Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved ferroelectric properties and band-gap tuning in BiFeO3 films via substitution of Mn
Song Yang ,
Guobin Ma,
Lei Xu,
Chaoyong Deng and
Xu Wang*
,
Guobin Ma,
Lei Xu,
Chaoyong Deng and
Xu Wang*
Guizhou Key Laboratory for Characteristics of Electronic Functional Composites, College of Big Data and Information Engineering, Guizhou University, Guiyang 550025, P. R. China. E-mail: SongYang_gzu@126.com
First published on 17th September 2019
Abstract
Multiferroic BiFe1−xMnxO3 (x = 0, 0.04, 0.08, 0.12) films have been prepared on Pt/Ti/SiO2/Si and ITO/glass substrates via the solution-gelation technique. The impacts of Mn doping of BFO thin films on the structure, morphology, leakage current, ferroelectric properties and optical band gap have been systematic investigated. From the XRD patterns, all samples match well with the perovskite structure without an impurity phase and the thin films exhibit dense and smooth microstructure. A leakage current density of 1.10 × 10−6 A cm−2 which is about four orders of magnitude lower than that of pure BiFeO3 was observed for the 8% Mn doped BFO thin film at an external electric field <150 kV cm−1. An increase in the remnant polarization with Mn substitution was observed, with a maximum value of ∼19 μC cm−2 for the 8% Mn-substituted film. Moreover, optical absorption spectra indicate that the doping of Mn has an effect on the energy band structure. Compared with pure BiFeO3, Mn doped thin films present an intense red shift as shown in the UV-visible diffuse absorption together with the decreased direct and indirect optical band gaps. In addition, this work gives insight into the relationship between ferroelectric remnant polarization and band-gap and finds that the optical band gap decreases with the increase of residual polarization.
1. Introduction
Multiferroic materials, which possess simultaneous ferroelectric, ferromagnetic, and ferroelastic ordering, have attracted much attention. Among these materials, BiFeO3 (BFO) is the only single phase multiferroic material with both a high ferroelectric Curie temperature (TC ∼ 1103 K) and a high antiferromagnetic Néel temperature (TN ∼ 643 K).1–3 It is also a known Pb-free and environmentally friendly material.4–6 Additionally, BFO thin films exhibit remarkable ferroelectric photovoltaic effects.7 Compared to most classical ferroelectric materials, BFO has a small band gap with reported values in the range of 2.6–3.0 eV,8 and a very large remnant ferrroelectric polarization, which can offer exciting opportunities for use in both optoelectronics and solar energy devices towards abundant renewable clean energy harvesting.9 However, the application of BFO is seriously hindered due to its high leakage current.4 Some reports also showed weak polarization in BFO thin films, which is attributed to the presence of impurity phases and oxygen vacancies.10 To overcome these problems significant efforts have been made, the substitution technology at the A or B position of perovskite crystals is the most widely used.11–13 Meanwhile, further improve the photovoltaic characteristics, it is necessary to narrow band-gap of the ferroelectric materials with larger polarization and smaller leakage. Therefore, in this work, thin films BiFe1−xMnxO3 (BFMO, x = 0, 0.04, 0.08, 0.12) were prepared on Pt/Ti/SiO2/Si and ITO/glass substrates by the solution-gelation technique. The microstructure, surface morphologies, ferroelectric and photovoltaic band-gap of BFO and Mn-doped BFO were discussed in detail.2. Experiment
The BFMO thin films were prepared on Pt/Ti/SiO2/Si and ITO/glass substrates by the solution-gelation technique. The precursor solutions were fabricated by using bismuth nitrate [Bi(NO3)3·5H2O] (99%), iron nitrate [Fe(NO3)3·9H2O] (98.5%) and manganese acetate [Mn(CH3COO)2·4H2O] (99%) as starting materials and ethylene glycol methyl ether (99.5%) as solvent. Acetic anhydride was also added as dehydrating agent. 10 mol% of excess Bi was added to compensate for bismuth loss during the heat treatment. Then the solution was stirred at the right temperature for several hours to form a homogeneous sol. The resultant solutions with concentration of 0.3 mol L−1 were deposited on substrates by spin-coating with the velocity 4000 rpm for 30 s. The as-deposited wet thin films were pre-annealed at 450 °C for 180 s and crystallized at 600 °C for 300 s in a rapid thermal process furnace. The spin-coating and thermal treatments were repeated several times to obtain the desired film thickness.The crystalline structures of the films were analyzed by X-ray diffraction (XRD, D/max-2500 V Rigaku) with Cu-Kα radiation. The surface morphologies were investigated by an atomic force microscope (AFM, Bruker MultiMode 8). The ferroelectric and leakage current properties were measured using a multiferroic tester system (MultiFerroic 200 V, Radiant Technologies). The optical absorption spectrum was measured by an ultraviolet-visible spectrophotometer (Hitachi U-4100).
3. Results and discussion
3.1. XRD analysis
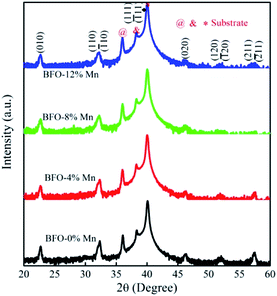
Fig. 1 shows the X-ray diffraction patterns (XRD) of Mn substituted BFO thin films grown on the Pt/Ti/SiO2/Si substrates. All diffraction peaks of the thin films exhibit a rhombohedral perovskite structure with (111) orientation, which is good agreement with the ICDD (PDF # 72-2112). No any other intermediate phases like Bi2Fe4O9 (ref. 14) has been found because the ionic radii of manganese are similar to that of Fe3+ (demonstrated in Table 1 (ref. 15)). It indicates that all these thin films are single-phase BiFeO3 materials in the same structure and the doping does not affect the lattice symmetry. In fact, their extranuclear structure of the outermost two layers exhibits high similarity.16 The (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) peaks overlap completely and the intensity remains roughly the same, while the (120) and (
11) peaks overlap completely and the intensity remains roughly the same, while the (120) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 20) peaks also overlap completely and the intensity is decreasing gradually. Since that the dopants with different ionic radius could cause the distortion of crystal lattice to various degrees and even change their syngony.17 Futhermore, all films show the (111) preferred orientation for the (111)-Pt/Ti/SiO2/Si substrate.
20) peaks also overlap completely and the intensity is decreasing gradually. Since that the dopants with different ionic radius could cause the distortion of crystal lattice to various degrees and even change their syngony.17 Futhermore, all films show the (111) preferred orientation for the (111)-Pt/Ti/SiO2/Si substrate.
| Ion | Bi3+ | Fe3+ | Mn3+ | O2− |
| Radius/nm | 0.096 | 0.055 | 0.058 | 0.132 |
The average crystallite size of the samples were calculated by using Scherer's formula:18
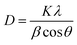 | (1) |
| Mn concentration (x) | Average crystallite size (D) nm | RMS roughness (Rq) nm |
|---|---|---|
| 0 | 19.7 | 15.6 |
| 0.04 | 22.65 | 11.2 |
| 0.08 | 23.96 | 8.6 |
| 0.12 | 21.08 | 10.4 |
3.2. Surface morphology and domain structure analysis
Fig. 2(a)–(d) show the surface topographic images of Mn doped BFO thin films test by AFM. It shows that the undoped BFO film is uneven and has a little undulating surface, which could impair the properties of the films severely. By contrast, the BFMO thin films exhibit very dense, pore free and uniform microstructure. Thus the substitution of Mn is found to be beneficial to enhance the microstructure of the films. The surface of Mn-doped BFO films were the root-mean-square (RMS) surface roughness values observed for Mn-doped BFO films (with x = 0.00, 0.04, 0.08 and 0.12) to be 15.6, 11.2, 8.6 and 10.4 nm respectively. The values of average grain size obtained from the AFM study are about 34.5, 37.7, 46.8 and 35.6 nm for BiFe1−xMnxO3 (x = 0, 4%, 8% and 12%) thin films. The grain size of the BFO film trend with Mn-doping, which was in agreement with the XRD results (Table 2). The grains of BFMO thin films was found to be increased with increase in Mn doping content (x = 0, 0.04, 0.08). A similar increase in grain size on Mn substitution was reported by Kim et al. and Choi et al.19–21 Obviously, a sudden decrease in the grain size was observed in BiFe0.88Mn0.12O3 thin film. This is because in 12% Mn doped BFO thin films, excessive addition of Mn may cause densification problems, thus reducing grain growth.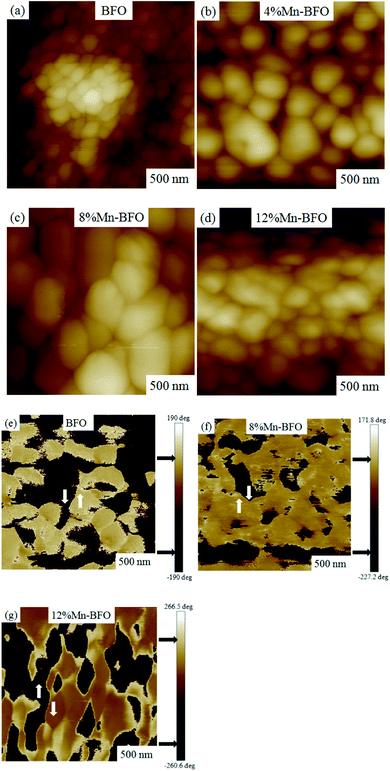 | ||
