Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sequestration of trivalent americium and lanthanide nitrates with bis-lactam-1,10-phenanthroline ligand in a hydrocarbon solvent†
Yana Karslyan ab,
Frederick V. Sloop
ab,
Frederick V. Sloop b,
Laetitia H. Delmau
b,
Laetitia H. Delmau c,
Bruce A. Moyer
c,
Bruce A. Moyer b,
Ilja Popovs
b,
Ilja Popovs b,
Alena Paulenova
b,
Alena Paulenova a and
Santa Jansone-Popova
a and
Santa Jansone-Popova *b
*b
aLaboratory of Transuranic Elements, Department of Chemistry, Oregon State University, Corvallis, OR 97331, USA
bChemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA. E-mail: jansonepopos@ornl.gov
cIsotope and Fuel Cycle Technology Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
First published on 23rd August 2019
Abstract
Efficient separation of minor actinides and lanthanides from used nuclear fuel could potentially lead to the development of sustainable nuclear fuel cycles. Herein, we report an in-depth study on selectivity and speciation in the extraction of the trivalent minor actinide Am and rare earth metal ions with a pre-organized phenanthroline-based ligand in a hydrocarbon solvent system relevant to nuclear fuel reprocessing. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal complexes dominate the speciation in the organic solvent over a range of ligand-to-metal concentrations, as evidenced by experimental data and supported by modeling.
1 ligand-to-metal complexes dominate the speciation in the organic solvent over a range of ligand-to-metal concentrations, as evidenced by experimental data and supported by modeling.
Introduction
The minor actinides (MA) americium, curium, and neptunium have high radiotoxicities and americium is the main heat contributor present in used nuclear fuel after the fission products 137Cs and 90Sr have decayed in the first 300 years. Lanthanides (Ln) on the other hand, being neutron absorbers, hamper the transmutation of actinides and must be removed for MA recycling in mixed oxide fuel. The selective partitioning and transmutation of minor actinides following the Plutonium Uranium Redox Extraction (PUREX) process will therefore decrease the heat generation and long-term radiotoxicity of the used nuclear fuel, enabling safer long-term storage.1 A single process of MA separation is desired ideally; however, due to similar chemical and physical properties of MA and Ln, it is rather a challenging task to realize. Alternatively, a coextraction of MA and Ln by a mixture of neutral and acidic extractants in organic solvent, followed by selective back-extraction of MA using aqueous aminopolycarboxylate complexants, has been recognized as a robust and effective process to achieve mutual separation.2 The selective isolation of f-elements is dictated by the modes of interaction with the ligands, and the choice of media used in liquid–liquid separation system. According to hard and soft Lewis acid and base theory, the covalency in complexes between 5f element and ligand is greater than in 4f element complexes and is strongly dependent on the nature of ligand.3 The number of potential coordination sites and the level of preorganization in ligand are important factors that can influence the affinity and selectivity towards particular f-block metal ions. In comparison to conformationally flexible chelators, preorganized multidentate ligands show improved affinity for f-elements, owing to convergent positioning of the donor atoms at the metal binding site.4–8 Performance of new unsaturated heterocyclic extractants is usually evaluated in polar chlorinated or aromatic solvents. This is in large part due to limited solubility in non-polar aliphatic diluents such as n-dodecane, Isopar, and kerosene, despite their low toxicity, low viscosity, high flash point, and unsuitability for an industrial-scale extractions.9Phenanthroline-based ligands have received increased amount of attention due to their excellent ability to separate trivalent actinides and lanthanides.6,10 Previously, we have demonstrated application of the preorganized bis-lactam-1,10-phenanthroline (BLPhen) ligand in efficient separation of americium from europium7 and showing preferential selectivity for light-versus-heavy trivalent lanthanides.8 However, unsatisfactory solubility of BLPhen ligand 1 in non-polar diluents required the use of 1,2-dichloroethane-based extraction system. This, in turn, resulted in complete extraction of both Am and Eu from aqueous solution due to abnormally high binding affinity of this ligand, hampering the elucidation of both selectivity and speciation in the organic phase. Herein, we present a study that addresses these shortcomings by using a new branched-chain ligand from this family, BLPhen ligand 2. This ligand has a markedly better solubility in hydrocarbon diluent, therefore allowing its performance to be evaluated with the emphasis on speciation of f-element complexes with BLPhen ligand in more appropriate hydrocarbon solvent, which is significantly less toxic and is not a suspected carcinogen.
Results and discussion
The phenanthroline-based ligands usually have very limited solubility in hydrocarbon solvents due to increased polarity and likely π–π stacking due to the presence of extended π-surfaces. The solubility in non-polar diluents can be significantly improved by introducing long and branched alkyl substituents that disrupt the π–π stacking. Indeed, the introduction of a 2-octyldecyl group on the two lactam nitrogens resulted in ligand 2, shown in Fig. 1, which in comparison to bis-lactam-1,10-phenanthroline ligand with n-hexyl substituents (1),7 showed markedly improved solubility in organic solvents.11The solubility and third-phase formation compatibility tests were performed to determine the concentration limits of ligand 2 and solvent modifier in the hydrocarbon diluent Isopar L. As can be seen in Fig. 2a, the solubility behaviour follows the expectations of classical solution theory12 in that an increase in solubility of 2 is manifested with the addition of solvent modifier Exxal 13 (11-methyl-1-dodecanol with >87 wt% purity). To increase ligand 2 concentration in Isopar L, an addition of higher volume fraction (vol%) of solvent modifier is necessary to attain a homogeneous solution (blue region in Fig. 2a). Although at a lower concentration (5 mM), 2 completely dissolves in Isopar L–Exxal 13 (2.5 vol%) mixture, upon contact with 1 mM Ln(III) in 3 M nitric acid solution, the organic phase undergoes phase disengagement forming a precipitate (green region in Fig. 2a). The orange region in Fig. 2a represents the incomplete dissolution of 2 in Isopar L–Exxal 13 mixture. The higher concentration of modifier that contains a hydrogen-bond accepting and donating hydroxyl group helps to solubilize both the free ligand and the ligand–metal nitrate complexes in the organic phase. Hence, the increase in volume fraction of modifier results in co-extraction of water, which in turn can influence the separation of Ln(III). We demonstrate this with Nd(III) and Eu(III) extraction using ligand 2 in Isopar L–Exxal 13/nitric acid system. As presented in Fig. 2b, a slight decrease in distribution ratios for Nd(III) is observed when increasing Exxal 13 concentration above 10 vol% and with it the water concentration in organic phase. Therefore, to prevent the precipitation, while reaching an adequate ligand solvation within the range of ligand concentrations feasible for extraction, and to attain the most efficient separation of f-block metal ions, Isopar L with 10 vol% of Exxal 13 was chosen as the optimal organic medium for the evaluation of ligand 2 complexation with 241Am(III) and Ln(III) nitrates.
The performance evaluation of ligand 2 in this solvent system revealed its strong preference for both 241Am and light Ln(III). First, a solution of 4 mM ligand 2 in Isopar L with 10 vol% Exxal 13 was contacted with 3 M nitric acid containing 0.1 mM Eu(NO3)3 spiked with 241Am and 152Eu radiotracers. The results revealed that both metals are equally well extracted into the organic phase by ligand 2 (DAm = 268 ± 13, DEu = 279 ± 14), with no apparent selectivity for one or the other. While this result may seem surprising in the view of the presence of two N-donor atoms in ligand 2, we had shown earlier7 that the selectivity between trivalent 4f- and 5f-metals results from an interplay involving structural and electronic-structure effects in the family of bis-amide phenanthroline ligands. In fact, by lowering the electron density on the amide oxygens, by the virtue of incorporating electron-withdrawing groups on the lactam rings, and ultimately increasing the softness of the oxygen donors, excellent selectivity for Am(III) over Eu(III) was achieved.7
The pre-organization of the four-donor binding site in 2 leads to an efficient differentiation among the 14 lanthanides tested (excluding Pm) and yttrium, with strong preference for metal ions with larger ionic radii. As shown in Fig. 2c, under loading conditions ligand 2 nearly quantitatively extracts La and Ce, showing steady decrease in extraction from Pr to Dy, and limited complexation with Ho–Lu. These results are consistent with ligand 1 performance in 1,2-dichloroethane–nitric acid system, implying that the size of the binding cavity is solely responsible for the observed intra-lanthanide selectivity.8 This also highlights the efficiency of this ligand for recovery of Ln, since La alone can comprise up to 30% by mass of all Ln present in used nuclear fuel.13 Other neutral extractants, such as TODGA and CMPO show the reversed selectivity trend in the extraction across the trivalent Ln series.14
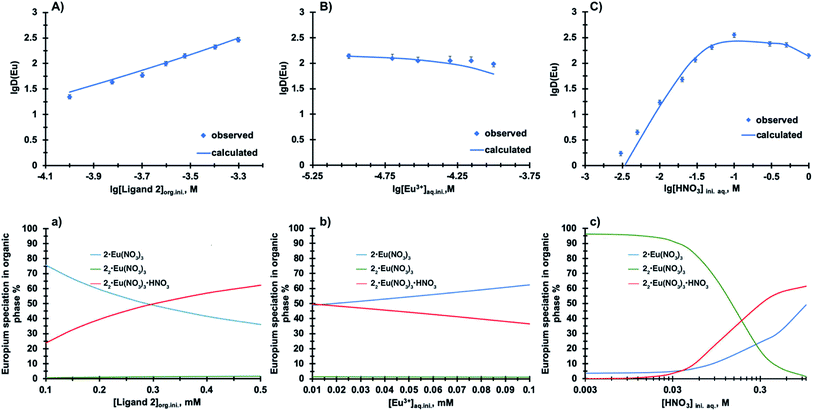
To probe the stoichiometry of the complexes in the organic phase, we chose two metal ions Nd(III) and Tm(III) representing early and late lanthanides. The ligand 2 concentration was varied from 0.5 mM to 2 mM at constant 1 mM Ln(III) loading to cover a range of ligand to metal ratios. The results presented in Fig. SI-7† show that the slope for Nd(III) and Tm(III) is 2.02 and 2.07 at 1–2 mM 2, respectively, suggesting a uniform complexation of 2 with lanthanides across the series. To support this finding, the extraction equilibrium analysis was performed based on 152Eu distribution ratios summarized in Table SI-4.† These values were obtained from three extraction series where the concentration of components (ligand 2, nitric acid and Eu3+) was varied one at a time. Utilizing the distribution ratios as input numbers, the equilibrium constants were modelled for plausible complexes formed during Eu3+ extraction using the solvent-extraction modelling program SXLSQI.15 The results from SXLSQI model with five chemical equilibria eqn (1)–(5) are compared to the experimental values in Fig. 3(A–C). The comparison between calculated values and experimental data results in agreement factor of 1.93.16 From the multiple chemical equilibria involved in the Eu3+ extraction, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to Eu(NO3)3 complexes dominate the speciation in the organic phase that is composed of compound 2 in n-dodecane with 10 vol% 1-octanol.17 Note that the formation of the nitric acid adducts in eqn (1) and (2) is inferred, as acid extraction by 2 was not measured.
1 ligand to Eu(NO3)3 complexes dominate the speciation in the organic phase that is composed of compound 2 in n-dodecane with 10 vol% 1-octanol.17 Note that the formation of the nitric acid adducts in eqn (1) and (2) is inferred, as acid extraction by 2 was not measured.
 | (1) |
 | (2) |
 | (3) |
 | (4) |
 | (5) |
At a low ligand 2 concentration, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to Eu(NO3)3 complexes are the predominant species present in the solution (eqn (3), Fig. 3a). Formation of 2
1 ligand to Eu(NO3)3 complexes are the predominant species present in the solution (eqn (3), Fig. 3a). Formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to Eu(NO3)3 complexes increases with increase in ligand 2 concentration at constant metal ion loading (eqn (4) and (5)). The reverse trend is observed with increasing Eu(NO3)3 concentration, where likely one ligand molecule dissociates from the 2
1 ligand to Eu(NO3)3 complexes increases with increase in ligand 2 concentration at constant metal ion loading (eqn (4) and (5)). The reverse trend is observed with increasing Eu(NO3)3 concentration, where likely one ligand molecule dissociates from the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex to bind with an incoming metal ion (Fig. 3b). At a low to moderate nitric acid concentrations (Fig. 3c), the 2
1 complex to bind with an incoming metal ion (Fig. 3b). At a low to moderate nitric acid concentrations (Fig. 3c), the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand 2 to Eu(NO3)3 complexes represent the dominant species in the organic phase, but with the increase in aqueous acidity, the speciation changes and is dominated by 1
1 ligand 2 to Eu(NO3)3 complexes represent the dominant species in the organic phase, but with the increase in aqueous acidity, the speciation changes and is dominated by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, as well as 2
1 complexes, as well as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HNO3 adduct. These findings are supported by the other phenanthroline-based ligand systems, where the formation of 2
1 HNO3 adduct. These findings are supported by the other phenanthroline-based ligand systems, where the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ion complexes has been elucidated.6,18
1 ligand to metal ion complexes has been elucidated.6,18
Conclusions
The obtained results show that the new bis-lactam-1,10-phenanthroline (BLPhen) ligand efficiently extracts trivalent Am and light Ln from aqueous acidic medium into aliphatic solvent. The analysis comparison of the SXLSQI model with the experimentally measured data confirms the presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ion complex species in the organic phase of Isopar L–Exxal 13/nitric acid system. Our results present a deeper insight into relations between the performance, functionality and structure of phenanthroline-based ligands, and will guide the rational design of new, tailor-made ligands with desirable properties for f-element extraction and separation, ushering the development of sustainable nuclear fuel cycles.
1 ligand to metal ion complex species in the organic phase of Isopar L–Exxal 13/nitric acid system. Our results present a deeper insight into relations between the performance, functionality and structure of phenanthroline-based ligands, and will guide the rational design of new, tailor-made ligands with desirable properties for f-element extraction and separation, ushering the development of sustainable nuclear fuel cycles.
Experimental
Synthesis
To BLPhen precursor (1.0 g, 2.22 mmol) dissolved in dry DMF (22 mL, 0.1 M) was added NaH (0.22 g, 5.55 mmol) under inert atmosphere. After 20 minutes, 2-octyl-1-bromododecane (2.26 mL, 6.66 mmol) was added and the reaction mixture was heated at 85 °C for 12 hours. Afterwards, the reaction mixture was allowed to cool to room temperature, before being diluted with water (∼0.01 M). The formed precipitate was filtered off, washed with water and dried to yield crude product. The product was purified on CombiFlash Rf automated flash chromatography system using normal phase silica gel as a stationary phase and gradient from 0% to 20% of MeOH in CH2Cl2 as an eluent system. The product was purified one more time using isocratic 3% of MeOH in CH2Cl2 as an eluent system. Compound 2 was obtained as brown oil (0.40 g, 19% yield). 1H NMR (400 MHz, CDCl3) 8.05 (s, 2H), 7.73 (s, 2H), 4.50–4.35 (m, 2H), 3.80–3.72 (m, 2H), 3.55–3.47 (m, 2H), 2.85–2.72 (m, 2H), 2.61–2.54 (m, 2H), 2.37–2.29 (m, 2H), underneath 2.2–2.1 signal (m, 2H), 2.04–1.92 (m, 2H), 178–1.66 (m, 4H), 1.66–1.57 (m, 2H), 1.54–1.46 (m, 2H), 1.4–1.1 (m, 56H), 0.91–0.78 (m, 12H). 13C NMR (100.66 MHz, CDCl3) 160.6 (CH0), 145.8 (CH0), 144.1 (CH0), 135.9 (CH1), 134.5 (CH0), 130.2 (CH0), 127.2 (CH1), 63.7 (CH1), 51.0 (CH2), 48.9 (CH1), 44.7 (CH1), 42.3 (CH1), 35.3 (CH1), 32.0 (CH2), 31.8 (CH2), 31.6 (CH2), 30.3 (CH2), 30.2 (CH2), 29.8 (CH2), 29.5 (CH2), 29.2 (CH2), 27.7 (CH2), 26.6 (CH2), 22.8 (CH2), 14.3 (CH3). Additionally, the proton and carbon assignments were verified with COSY, HMQC and NOESY NMR experiments. HRMS m/z [M + H]+ and [2M + K + H]2+, calculated for C64H98N4O2, 955.7763 and C128H197KN8O4, 974.75420; found 955.7758 and 974.75080, respectively.Solvent extraction experiments
(A) All experimental work with 241Am was conducted in radiological facility. A 500 microliter (μL) aqueous phase consisting of 0.1 mM Eu(NO3)3 in 3 M HNO3-spiked with 5 μL of 1.85 × 103 kBq each 241Am and 152Eu-was contacted with an equal volume of organic phase containing 1.0 mM of ligand 2 in Isopar L with 10 vol% Exxal 13. The two phases were contacted at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of organic/aqueous by end-over-end rotation in individual 1.8 mL capacity snap-top Eppendorf tubes using a rotating wheel in an air box set at 25.5 °C ± 0.5 °C. Contacts were performed in duplicate using contact time of 1 hour. Following contacting, the duplicate samples were subjected to centrifugation at 1811 × g for five minutes at 25 °C to separate the phases. Each phase was then subsampled, with 250 μL volumes isolated from each phase and transferred to polypropylene tubes for counting using a Canberra Gamma Analyst Integrated Gamma Spectrometer.
1 ratio of organic/aqueous by end-over-end rotation in individual 1.8 mL capacity snap-top Eppendorf tubes using a rotating wheel in an air box set at 25.5 °C ± 0.5 °C. Contacts were performed in duplicate using contact time of 1 hour. Following contacting, the duplicate samples were subjected to centrifugation at 1811 × g for five minutes at 25 °C to separate the phases. Each phase was then subsampled, with 250 μL volumes isolated from each phase and transferred to polypropylene tubes for counting using a Canberra Gamma Analyst Integrated Gamma Spectrometer.
(B) Equal volumes of aqueous and organic phases, 750 microliter (μL) each, were contacted for 1 hour in individual 1.8 mL capacity snap-top Eppendorf tubes using a rotating wheel set at 60 rpm and placed in an air box set at 25 °C ± 0.5 °C. Following contacting, the samples were subjected to centrifugation for 5 minutes at 1811 × g. Then, the samples were subsampled by removing 600 μL of the organic phase, which was subjected to Karl Fischer titration. The interface layer, if any, was removed using a plastic pipette, prior to subsampling the lower aqueous phase. The remaining aqueous layer was then analyzed by ICP-OES and/or IC to determine the metal and nitrate concentrations in the organic phase. All extractions were performed in duplicate.
Associated content
Experimental and modeling details and NMR data (PDF).Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This research was sponsored by the Fuel Cycle Research and Development program, Office of Nuclear Energy, U.S. Department of Energy. Authors thank Dr Neil J. Williams for performing IC experiments and Dr Aleksandr S. Ivanov for generating a structure for TOC graphic. This manuscript has been authored in part by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).Notes and references
- (a) J. Veliscek-Carolan, J. Hazard. Mater., 2016, 318, 266–281 CrossRef CAS PubMed; (b) M. Salvatores, Nucl. Eng. Des., 2005, 235(7), 805–816 CrossRef CAS; (c) T. A. Todd and R. A. Wigeland, in Separations for the Nuclear Fuel Cycle in the 21st Century, ed. G. J. Lumetta, K. L. Nash, S. B. Clark and J. I. Friese, American Chemical Society, 2006, vol. 933, pp. 41–55 Search PubMed; (d) C. Hill, in Ion Exchange and Solvent Extraction, ed. B. A. Moyer, CRC Press, Boca Raton, FL, 2010, pp. 119–195 Search PubMed.
- (a) A. V. Gelis and G. J. Lumetta, Ind. Eng. Chem. Res., 2014, 53(4), 1624–1631 CrossRef CAS; (b) K. L. Nash, Solvent Extr. Ion Exch., 2015, 33(1), 1–55 CrossRef CAS; (c) T. S. Grimes, C. R. Heathman, S. Jansone-Popova, V. S. Bryantsev, S. Goverapet Srinivasan, M. Nakase and P. R. Zalupski, Inorg. Chem., 2017, 56(3), 1722–1733 CrossRef CAS PubMed.
- (a) K. L. Nash, Solvent Extr. Ion Exch., 1993, 11(4), 729–768 CrossRef CAS; (b) M. P. Jensen and A. H. Bond, J. Am. Chem. Soc., 2002, 124(33), 9870–9877 CrossRef CAS PubMed; (c) A. Chandrasekar and T. K. Ghanty, Inorg. Chem., 2019, 58(6), 3744–3753 CrossRef CAS PubMed; (d) J. A. Drader, M. Luckey and J. C. Braley, Solvent Extr. Ion Exch., 2016, 34(2), 114–125 CrossRef CAS.
- H. H. Dam, D. N. Reinhoudt and W. Verboom, Chem. Soc. Rev., 2007, 36(2), 367–377 RSC.
- (a) G. J. Lumetta, B. M. Rapko, P. A. Garza, B. P. Hay, R. D. Gilbertson, T. J. R. Weakley and J. E. Hutchison, J. Am. Chem. Soc., 2002, 124(20), 5644–5645 CrossRef CAS PubMed; (b) H. V. Lavrov, N. A. Ustynyuk, P. I. Matveev, I. P. Gloriozov, S. S. Zhokhov, M. Y. Alyapyshev, L. I. Tkachenko, I. G. Voronaev, V. A. Babain, S. N. Kalmykov and Y. A. Ustynyuk, Dalton Trans., 2017, 46(33), 10926–10934 RSC.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T.-H. Vu and J.-P. Simonin, J. Am. Chem. Soc., 2011, 133(33), 13093–13102 CrossRef CAS PubMed.
- S. Jansone-Popova, A. S. Ivanov, V. S. Bryantsev, F. V. Sloop, R. Custelcean, I. Popovs, M. M. Dekarske and B. A. Moyer, Inorg. Chem., 2017, 56(10), 5911–5917 CrossRef CAS PubMed.
- M. R. Healy, A. S. Ivanov, Y. Karslyan, V. S. Bryantsev, B. A. Moyer and S. Jansone-Popova, Chem.–Eur. J., 2019, 25, 6326–6331 CrossRef CAS PubMed.
- K. L. Nash, C. Madic, J. N. Mathur and J. Lacquement, in The Chemistry of the Actinides and Transactinide Elements, ed. L. R. Morss, N. M. Edlestein and J. Fuger, Springer, Dordrecht, The Netherlands, 3rd edn, 2006, vol. 4, p. 2622 Search PubMed.
- (a) L. Xu, N. Pu, Y. Li, P. Wei, T. Sun, C. Xiao, J. Chen and C. Xu, Inorg. Chem., 2019, 58(7), 4420–4430 CrossRef CAS PubMed; (b) C. L. Xiao, C. Z. Wang, L. Y. Yuan, B. Li, H. He, S. Wang, Y. L. Zhao, Z. F. Chai and W. Q. Shi, Inorg. Chem., 2014, 53, 1712–1720 CrossRef CAS PubMed; (c) A. Afsar, J. Cowell, P. Distler, L. M. Harwood, J. John and J. Westwood, Synlett, 2017, 28(20), 2795–2799 CrossRef CAS.
- Ligand 1 is insoluble in Isopar L. See the ESI† for additional details.
- J. H. Hilderbrand and R. L. Scott, The solubility of nonelectrolytes, Reinhold, New York, 3rd edn, 1950, p. 488 Search PubMed.
- M. Regalbuto, in Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment, ed. K. L. Nash and G. J. Lumetta, Woodhead Publishing, Oxford, U.K., 2011, p. 176 Search PubMed.
- (a) G. J. Lumetta, A. V. Gelis, J. C. Carter, C. M. Niver and M. R. Smoot, Solvent Extr. Ion Exch., 2014, 32(4), 333–347 CrossRef CAS; (b) R. J. Ellis, D. M. Brigham, L. Delmau, A. S. Ivanov, N. J. Williams, M. N. Vo, B. Reinhart, B. A. Moyer and V. S. Bryantsev, Inorg. Chem., 2017, 56(3), 1152–1160 CrossRef CAS PubMed; (c) L. H. Delmau, N. Simon, M.-J. Schwing-Weill, F. Arnaud-Neu, J.-F. Dozol, S. Eymard, B. Tournois, C. Grüttner, C. Musigmann, A. Tunayar and V. Böhmer, Sep. Sci. Technol., 1999, 34(6–7), 863–876 CrossRef CAS.
- C. F. Baes Jr, Oak Ridge National Laboratory Report, ORNL/TM-13604, Oak Ridge National Laboratory, Oak Ridge, TN, December 1998 Search PubMed.
- Goodness-of-Fit Tests and Model Validity, ed. C. Huber-Carol, N. Balakrishnan, M. S. Nikulin and M. Mesbah, Springer, 2002 Search PubMed.
- The 1-octanol was included in the calculations as solvent but not as an extracting agent.
- Y. Liu, X. Yang, S. Ding, Z. Wang, L. Zhang, L. Song, Z. Chen and X. Wang, Inorg. Chem., 2018, 57, 5782–5790 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06115k |
| This journal is © The Royal Society of Chemistry 2019 |