.-Q. Zang, Dalton Trans., 2013, 42, 10579 RSC.
- S. Song, X. Li and Y.-H. Zhang, Dalton Trans., 2013, 42, 10409 RSC.
- M. Reddy and S. Sivakumar, Dalton Trans., 2013, 42, 2663 RSC.
- N. M. Shavaleev, S. V. Eliseeva, R. Scopelliti and J.-C. G. Bünzli, Inorg. Chem., 2010, 49, 3927 CrossRef CAS.
- Y. Liu, J. Ma, C. Xu, Y. Yang, M. Xia, H. Jiang and W. Liu, Dalton Trans., 2018, 47, 13543 RSC.
- S. G. Liu, D. Luo, N. Li, W. Zhang, J. L. Lei, N. B. Li and H. Q. Luo, ACS Appl. Mater. Interfaces, 2016, 8, 21700 CrossRef CAS.
- J. Li, B. Li and M. Liu, Sens. Actuators, B, 2019, 280, 171 CrossRef CAS.
- S. G. Liu, N. Li, Y. Ling, B. H. Kang, S. Geng, N. B. Li and H. Q. Luo, Langmuir, 2016, 32, 1881 CrossRef CAS.
- D. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204 CrossRef CAS.
- W. I. Lee, Y. Bae and A. J. Bard, J. Am. Chem. Soc., 2004, 126, 8358 CrossRef CAS.
- L. Pastor-Pérez, Y. Chen, Z. Shen, A. Lahoz and S. E. Stiriba, Macromol. Rapid Commun., 2007, 28, 1404 CrossRef.
- L. Guo, S. Wu, F. Zeng and J. Zhao, Eur. Polym. J., 2006, 42, 1670 CrossRef CAS.
- I. Lee, S. Kim, S.-n. Kim, Y. Jang and J. Jang, ACS Appl. Mater. Interfaces, 2014, 6, 17151 CrossRef CAS.
- S. A. Lee, G. R. You, Y. W. Choi, H. Y. Jo, A. R. Kim, I. Noh, S.-J. Kim, Y. Kim and C. Kim, Dalton Trans., 2014, 43, 6650 RSC.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Chem. Rev., 2013, 114, 4496 CrossRef.
- H. Agarwalla, K. Jana, A. Maity, M. K. Kesharwani, B. Ganguly and A. Das, J. Phys. Chem. A, 2014, 118, 2656 CrossRef CAS.
- T. S. Mahapatra, S. Chaudhury, S. Dasgupta, V. Bertolasi and D. Ray, New J. Chem., 2016, 40, 2268 RSC.
- T. S. Mahapatra, H. Singh, A. Maity, A. Dey, S. K. Pramanik, E. Suresh and A. Das, J. Mater. Chem. C, 2018, 6, 9756 RSC.
- J. Pei, X. L. Liu, W. L. Yu, Y. H. Lai, Y. H. Niu and Y. Cao, Macromolecules, 2002, 35, 7274 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, synthesis and figures (S1). See DOI: 10.1039/c9ra06476a |
| This journal is © The Royal Society of Chemistry 2019 |