| Fig. 2 (a)–(d) Surface morphologies of 0–12% Mn doped BFO thin films measured by AFM. (e)–(g) PFM phase images of BFMO thin films. | ||
The out-of-plane PFM phase images of the BFO, 8% Mn–BFO and 12% Mn–BFO films are shown in Fig. 2(e)–(g), respectively. The ferroelectric domain structures of the BFO and 12% Mn–BFO exhibit a fractal growth habit, a domain size similar to the grain size and upward or downward polarization within each domain (Fig. 2(e)). The domain structures of the 8% Mn–BFO show more homogeneous domains and the domains are pinned at the ground boundaries (Fig. 2(f)). The density of the domain walls in the 8% Mn–BFO is less than in the BFO and 12% Mn–BFO. Therefore, it is expected that there is a lower leakage of current and large residual polarization in the 8% Mn–BFO (Fig. 4 and 5) since certain domain walls in the BFO and 12% Mn–BFO are much more conductive than the domains themselves.22 In addition, there exist three types of domain walls, namely those that separate domains 71°, 109° and 180° different in polarization.23 Domain patterns can develop with either a (100)-type plane for 109° walls or a (101)-type plane for 71° walls, respectively.24 As shown from the XRD spectra, the polycrystalline films should be a mixture of all three types of domain wall.25 The domain size in the BiFe0.92Mn0.08O3 film is larger than that in the BiFeO3 film, which could originate from the tensile strain enhanced by the Mn doping, the bigger grain size or even the misfit dislocation between the film and the substrate.26 The domain structure and the density of the domain walls of the BFMO also suggests that it is easier to polarize than the BFO.
 | ||
| Fig. 5 Piezoresponse phase (a) and piezoresponse amplitude (b) versus applied voltage loops, measured with an AC driving voltage Vac = 5 V. | ||
3.3. Leakage current analysis
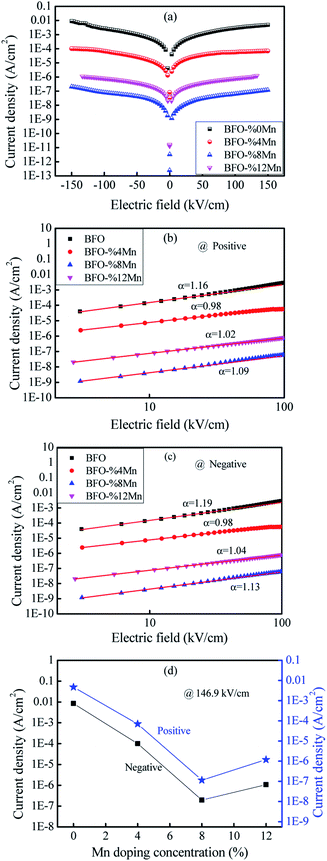
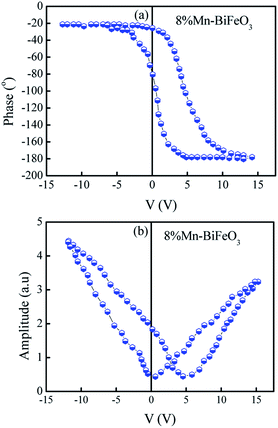
Fig. 3(a) presents the typical leakage current behavior (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J–E curves) of BFMO films. The measured leakage current density of BFMO (x = 0, 0.04, 0.08, 0.12) thin films is 9.04 × 10−2 A cm−2, 1.02 × 10−4 A cm−2, 1.14 × 10−7 A cm−2 and 1.10 × 10−6 A cm−2 at an applied electric field of 146.9 kV cm−1, repectively. The doped BFMO (x = 0.04, 0.08) thin films exhibit lower leakage current density compared to the undoped BFO thin film. Obviously, as the applied electrical field increases, the leakage current increases. It is worth noting that with the increase of Mn content (0–8%), the leakage current density curves gradually decrease. The two following factors can explain this phenomenon: (i) with the increase of Mn doping, the conversion of Fe2+ to Fe3+ ions may be promoted. Generally speaking, the less Fe2+ ions, the smaller the oxygen vacancy concentration corresponding to the film, which is similar to the results reported by Yoo et al.27 With the addition of Mn into BFO cells, the valence states of Mn, Fe and O ions react in BFO-based thin films. Oxygen vacancies act as “transition bridges” to achieve charge balance in BFO-based thin films and the reaction equation between them is as follows:28
J–E curves) of BFMO films. The measured leakage current density of BFMO (x = 0, 0.04, 0.08, 0.12) thin films is 9.04 × 10−2 A cm−2, 1.02 × 10−4 A cm−2, 1.14 × 10−7 A cm−2 and 1.10 × 10−6 A cm−2 at an applied electric field of 146.9 kV cm−1, repectively. The doped BFMO (x = 0.04, 0.08) thin films exhibit lower leakage current density compared to the undoped BFO thin film. Obviously, as the applied electrical field increases, the leakage current increases. It is worth noting that with the increase of Mn content (0–8%), the leakage current density curves gradually decrease. The two following factors can explain this phenomenon: (i) with the increase of Mn doping, the conversion of Fe2+ to Fe3+ ions may be promoted. Generally speaking, the less Fe2+ ions, the smaller the oxygen vacancy concentration corresponding to the film, which is similar to the results reported by Yoo et al.27 With the addition of Mn into BFO cells, the valence states of Mn, Fe and O ions react in BFO-based thin films. Oxygen vacancies act as “transition bridges” to achieve charge balance in BFO-based thin films and the reaction equation between them is as follows:28
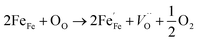 | (2) |
| Mn3+ + Fe2+ → Mn2+ + Fe3+ | (3) |
It can be seen from the formula that Fe3+ becomes more stable after Mn doping and limit the movement of oxygen vacancies.29 (ii) The increase of (MnFe3+3+)* can suppress Fe3+ turning into Fe2+ by doping of trivalent ions (Mn3+) in BFO films.30 More significantly, the leakage current density does not present a monotonous decreasing trend with the increase of Mn doping content. As Mn doping content increased from 8% to 12%, the leakage current density began to become larger for 1.10 × 10−6 A cm−2. This is because the increase in Mn content causes a gradual transformation of BFMO thin films from an insulator to semiconductor and also increase its density of free electrons in same fashion.31
Noticing that, the BiFe0.92Mn0.08O3 thin film showed the lowest current density in the low electric field region (E < 150 kV cm−1) as shown in the Fig. 3(d). Based on the AFM results, a flat and dense surface should be a source of lower leakage current in low electric field. From internal factors, a flat and a dense surface indicate that the grain size is more uniform, so the defect can be reduced. From extrinsic factors, the flat thin films surface could result to a better Pt/BFMO interface leading to the decrement of the leakage current.29 We also found that in the low electric field area, the leakage current of Mn-doped BFO films are lower than the undoped one.
To further study the source of leakage current, we analyzed the leakage mechanism of BFMO films. Fig. 3(b) shows the J–E plots of BFMO thin films in logarithmic scales during the positive process. The plots reveals a near linearity in the range of applied electric fields and they obey the power law of J ∝ Eα. We can judge the leakage mechanism of the samples by the slope of α.32,33 Ohmic conduction and space charge limited conduction (SCLC) are the two most common leakage mechanism with the fitting slope of 1 and 2, respectively.34 The slope under the low electric field is 1.22, 0.98, 1.03, 1.19 for the samples with x = 0%, 4%, 8%, 12% respectively. Which indicates that the ohmic conduction is dominant conduction mechanism in these samples. Similar analyses were conducted to determine the dominant leakage mechanism in the samples during the negative. The fitting results for BFMO (x = 0, 0.04, 0.08, 0.12) thin films are show in Fig. 3(c), in which a linear fitting with slope ≈ 1 would suggest the ohmic mechanism during the negative. It is shown that the free carriers in BFMO films play an important role in this stage, and there is no space charge effect.35 Therefore, at low electric field, the decrease of leakage current is not due to the decrease of BFO defect number, but mainly due to the inhibition the transformation of Fe3+ into Fe2+ by Mn doping, and the interface effect plays a major role.
3.4. Hysteresis analysis
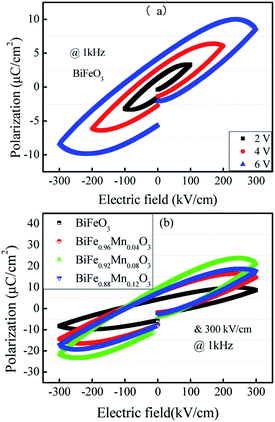
Fig. 4(a) shows the hysteresis loops at different applied voltages for same frequency. It can be seen that the hysteresis loop is strongly dependent on the applied voltage. For the applied voltage lower than 6 V (∼300 kV cm−1), although a hysteresis loop could be observed, the polarization was not saturated around the maximum of the applied voltage, indicating that polarization switching is incomplete. As the applied voltage increased, the reversal proceeded more completely. At 6 V (∼300 kV cm−1), the relatively saturated loop was observed.Fig. 4(b) shows the P–E hysteresis curves of Mn-doped BFO films with different Mn concentrations at the same electric field. The ferroelectric polarization and other parameters have been summarized in Table 3. It is evident that ferroelectricity of BFMO thin films has been greatly enhanced with the increase in Mn substitution from 0% to 8%, whereas the Pr of 12% Mn substituted BFO thin films is smaller. The lower residual polarization value and non saturated behavior of 12% Mn doped BFO thin films is associated with the existence of large leakage current component.21 It also reported by Das and his co-workers that high leakage characteristics of BFO ceramics could produce difficulty in attaining saturated polarization.36
| Samples | 2Ps (μC cm−2) | 2Pr (μC cm−2) | 2Ec (kV cm−1) | Direct band gap (eV) | Indirect band gap (eV) |
|---|---|---|---|---|---|
| BFO | 19.86 | 9.8 | 191.54 | 2.12 | 2.26 |
| BF0.96M0.04O | 33.02 | 15.5 | 151.96 | 2.0 | 2.22 |
| BF0.92M0.08O | 47.12 | 19 | 134.76 | 1.92 | 2.20 |
| BF0.88M0.12O | 37.84 | 14.6 | 196 | 1.97 | 2.23 |
The remnant polarization (2Pr) and coercive field (Ec) increase with the elevation of Mn content from 0% to 8% in BFMO thin films. This increase in 2Pr value with the increase in Mn content from 0 to 8% can be attributed to combine defect of (i) lowering of defects and (ii) increase in the grain size. Space charges such as oxygen vacancies 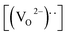 and acceptors [(AFe3+2+)*] such as [(FeFe3+2+)*] form a defect polarization which can align along the direction of spontaneous polarization alleviates the domain pinning.37,38 The substitution of Mn at B site in BFO matrix suppresses the content of defect complexes of
and acceptors [(AFe3+2+)*] such as [(FeFe3+2+)*] form a defect polarization which can align along the direction of spontaneous polarization alleviates the domain pinning.37,38 The substitution of Mn at B site in BFO matrix suppresses the content of defect complexes of 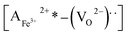 by suppressing oxygen vacancy concentration, which in turn resulted in higher 2Pr value.39 It is well known that grain size dependent domain structure, domain nucleation and domain mobility greatly influence the ferroelectric properties of ferroelectric thin films.40 The grain boundaries act as a pinning center for polarization and produce hindrance of polarization switching. Thus, the small grain size BFMO thin films experience more suppression of ferroelectric character whereas polarization switching is much easier inside the larger grain. The increase in the tetragonality of a crystal structure with increase in Mn doping could be another reason for the enhancement of ferroelectricity as propounded by Ederer et al.41 This possibility can be easily ruled out as in our experiment, no other phases appeared, as shown in Fig. 1 XRD. Table 3 summarizes the different parameters obtained from the (P–E) loops of BFMO thin films. The sudden decrease in the remnent polarization 2Pr value of 12% Mn doped BFO thin film is attributed to decrease in the grain size. A small conveyance in polarization hysteresis loops along the direction of the positive field in BFMO thin films can be imputed of actors like crystallographic defects, work function difference and thermal history between top and bottom electrodes.42,43
by suppressing oxygen vacancy concentration, which in turn resulted in higher 2Pr value.39 It is well known that grain size dependent domain structure, domain nucleation and domain mobility greatly influence the ferroelectric properties of ferroelectric thin films.40 The grain boundaries act as a pinning center for polarization and produce hindrance of polarization switching. Thus, the small grain size BFMO thin films experience more suppression of ferroelectric character whereas polarization switching is much easier inside the larger grain. The increase in the tetragonality of a crystal structure with increase in Mn doping could be another reason for the enhancement of ferroelectricity as propounded by Ederer et al.41 This possibility can be easily ruled out as in our experiment, no other phases appeared, as shown in Fig. 1 XRD. Table 3 summarizes the different parameters obtained from the (P–E) loops of BFMO thin films. The sudden decrease in the remnent polarization 2Pr value of 12% Mn doped BFO thin film is attributed to decrease in the grain size. A small conveyance in polarization hysteresis loops along the direction of the positive field in BFMO thin films can be imputed of actors like crystallographic defects, work function difference and thermal history between top and bottom electrodes.42,43
The piezoelectric response of the 8% Mn–BFO thin film has been studied simply by piezoresponse force microscopy. Typical butterfly loops were observed for 8% Mn–BFO sample, as presented in Fig. 5(a) and (b). Local piezoelectric phase hysteresis loops and amplitude–voltage loops were also recorded at fixed location as a function of ±15 dc bias superimposed on ac modulating voltage. Complete phase reversal of about 180° and piezo-actuation amplitude variation of more than 4.5 mV under the dc bias of ±5 V reflects the better ferroelectric properties of 8% Mn–BFO thin films important for practical applications of interest.44
3.5. Band-gap analysis
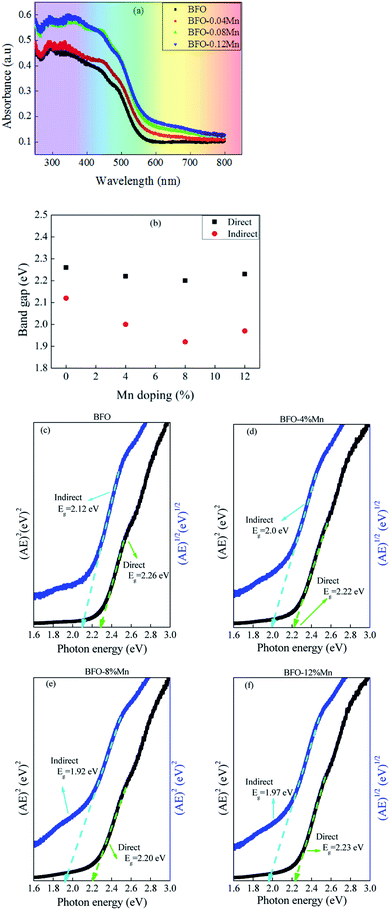
The most direct and perhaps the simplest method for probing the band structure of semiconductors is to measure the absorption spectrum. Fundamental absorption, which manifests itself by a rapid rise in absorption, can be used to determine the energy gap of the semiconductor. To investigate the influence of Mn doping on the optical absorption of BFO, the absorption optical spectra of pure BFO and Mn-doped BFO samples were measured at room temperature, as shown in Fig. 6(a). The absorption band edge of BFO thin film appeared at 572 nm, which was similar to those previously reported,45,46 indicating that BFO could respond to visible light for photocatalytic reaction. The fundamental absorption edge is seen to be shifted towards higher wavelength with increasing Mn-doping concentration of the BFMO thin films (Fig. 6(a)). Compared to pure BFO, the Mn-doped BFO samples exhibited enhanced absorption capability especially in the visible light region, and the absorption intensity became gradually stronger as increasing the Mn dopant content. Moreover, the band gap could be calculated from the plot of the Kubelka–Munk function:47| (αhν)n = A(hν − Eg) | (4) |
4. Conclusions
In summary pure and Mn doped BFO thin films were successfully prepared on Pt/Ti/SiO2/Si and ITO/glass substrates by the solution-gelation technique. Structural characterization by X-ray diffraction revealed that all samples exhibited a rhombohedral structure with (111) preferred orientation and without impurity phase. It is observed that improved ferroelectric property and leakage current density in 8% Mn-doped BFO thin film due to a decrease in the oxygen vacancy density, a stabilization of the perovskite structure, and increase in the grain size. The absorption spectrum for BFMO (x = 0, 0.04, 0.08) thin films presents a significant red shift and moves towards the visible region. Thus, photovoltaic behaviors are attributed to the narrower band-gap. This work gives insight into the relationship between ferroelectric remnant polarization and band-gap and found that the optical band gap decreases with the increase of residual polarization. This also provides an available way to exploring the mechanism of ferroelectric photovoltaic, getting more extensively applied in the new photovoltaic cells and other novel photoelectronic devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the Natural Science Foundation of Guizhou Province of China (Grant No. 61404033), the Natural Science Foundation of Guizhou Province of China (Grant No. 201813) and Opening Project of Guizhou Public Key Laboratory of Big Data (Grant No. 2017BDKFJJ).References
- J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Schlom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M. Wuttig and R. Ramesh, Science, 2003, 299, 1719–1722 CrossRef CAS PubMed.
- H. R. Yan, H. M. Deng, N. F. Ding, J. He, L. Peng, L. Sun, P. X. Yang and J. H. Chu, Mater. Lett., 2013, 111, 123–125 CrossRef CAS.
- L. Yu, H. M. Deng, W. L. Zhou, Q. Zhang, P. X. Yang and J. H. Chu, Mater. Lett., 2016, 170, 85–88 CrossRef CAS.
- A. Kumar, J. F. Scott, R. Martínez, G. Srinivasan and R. S. Katiyar, Phys. Status Solidi A, 2012, 209, 1207–1212 CrossRef CAS.
- G. D. Hu, S. H. Fan, C. H. Yang and W. B. Wu, Appl. Phys. Lett., 2008, 92, 192905 CrossRef.
- S. Dutta, A. Pandey, I. Yadav, O. P. Thakur, R. Laishram, R. Pal and R. Chatterjee, J. Appl. Phys., 2012, 112, 084101 CrossRef.
- S. Y. Yang, L. W. Martin, S. J. Byrnes, T. E. Conry, S. R. Basu, D. Paran, L. Reichertz, J. Ihlefeld, C. Adamo, A. Melville, Y. H. Chu, C. H. Yang, J. L. Musfeldt, D. G. Schlom, J. W. Ager and R. Ramesh, Appl. Phys. Lett., 2009, 95, 062909 CrossRef.
- D. Sando, A. Barthélémy and M. Bibes, J. Phys.: Condens. Matter, 2014, 26, 473201 CrossRef CAS PubMed.
- S. Gupta, M. Tomar and V. Gupta, J. Appl. Phys., 2014, 115, 014102 CrossRef.
- Q. Yun, W. Y. Xing, J. Y. Chen, W. Gao, Y. L. Bai and S. F. Zhao, Thin Solid Films, 2015, 584, 103–107 CrossRef CAS.
- Y. Wang, J. Li, J. Y. Chen and Y. Deng, J. Appl. Phys., 2013, 113, 103904 CrossRef.
- S. K. Singh, Thin Solid Films, 2013, 527, 126–132 CrossRef CAS.
- G. N. Sharma, S. Dutta, A. Pandey, S. K. Singh and R. Chatterjee, Mater. Res. Bull., 2017, 95, 223–228 CrossRef CAS.
- H. P. Gao, J. J. Tian, H. W. Zheng, F. R. Tan and W. F. Zhang, J. Mater. Sci., 2015, 26, 700–704 CAS.
- J. A. Dean, Lange's Chemistry Handbook, McGraw-Hill Professional, New York, 2007, p. 15 Search PubMed.
- Q. Y. Rong, W. Z. Xiao, G. Xiao, A. M. Hu and L. L. Wang, J. Alloys Compd., 2016, 674, 463 CrossRef CAS.
- G. L. Song, J. Su, Z. Na and F. G. Chang, Phys. B, 2016, 493, 47–52 CrossRef CAS.
- P. Mathur, A. Thakur and M. Singh, J. Mater. Sci., 2007, 42, 8189–8192 CrossRef CAS.
- J. K. Kim, S. S. Kim, E. J. Choi, W. J. Kim, A. S. Bhalla and T. K. Song, J. Korean Phys. Soc., 2006, 49, S566 CAS.
- E. J. Choi, S. S. Kim, J. W. Kim, M. H. Park, H. K. Cho, W. J. Kim, S. Yi and D. Yoo, J. Korean Phys. Soc., 2007, 51, S138 CrossRef CAS.
- K. Singh, S. K. Singh and D. Kaur, Ceram. Int., 2016, 42, 13432–13441 CrossRef CAS.
- G. Catalan and J. F. Scott, Adv. Mater., 2009, 21, 2463–2485 CrossRef CAS.
- L. W. Martin, S. P. Crane, Y. H. Chu, M. B. Holcomb, M. Gajek, M. Huijben, C. H. Yang, N. Balke and R. Ramesh, J. Phys.: Condens. Matter, 2008, 20, 434220 CrossRef.
- Y. H. Chu, Q. He, C. H. Yang, P. Yu, L. W. Martin, P. Shafer and R. Ramesh, Nano Lett., 2009, 9, 1726–1730 CrossRef CAS PubMed.
- F. Yan, T. J. Zhu, M. O. Lai and L. Lu, Scr. Mater., 2010, 63, 780–783 CrossRef CAS.
- S. Venkatesan, C. Daumont, B. J. Kooi, B. Noheda and J. T. M. D. Hosson, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 214111 CrossRef.
- Y. J. Yoo, J. S. Hwang, Y. P. Lee, J. S. Park, J. H. Kang, J. Kim, B. W. Lee and M. S. Seo, J. Appl. Phys., 2013, 114, 163902 CrossRef.
- S. J. Clark and J. Robertson, Appl. Phys. Lett., 2009, 94, 022902 CrossRef.
- S. J. Yang, F. Q. Zhang, X. B. Xie, H. J. Sun, L. P. Zhang and S. H. Fan, J. Alloys Compd., 2017, 734, 243–249 CrossRef.
- F. T. Lin, Q. Q. Yu, L. Y. Deng, Z. J. Zhang, X. Y. He, A. Y. Liu and W. Z. Shi, J. Mater. Sci., 2017, 52, 7118–7129 CrossRef CAS.
- Z. Y. Zhong and H. S. Ishiwara, Appl. Phys. Lett., 2009, 95, 112902 CrossRef.
- A. R. Chaudhuri and S. B. Krupanidhi, J. Appl. Phys., 2005, 98, 094112 CrossRef.
- X. Qi, J. Dho, R. Tomov, M. G. Blamire and J. L. MacManusDriscoll, Appl. Phys. Lett., 2005, 86, 062903 CrossRef.
- J. G. Wu, J. Wang, D. Q. Xiao and J. G. Zhu, AIP Adv., 2011, 1, 022138 CrossRef.
- W. Sun, Z. Zhou, J. Luo, K. Wang and J. F. Li, J. Appl. Phys., 2017, 121, 1011–1018 Search PubMed.
- S. R. Das, R. N. P. Choudhary, P. Bhattacharya, R. S. Katiyar, P. Dutta, A. Manivannan and M. S. Seehra, J. Appl. Phys., 2007, 101, 034104 CrossRef.
- G. D. Hu, S. H. Fan, C. H. Yang and W. B. Wu, Appl. Phys. Lett., 2008, 92, 192905 CrossRef.
- L. X. Zhang and X. B. Ren, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 094121 CrossRef.
- Z. Wen, G. D. Hu, S. H. Fan, C. H. Yang, W. B. Wu, Y. Zhou, X. M. Chen and S. G. Cui, Thin Solid Films, 2009, 517, 4497–4501 CrossRef CAS.
- C. C. Leu, C. Y. Chen, C. H. Chien and M. N. Chang, Appl. Phys. Lett., 2003, 82, 3493–3495 CrossRef CAS.
- C. Ederer and N. A. Spaldin, Phys. Rev. Lett., 2005, 95, 257601 CrossRef PubMed.
- Y. Liu, C. N. Xu and T. Watanabe, J. Mater. Sci., 1999, 34, 4129–4132 CrossRef CAS.
- Q. Zhang and R. R. Whatmore, J. Eur. Ceram. Soc., 2004, 24, 277–282 CrossRef CAS.
- S. Gupta, R. Medwal, T. B. Limbu, R. K. Katiyar, S. P. Pavunny, M. Tomar, G. Morell, V. Gupta and R. S. Katiyar, Appl. Phys. Lett., 2015, 107, 062902 CrossRef.
- J. Y. Wang, Y. W. Wei, J. J. Zhang, L. D. Ji, Y. X. Huang and Z. Chen, Mater. Lett., 2014, 124, 242–244 CrossRef CAS.
- N. Zhang, D. Chen, F. Niu, S. Wang, L. S. Qin and Y. X. Huang, Sci. Rep., 2016, 6, 26467 CrossRef CAS PubMed.
- C. Clementi, C. Miliani, G. Verri, S. Sotiropoulou, A. Romani, B. G. Brunetti and A. Sgamellotti, Appl. Spectrosc., 2009, 63, 1323–1330 CrossRef CAS PubMed.
- J. I. Pankove, Optical processes in semiconductors, Prentice-Hall, New Jersey, 1971, p. 92 Search PubMed.
- L. You, F. Zheng, L. Fang, Y. Zhou, L. Z. Tan, Z. Y. Zhang, G. H. Ma, D. Schmidt, A. Rusydi, L. Wang, L. Chang, A. M. Rappe and J. Wang, Sci. Adv., 2018, 4, eaat3438 CrossRef PubMed.
- P. K. Gogoi and D. Schmidt, Phys. Rev. B, 2016, 93, 075204 CrossRef.
- S. J. Clark and J. Robertson, Appl. Phys. Lett., 2007, 90, 132903 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |