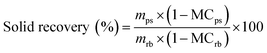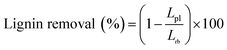Open Access Article
Open Access ArticleEffects of reduced severity of ammonium sulfite pretreatment on bamboo for high cellulose recovery
Liyue
Zhang
,
Yue
Liu
and
Zhiqiang
Li
 *
*
Key Laboratory of National Forestry and Grassland Administration, Beijing Co-built on Bamboo and Rattan Science and Technology, International Centre for Bamboo and Rattan, Beijing 100102, China. E-mail: lizq@icbr.ac.cn
First published on 25th September 2019
Abstract
In this study, the conditions for the pretreatment of bamboo by ammonium sulfite to achieve high cellulose recovery were investigated and optimized. To obtain higher cellulose recovery under low-severity pretreatment conditions such as ammonia sulfite concentration, pretreatment time and pretreatment temperature, three-factor and three-level experiments were designed by the Box–Behnken design based on response surface methodology. The results showed that the cellulose recovery yield after 48 h enzymatic hydrolysis could reach 58.36–59.87%; moreover, the recovered cellulose was pretreated with 20% ammonium sulfite at 150 °C for 6 h, and the obtained yield was in agreement with the predicted yield (58.87%). It was about 13-fold higher than that of the untreated bamboo (4.41%). Pretreatment temperature and ammonia sulfite concentration are significantly important factors than pretreatment time in the design space for achieving high cellulose recovery. Moreover, SEM analysis of the pretreated bamboo substrate under optimized conditions illustrated that the biomass surface had become more rough and porous after pretreatment.
1. Introduction
Due to concerns related to the increasing depletion of petroleum resources and climate change because of greenhouse gas emissions, the development of environmentally friendly and alternative energy sources has attracted special interest. Lignocellulosic biomass is the most abundant renewable and low-cost material with 30–40% cellulose, which is composed of β-D-glucan connected by β-1,4-glycosidic bonds and can be converted to a variety of renewable fuels and value-added chemicals by different technologies.1–3 Thus, lignocellulosic biomass is a primary candidate for alternative energy, and its conversion it into liquid fuel, valuable chemicals and materials has become a research hotspot in recent years.4Bamboo is one of the most abundant renewable lignocellulose resources; it usually contains 40–60% cellulose and 20–32% hemicellulose, which can be converted to fermentable sugars; moreover, it has 20–30% lignin that resists the accessibility of enzymes to cellulose. Therefore, pretreatment is a necessary process in biorefinery to increase the conversion efficiency via the removal of lignin and improvement of enzyme accessibility.5 However, pretreatment is also the most expensive process in the biomass utilization project,6 and the development of low-cost and high value-added pretreatment method is of great importance for the further use of cellulose.
Numerous pretreatment technologies, such as acid-based pretreatments,7 alkali-based pretreatments,8 organosolv pretreatments,9 ionic liquid pretreatments,10 and physically assisted chemical pretreatments,11 have been studied to remove lignin, break the resistance and depolymerize cellulose for fermentable sugar production.
Sulfite pretreatment is a traditional way in the pulping industry for papermaking; it can be conducted in a wide range of pH and temperature, which has been described in a textbook.12 The goal of pulping is to remove lignin as much as possible without the concurrent loss and degradation of hemicellulose and cellulose; this would lead to a pulp with high yield and strength.13 Sulfite process has also been used for pretreating wood chips,12 and it has been first used for softwoods (spruce and red pine) through enzymatic saccharification. The result of the study showed that the sulfite-treated softwood chips could significantly become soft, and the enzymatic cellulose conversion yield of over 90% was achieved.13 Then, alkaline sulfite or acid sulfite has been used for the pretreatment of other lignocellulosic biomass such as bamboo14 and switchgrass.15 In the pretreatment process, the active reagents could be sulfite (SO32−), bisulfite (HSO3−), or a combination of two of the three reagents sulfite (SO32−), bisulfite (HSO3−), and sulfur dioxide (SO2, or H2SO3) depending on the pH value of the pretreatment liquor at pretreatment temperature.16
Ammonium sulfite as a kind of neutral sulfite has been used for papermaking for a long time17 and can be easily decomposed into ammonia and sulfite at about 70 °C. Therefore, the effects of the pretreatment of ammonium sulfite and sodium sulfite can be significantly different. Ammonium sulfite may exhibit the effects of both ammonia and sulfite on the pretreated bamboo. However, only few studies have been reported on the pretreatment of lignocellulosic biomass. It was first used for wheat straw, and the result demonstrated that ammonium sulfite could significantly improve the enzymatic hydrolysis but under a more severe pretreatment condition; since bamboo is more rigid and compact than wheat straw, harsh pretreatment conditions would be needed.18 Hence, considering the pretreatment cost and the final value of the produced glucose, the ammonia sulfite pretreatment conditions of bamboo need to be optimized. The aim of this study was to find low-severity pretreatment parameters (pretreatment temperature, time, and ammonia sulfite concentration) to achieve higher cellulose recovery yield. Milled bamboo was selected as feedstock, and a series of pretreatments were performed based on the Box–Behnken design involving three variables: pretreatment temperature, time, and ammonia sulfite concentration. The total cellulose recovery yield (TCRY) was calculated by enzymatic hydrolysis efficiency obtained after 48 h enzymatic hydrolysis multiplied with the solid recovery rate after the ammonia sulfite pretreatment. Environmental scanning electron microscopy was conducted to compare the structural changes of raw bamboo and the preferred pretreated substrate.
2. Materials and methods
2.1. Raw materials and reagents
The raw moso bamboo was four-year-old, obtained from the Guangxi Zhuang Autonomous Region, China. The air-dried bamboo was ground and passed through a 40–60 mesh sieve (the length ≤ 2 mm); then, the milled bamboo powder was stored in a sealed plastic bag at room temperature until pretreatment. The moisture contents of the ground bamboo powder and pretreated substrates were determined by drying them in an oven at 103 ± 2 °C for 24 h. Chemically pure ammonium sulfite was obtained from Rhawn Reagent Company (Shanghai, China). All the other chemicals in this study were purchased from Sinopharm Chemical Reagent (Beijing) Co., Ltd. (China) and were of analytical grade. Commercial enzymes, i.e. Celluclast 1.5 L (cellulase) and Novozyme 188 (β-glucosidase), were received from Novozymes. All the analysis and experiments were conducted in duplicate.2.2. Pretreatment
Pretreatments were conducted in a 100 mL hydrothermal reactor with a Teflon-lined steel autoclave; herein, 5 g raw bamboo material and 30 mL aqueous ammonium sulfite were mixed and poured into the reactor, and then, the reactor was put in an oven that was already preheated to the target temperature for a certain time period according to the experimental design. After pretreatment, until the mixture was air-cooled to room temperature, the bamboo substrate and spent liquid were separated by vacuum filtration using a water-circulation multifunction vacuum pump. The pretreated substrate was washed several times with distilled water until the washing water became colourless, then weighed, and stored in a sealed plastic bag at 4 °C for further analysis and enzymatic hydrolysis. Some of the pretreated biomass was oven dried, and the moisture content was calculated. The pretreatment experiments were conducted in duplicate. The solid recovery was determined by the following equation:where mps = the mass weight of the pretreated substrate, g; MCps = the moisture content of the pretreated substrate, %; mrb = the mass weight of raw bamboo, g; MCrb = the moisture content of raw bamboo, %.
2.3. Enzymatic hydrolysis
Enzymatic hydrolysis was carried out in a 50 mL plastic centrifuge tube with a certain weighed substrate that contained equivalent to 0.44 g glucan, 20 mL acetic acid/sodium acetate buffer (pH = 4.8), tetracycline (that was placed to prevent microbial growth) and cellulose; β-glucosidase was added at 15 FPU per g glucan and 30 IU per g glucan. The mixture was then put in an incubator shaker at 50 °C with 120 rpm for 48 h. Then, the supernatant was obtained and filtered through a membrane (pore size of 0.45 μm) for the further analysis of glucose content. The data are presented as the average of duplicates from each experimental substrate.The enzymatic hydrolysis efficiency was calculated by the following equation:
| Enzymatic hydrolysis efficiency (%) = (glucose content in the liquid supernatant after enzymatic hydrolysis × 0.9/glucose content in liquid after pretreatment) × 100 |
2.4. Chemical composition analysis
The chemical composition analysis of raw bamboo, pretreated and washed bamboo substrates and spent liquors was conducted by a two-step sulfuric acid hydrolysis method following the National Renewable Energy Laboratory (NREL) analytical procedure.19 The substrate was prehydrolyzed by 72% sulfuric acid for 2 h at room temperature, and then, the mixture was transferred to a conical flask and diluted to 3% sulfuric acid with 287.5 mL distilled water for further hydrolysis for 1 h at 121 °C in an autoclave. The pretreated spent liquor was also hydrolyzed by 3% sulfuric acid under the same condition. The liquid after hydrolysis was obtained for monosaccharide and acid-soluble lignin analysis. The lignin removal was determined by the following equation:where Lpl = the lignin content of the pretreated bamboo substrates, g; Lrb = the lignin content of raw bamboo, g.
The monosaccharide concentrations were analyzed by ion chromatography using an amperometric detector (Metrohm Corporation, Switzerland). Detection was performed at 32 °C using the Hamilton RCX-30 column and Metrosep RP2 guard column. The cellulose content was presented by the glucose concentration. Moreover, the hemicellulose content was the combination of the concentrations of xylose, arabinose, galactose and mannose.
The acid-soluble lignin was analyzed at 205 nm via a UV-visible spectrophotometer using 3% sulfuric acid as the control blank. Acid-insoluble lignin of all the samples was determined by the weight of the residues after the two-step sulfuric acid hydrolysis. Fermentation inhibitors, including acetic acid, formic acid, furfural, levulinic acid and 5-hydroxylmethylfurural (HMF), were degraded from cellulose and hemicellulose during the pretreatment process. They were analyzed using a high-performance liquid chromatograph (HPLC) equipped with the Aminex HPX-87H (30 cm × 7.8 mm) column at the temperature of 25 °C and a UV detector at 210 nm. Eluent was 0.1% phosphoric acid at the rate of 0.7 mL min−1.
2.5. Experimental design and statistical analysis
| Coded levels of factors | Factors | ||
|---|---|---|---|
| Ammonium sulfite concentration (wt%) | Time (h) | Temperature (°C) | |
| Low level (−1) | 10 | 3 | 120 |
| Central level (0) | 20 | 6 | 150 |
| High level (1) | 30 | 9 | 180 |
2.6. Scanning electron microscopy (SEM)
The changes in the surface morphologies and characteristics of the untreated and pretreated bamboo under optimized pretreatment condition were observed using an environmental scanning electron microscope (XL30 ESEM-FEG, Philips, the Netherlands). Dried samples were coated with platinum using an ion sputter coater and placed on aluminum stubs; all the images were obtained at the magnification of 500 times.3. Results and discussion
3.1. The chemical composition of pretreated bamboo substrates and spent liquor at different severity levels
The chemical composition of all the pretreated substrates and the response surface experimental design are listed in Table 2. The pretreatment could change the chemical composition of the raw lignocellulose material and increase the enzymatic hydrolysis efficiency. The content of cellulose could increase from 42.22% to 64.27%, and 46.24% lignin was removed accordingly using a 20% ammonium sulfite for 3 h at 180 °C (trail 11); moreover, the enzymatic hydrolysis efficiency could reach 50.24%. Unfortunately, the solid substrate recovery rate was only 56.21%; this might be because most of the carbohydrates were degraded to the fermentation inhibitor, as presented in Table 3.| Run | Factors | Composition (%) | Removal (%), lignin | Solid recovery rate (%) | Enzymatic hydrolysis efficiency (%) | Cellulose recovery yield (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | T | H | Glucan | Xylan | Lignin | bExp | cPred | ||||
| a CK-control check (untreated raw bamboo). C-concentration (%). T-temperature (°C). H-time (h). b Exp-experimental. c Pred-predicted. | |||||||||||
| CK | — | — | — | 42.22 ± 0.23 | 17.51 ± 0.36 | 29.37 ± 0.02 | — | 100 | 4.41 | — | — |
| 1 | 20 | 6 | 150 | 55.66 ± 1.02 | 17.76 ± 0.12 | 11.58 ± 0.01 | 37.08 | 71.30 | 83.97 | 59.87 | 58.87 |
| 2 | 20 | 6 | 150 | 57.12 ± 0.84 | 18.21 ± 0.48 | 11.09 ± 0.00 | 37.83 | 70.54 | 82.73 | 58.36 | 58.87 |
| 3 | 10 | 6 | 120 | 46.43 ± 0.62 | 19.02 ± 0.29 | 25.87 ± 0.01 | 10.49 | 92.56 | 38.22 | 35.38 | 36.39 |
| 4 | 20 | 9 | 180 | 57.89 ± 0.24 | 15.05 ± 0.35 | 15.99 ± 0.00 | 39.08 | 48.28 | 71.54 | 34.54 | 35.59 |
| 5 | 20 | 6 | 150 | 56.25 ± 0.48 | 17.64 ± 0.27 | 11.32 ± 0.01 | 37.54 | 71.24 | 81.92 | 58.36 | 58.87 |
| 6 | 20 | 6 | 150 | 56.95 ± 0.15 | 17.41 ± 1.12 | 11.43 ± 0.01 | 37.68 | 70.71 | 82.80 | 58.55 | 58.87 |
| 7 | 10 | 3 | 150 | 49.43 ± 0.21 | 19.21 ± 1.14 | 23.81 ± 0.01 | 17.86 | 82.33 | 34.69 | 28.56 | 28.60 |
| 8 | 30 | 6 | 180 | 48.76 ± 0.37 | 14.97 ± 0.89 | 1.22 ± 0.01 | 64.51 | 49.72 | 63.68 | 31.66 | 30.66 |
| 9 | 20 | 9 | 120 | 54.50 ± 1.29 | 17.34 ± 0.57 | 16.30 ± 0.00 | 13.74 | 79.34 | 53.47 | 42.42 | 42.05 |
| 10 | 30 | 9 | 150 | 60.30 ± 0.47 | 20.63 ± 0.31 | 7.82 ± 0.01 | 55.22 | 63.28 | 57.46 | 36.36 | 36.32 |
| 11 | 20 | 3 | 180 | 64.27 ± 1.23 | 17.36 ± 0.34 | 11.24 ± 0.02 | 46.24 | 56.21 | 50.24 | 28.24 | 28.61 |
| 12 | 20 | 3 | 120 | 43.32 ± 1.06 | 17.78 ± 0.41 | 25.27 ± 0.00 | 15.51 | 83.69 | 61.23 | 51.24 | 50.19 |
| 13 | 30 | 3 | 150 | 49.59 ± 0.56 | 19.33 ± 0.51 | 14.60 ± 0.01 | 30.71 | 77.24 | 56.40 | 43.56 | 44.19 |
| 14 | 30 | 6 | 120 | 47.37 ± 0.40 | 19.05 ± 0.21 | 16.80 ± 0.01 | 18.45 | 78.61 | 60.88 | 47.86 | 48.27 |
| 15 | 20 | 6 | 150 | 55.98 ± 1.04 | 18.35 ± 0.16 | 11.74 ± 0.01 | 37.92 | 71.04 | 83.35 | 59.21 | 58.87 |
| 16 | 10 | 6 | 180 | 57.17 ± 1.48 | 2.06 ± 0.20 | 38.78 ± 0.00 | 25.07 | 63.06 | 83.35 | 26.37 | 25.96 |
| 17 | 10 | 9 | 150 | 44.09 ± 0.65 | 4.52 ± 0.51 | 23.04 ± 0.03 | 28.23 | 65.88 | 54.58 | 35.96 | 35.32 |
| Pretreatment | Sum of arabinose and galactose (g L−1) | Xylose (g L−1) | Glucose (g L−1) | Soluble lignin (g L−1) | Formic acid (g L−1) | Acetic acid (g L−1) | Levulinic acid (g L−1) | Furfural (g L−1) | HMF (g L−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | T | H | |||||||||
| 20 | 6 | 150 | 0.48 ± 0.05 | 0.83 ± 0.01 | 2.11 ± 0.30 | 10.89 ± 0.01 | 3.99 ± 0.41 | 21.53 ± 1.34 | 17.69 ± 2.33 | 0.25 ± 0.12 | 0.00 ± 0.00 |
| 20 | 6 | 150 | 0.48 ± 0.08 | 0.79 ± 0.01 | 2.13 ± 0.32 | 11.11 ± 0.02 | 3.79 ± 0.25 | 18.72 ± 2.23 | 8.91 ± 1.05 | 0.18 ± 0.02 | 0.00 ± 0.00 |
| 10 | 6 | 120 | 0.11 ± 0.02 | 1.80 ± 0.14 | 0.08 ± 0.00 | 3.08 ± 0.01 | 1.42 ± 0.08 | 3.55 ± 0.21 | 1.74 ± 0.65 | 0.07 ± 0.01 | 0.37 ± 0.01 |
| 20 | 9 | 180 | 0.02 ± 0.00 | 0.06 ± 0.03 | 0.09 ± 0.00 | 11.47 ± 0.03 | 3.18 ± 0.34 | 6.37 ± 0.58 | 1.99 ± 0.26 | 0.07 ± 0.01 | 0.02 ± 0.00 |
| 20 | 6 | 150 | 0.49 ± 0.01 | 0.75 ± 0.08 | 2.21 ± 0.37 | 10.97 ± 0.01 | 3.38 ± 0.56 | 14.63 ± 0.51 | 6.70 ± 1.02 | 0.23 ± 0.00 | 0.01 ± 0.00 |
| 20 | 6 | 150 | 0.50 ± 0.01 | 0.62 ± 0.02 | 2.09 ± 0.21 | 10.01 ± 0.01 | 4.01 ± 0.16 | 19.49 ± 1.33 | 13.73 ± 2.04 | 0.18 ± 0.00 | 0.00 ± 0.00 |
| 10 | 3 | 150 | 0.40 ± 0.02 | 1.18 ± 0.03 | 0.80 ± 0.04 | 5.25 ± 0.05 | 0.47 ± 0.06 | 2.03 ± 0.31 | 15.81 ± 2.19 | 0.05 ± 0.00 | 0.07 ± 0.00 |
| 30 | 6 | 180 | 0.18 ± 0.03 | 0.28 ± 0.03 | 2.09 ± 0.05 | 18.95 ± 0.04 | 17.00 ± 1.25 | 9.57 ± 0.53 | 35.37 ± 2.67 | 0.05 ± 0.00 | 0.03 ± 0.00 |
| 20 | 9 | 120 | 0.09 ± 0.00 | 0.99 ± 0.01 | 0.13 ± 0.02 | 4.04 ± 0.06 | 1.97 ± 0.24 | 5.73 ± 0.23 | 14.64 ± 1.35 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 30 | 9 | 150 | 0.54 ± 0.02 | 0.53 ± 0.02 | 1.16 ± 0.15 | 16.22 ± 0.04 | 4.63 ± 0.01 | 11.88 ± 1.35 | 26.28 ± 2.35 | 0.02 ± 0.02 | 0.06 ± 0.00 |
| 20 | 3 | 180 | 0.52 ± 0.04 | 0.53 ± 0.01 | 4.47 ± 0.09 | 13.58 ± 0.02 | 13.67 ± 0.21 | 8.62 ± 0.34 | 26.70 ± 1.57 | 0.10 ± 0.0 | 0.01 ± 0.00 |
| 20 | 3 | 120 | 0.12 ± 0.00 | 1.83 ± 0.28 | 0.11 ± 0.01 | 4.55 ± 0.15 | 2.01 ± 0.34 | 9.12 ± 1.23 | 6.15 ± 0.95 | 0.08 ± 0.00 | 0.20 ± 0.00 |
| 30 | 3 | 150 | 0.20 ± 0.00 | 0.75 ± 0.01 | 0.17 ± 0.24 | 8.02 ± 0.04 | 4.02 ± 0.02 | 12.61 ± 1.55 | 8.88 ± 1.25 | 0.11 ± 0.00 | 0.17 ± 0.00 |
| 30 | 6 | 120 | 0.03 ± 0.00 | 1.19 ± 0.07 | 0.05 ± 0.00 | 5.42 ± 0.09 | 13.02 ± 1.58 | 9.47 ± 0.84 | 0.54 ± 0.11 | 0.28 ± 0.00 | 0.01 ± 0.00 |
| 20 | 6 | 150 | 0.47 ± 0.06 | 0.82 ± 0.05 | 2.22 ± 0.13 | 11.08 ± 0.06 | 3.27 ± 0.28 | 16.40 ± 2.01 | 13.89 ± 1.82 | 0.20 ± 0.00 | 0.00 ± 0.00 |
| 10 | 6 | 180 | 0.02 ± 0.00 | 0.26 ± 0.01 | 0.14 ± 0.00 | 7.36 ± 0.24 | 0.58 ± 0.09 | 6.58 ± 0.63 | 4.53 ± 0.56 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| 10 | 9 | 150 | 0.39 ± 0.01 | 0.52 ± 0.00 | 4.69 ± 0.36 | 8.29 ± 0.02 | 1.35 ± 0.08 | 3.08 ± 0.01 | 8.73 ± 1.62 | 0.01 ± 0.00 | 0.01 ± 0.00 |
The contents of arabinose, galactose, xylose, glucose, soluble lignin and fermentation inhibitor in all the pretreated liquors are listed in Table 3. It was clearly found that the content of carbohydrate was low in the spent liquor, and the soluble lignin content was in accordance with the enzymatic hydrolysis efficiency; this meant that the content of soluble lignin in the spent liquid was increased significantly (from 3.08% to 16.22%) after pretreatment, and the higher lignin removal could result in higher enzymatic hydrolysis efficiency.
Furfural and HMF were obtained from the dehydration of pentoses and hexoses, respectively; acetic acid was mainly obtained from the acetyl groups on hemicelluloses; moreover, levulinic and formic acids were the products of the successive decomposition of HMF.21 The contents of formic acid, acetic acid, and levulinic acid were much higher than those of furfural and HMF; this might be due to the long pretreatment time.
The chemical component in the spent liquor was extremely complicated according to the HPLC analysis. The spent liquor contained different kinds of monosaccharides, fermentation inhibitors, extractives, waxes, lignosulfonates, unreacted ammonium sulfite and so on. The cost of solvent recovery was really high. Normally, the spent liquor is used as a fertilizer.22 To fully utilize the pretreated liquor, it was subjected to pyrolysis with hydrogen to break lignin into flammable gas, whereas ammonium sulfite was decomposed into sulfur dioxide and ammonia.23
3.2. Model fitting and statistical analysis
The results of the experimental design are listed in Table 2. They clearly indicate that the total cellulose recovery yield for the 17 trails ranged from 26.37% to 59.87%, whereas the corresponding delignification rate was in the range from 10.49% to 64.51%. Significantly low reaction severity could cause higher solid recovery rate (as trial 3). Trial 1 with the solid recovery rate of 71.30% and enzymatic hydrolysis efficiency of 83.97% had the highest cellulose recovery yield of 59.87%. Trial 8 had the highest lignin removal rate of 64.51% but the lower enzymatic hydrolysis efficiency of 63.68%; this might be because most of the carbohydrates were degraded and transferred to the fermentation inhibitor, as presented in Table 3.
Y
TCRY was set as the responsive variable for modelling to reach the highest cellulose recovery yield, and the final quadratic polynomial model is shown by the following equation:
| YTCRY = +58.87 + 4.15A − 0.29B − 7.01C − 3.65AB − 1.80AC + 3.78BC − 13.28A2 − 9.48B2 − 10.28C2 |
In the abovementioned equation, positive coefficients indicated a synergistic effect, whereas negative signs suggested an antagonistic effect. The analysis of variance (ANOVA) showed the possible effects of all variables on YTCRY. The fit of the model was evaluated by comparing the R2 and the adjusted Radj2. The statistical significance was determined by an F-test, which should not be less than 0.80, and the P-value, which should be less than 0.05 for a good fit of the developed model.24,25 The ANOVA results of the developed model are listed in Table 4. The model fitted the data with an R2 of 0.9969 for YTCRY, which suggested a strong correlation coefficient between all the data. The model parameters, such as temperature and concentration, showed a high level of significance (p < 0.0001), whereas the time implied a low level of significance (p = 0.4507 > 0.05), and the quadratic terms A2, B2, and C2 and the interactions of AB, BC, and AC were significant with the P-value less than 0.05. The value of the predicted R-squared (Rpre2 = 0.9613) significantly suggested the strong predictive ability of the model. The value of lack of fit (p = 0.0954 > 0.05) further indicated model adequacy. In addition, the experimental values versus the predicted values showed a high quality of fit throughout the design space (Table 2).
| Source | Sum of squares | DF | Mean square | F value | P-value prob. > F | Significance |
|---|---|---|---|---|---|---|
| a DF-degree of freedom. A-concentration. B-time. C-temperature. **-denotes very significance difference at P < 0.01. — denotes not significance at P > 0.05. | ||||||
| Model | 2398.84 | 9 | 266.45 | 252.69 | <0.0001 | ** |
| A | 137.53 | 1 | 137.53 | 130.38 | <0.0001 | ** |
| B | 0.67 | 1 | 0.67 | 0.64 | 0.4507 | — |
| C | 393.26 | 1 | 393.26 | 372.82 | <0.0001 | ** |
| AB | 53.29 | 1 | 53.29 | 50.52 | 0.0002 | ** |
| AC | 12.92 | 1 | 12.92 | 12.25 | 0.0100 | ** |
| BC | 57.15 | 1 | 57.15 | 54.18 | 0.0002 | ** |
| A 2 | 742.14 | 1 | 742.14 | 703.57 | <0.0001 | ** |
| B 2 | 378.70 | 1 | 378.70 | 359.02 | <0.0001 | ** |
| C 2 | 444.64 | 1 | 444.64 | 421.53 | <0.0001 | ** |
| Residual | 7.38 | 7 | 1.05 | |||
| Lack of fit | 5.65 | 3 | 1.88 | 4.33 | 0.0954 | Not significant |
| Pure error | 1.74 | 4 | 0.43 | |||
| Corrected total | 2406.22 | 16 | ||||
| R 2 | 0.9969 | |||||
| R adj 2 | 0.9930 | |||||
| R pred 2 | 0.9613 | |||||
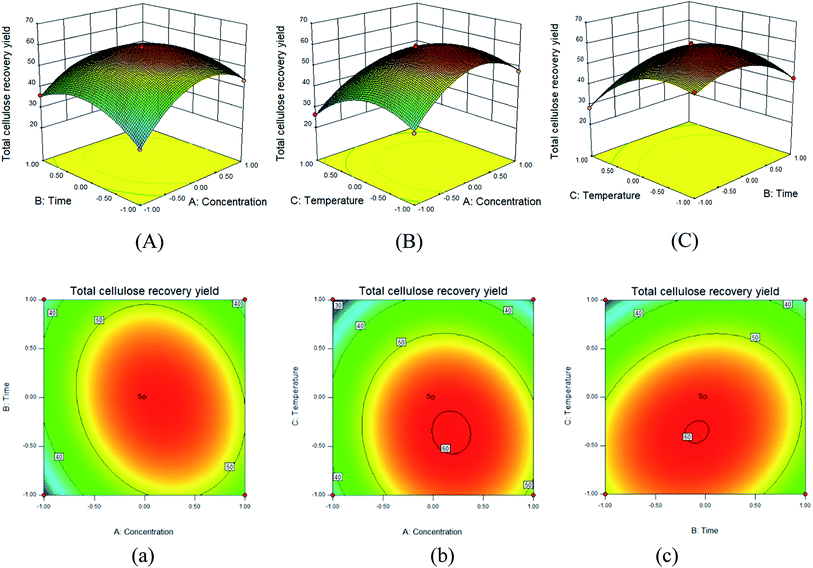
3.3. Impact of variables on the response
The 3D-response surfaces for the impacts of variables and their mutual effects on YTCRY are shown in Fig. 1(A–C), and the corresponding contour plots are presented in Fig. 1(a–c).The slope of the response surface could indicate the extent of the response under various pretreatment conditions, whereas the steep curved surface indicated that the response value was very sensitive to the relevant pretreatment conditions.20Fig. 1(A–C) show the effects of two parameters on the total cellulose recovery yield. The three steep slopes clearly suggested the relatively strong interaction effect between all the pretreatment conditions. Moreover, the shape of the contour plot could reflect the intensity of the interaction effects. In general, an oval shape revealed a strong interaction of the two factors, which was opposite to the case of the circular shape;26 the contour plot showed an apparent oval shape, which was in agreement with the result of the 3-D response surface.
3.4. Model validation
According to the model analysis, the optimized pretreatment conditions were a 20% ammonium sulfite concentration and the temperature of 150 °C with the pretreatment time of 6 h; moreover, the best combination of pretreatment parameter level for the model predicting the total cellulose recovery yield of around 59.87% was tested experimentally for model validation, and the result was 58.56%, which was in good agreement with the predicted value. In addition, the difference between the predicted and the actual values was within 3.3% (run 8) for all the validation treatments (Table 2).3.5. The surface structure of raw bamboo and the preferred substrate
The SEM images of the untreated bamboo and the pretreated bamboo substrate under optimal pretreatment conditions are shown in Fig. 2. The surface structure of the raw bamboo demonstrated more rigid and ordered fibrils. The fiber was slightly distorted, and the surfaces appeared rough, which increased the surface area and might contribute to the accessibility of enzyme to cellulose and improve the enzymatic hydrolysis efficiency.274. Conclusion
This study was performed to quantify the effect of different pretreatment temperatures, time durations, and ammonium sulfite concentrations on the total cellulose recovery yield during pretreatment by the Box–Behnken design based on the response surface methodology. The total cellulose recovery yield after the 48 h enzymatic hydrolysis (with the cellulose loading of 15 FPU per g glucan) could reach around 59.87% with the preferred pretreatment condition of 20% ammonium sulfite at 150 °C for 6 h.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Key Research and Development Program of China (Project No. 2017YFD0600805).References
- L. Jiang, A. Zheng, Z. Zhao, F. He, H. Li and N. Wu, Bioresour. Technol., 2015, 200, 8–13 CrossRef PubMed
.
- K. Zhang, Z. Pei and D. Wang, Bioresour. Technol., 2016, 199, 21–33 CrossRef CAS
.
- M. Rastogi and S. Shrivastava, Renewable Sustainable Energy Rev., 2017, 80, 330–340 CrossRef
.
-
L. R. S. Moreira, N. vG. Milanezi and E. X. F. Filho, in Routes to Cellulosic Ethanol, ed. M. S. Buckeridge and G. H. Goldman, Springer, New York, 2011, ch. 6, pp. 73–96 Search PubMed
.
- Y. Sun and J. Cheng, Bioresour. Technol., 2003, 83, 1–11 CrossRef
.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef CAS
.
- J. R. Jensen, J. E. Morinelly, K. R. Gossen, M. J. Brodeur-Campbell and D. R. Shonnard, Bioresour. Technol., 2010, 101, 2317–2325 CrossRef CAS
.
- N. S. Pooja, M. S. Sajeev, M. L. Jeeva and G. Padmaja, 3 Biotech, 2018, 8, 69 CrossRef CAS
.
- Z. Li, Z. Jiang and B. Fei, BioResources, 2012, 7, 3452–3462 CAS
.
- A. P. Dadi, S. Varanasi and C. A. Schall, Biotechnol. Bioeng., 2006, 95, 904–910 CrossRef CAS PubMed
.
- Z. Li, B. Fei and Z. Jiang, BioResources, 2014, 10, 1037–1047 Search PubMed
.
-
J. R. G. Bryce, in Pulp and Paper: Chemistry and Chemical Technology, ed. J. P. Casey, John Wiley & Sons, New York, 3rd edn, 1981, pp. 291–376 Search PubMed
.
- J. Y. Zhu, X. J. Pan, G. S. Wang and R. Gleisner, Bioresour. Technol., 2009, 100, 2411–2418 CrossRef CAS
.
- Z. Li, B. Fei, D. Qin and Z. Jiang, J. Biobased Mater. Bioenergy, 2017, 11, 521–526 CrossRef CAS
.
- D. S. Zhang, Q. Yang, J. Y. Zhu and X. J. Pan, Bioresour. Technol., 2013, 129, 127–134 CrossRef CAS
.
-
O. Ingruber, in Sulfite Science and Technology, ed. O. Ingruber, M. Kocurek and A. Wong, TAPPI/CPPA, Atlanta, 3rd edn, 1985, pp. 3–23 Search PubMed
.
- W. Hull, B. Smith, J. Hull and W. Holzer, Ind. Eng. Chem., 1954, 46, 1546–1557 CrossRef CAS
.
- G. Qi, L. Xiong, L. Tian, M. Luo, X. Chen, C. Huang, H. Li and X. Chen, Sustain Energy Techn., 2018, 29, 12–18 Search PubMed
.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, NREL/TP-510-42618, National Renewable Energy Laboratory, Golden, 2008.
- S. Chi, G. Yu, X. Zhang, Y. Zhang, C. Liu, Z. Li, B. Li and Q. Cui, Bioresour. Technol., 2019, 14, 4603–4622 CAS
.
- Z. Li, Q. Yang, Z. Jiang, B. Fei, Z. Cai and X. Pan, J. Biobased Mater. Bioenergy, 2012, 6, 544–551 CrossRef CAS
.
- S. Y. Bai and B. Y. Liu, Heilongjiang Pulp & Paper, 2008, 36(4), 24–27 Search PubMed
.
- A. V. Bridgwater, D. Meier and D. Radlein, Org. Geochem., 1999, 30, 1479–1493 CrossRef CAS
.
- A. Ruangmee and C. Sangwichien, Energy Convers. Manage., 2013, 73, 381–388 CrossRef CAS
.
- T. Auxenfans, S. Buchoux, D. Larcher, G. Husson, E. Husson and C. Sarazin, Energy Convers. Manage., 2014, 88, 1094–1103 CrossRef CAS
.
- H. Lu, C. Lv, M. Zhang, S. Liu, J. Liu and F. Lian, Energy Convers. Manage., 2017, 132, 251–260 CrossRef CAS
.
- K. E. Kang, G. T. Jeong, C. Sunwoo and D. H. Park, Bioprocess Biosyst. Eng., 2012, 35, 77–84 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |