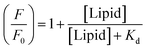Open Access Article
Open Access ArticleFluorescence-based ion sensing in lipid membranes: a simple method of sensing in aqueous medium with enhanced efficiency
Leena Sushmita
Barla
,
Gourab Prasad
Pattnaik
 ,
Geetanjali
Meher
,
Geetanjali
Meher
 ,
Subrata Kumar
Padhan
,
Subrata Kumar
Padhan
 ,
Satya Narayan
Sahu
,
Satya Narayan
Sahu
 * and
Hirak
Chakraborty
* and
Hirak
Chakraborty
 *
*
School of Chemistry, Sambalpur University, Jyoti Vihar, Burla, Odisha 768 019, India. E-mail: hirakchakraborty@gmail.com; snsahu.chem@gmail.com; Tel: +91-8008716419 Tel: +91-7008104352
First published on 1st October 2019
Abstract
Detection of ions in chemical, biological and environmental samples has gathered tremendous momentum considering the beneficial as well as adverse effects of the ions. Generally, most of the ions are beneficial up to an optimum concentration, beyond which they are toxic to human health. However, most of the fluorescence-based ion sensors are only active in non-aqueous solution because of the low solubility of the sensor molecules in aqueous buffer medium. In the present work, we have demonstrated that encapsulation of an aqueous insoluble thiocarbonohydrazone-locked salicylidene-based macrocyclic ligand in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membranes allows the selective detection of Zn2+ in aqueous medium with approximately 3-fold enhanced efficiency compared to its efficiency in DMSO medium. We have further modulated the charge of the membrane surface by adding various concentrations of a negatively charged lipid, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG), and showed that negative surface charge further enhances the Zn2+ sensing efficiency up to approximately 6-fold. This strategy opens up a new avenue of utilizing organic sensors to detect vital ions in aqueous medium.
Introduction
Detection of ions has gathered significant momentum in the last couple of decades owing to their toxic effects.1 While plants and animals are known to require ions as macro and micro nutrients, their concentration beyond an optimal level can be lethal. Therefore, a significant amount of effort has been applied to the detection and removal of excess and unnecessary ions. Fluorescence-based ion sensors have gained popularity over other counterparts due to their high sensitivity, tunability, efficacy in cellular environments, and cost effectiveness.2 However, the major challenge of fluorescence-based sensors is their poor solubility in aqueous medium. From biological perspective, application of these probes in the aqueous medium of cellular milieu would be extremely important, considering the high aqueous solubility of ions. Therefore, strategies need to be designed to enable non-aqueous fluorescence-based sensor molecules to be active in aqueous medium without undergoing any synthetic modification. Zinc is an extremely important ion, since its deficiency leads to neurological disorders like Alzheimer's disease, Parkinson's disease, epilepsy, and ischemic stroke while its excess cellular concentration contributes to hypoglycaemia-induced neuronal death.3–6Lipid membranes provide an inimitable environment for the solubilization of polar, nonpolar, and amphipathic molecules. The unique blending of polar headgroup and hydrophobic fatty acyl tail creates a nano-environment of graded polarity, mobility, and heterogeneity, which facilitates the solubilization of macrocyclic ligands of varied polarity.7–9 A recent report has shown that a thiocarbonohydrazone-locked salicylidene-based macrocycle ligand (Fig. 1) senses Zn2+ ions in dimethylsulfoxide (DMSO) medium with a very high affinity (Kd of 6 μM); however, the probe is insoluble in aqueous medium.10 In this work, we aimed to utilize membrane incorporation of the thiocarbonohydrazone-locked salicylidene-based macrocyclic ligand to make it an efficient Zn2+ sensor in aqueous buffered medium. We incorporated the macrocyclic probe (Fig. 1) in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) vesicles and evaluated its Zn2+ sensing ability in phosphate buffer at pH 7.4.
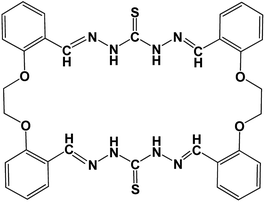 | ||
| Fig. 1 Chemical structure of thiocarbonohydrazone-locked salicylidene-based macrocyclic ligand (Probe). | ||
Material and methods
Materials
The thiocarbonohydrazone-locked salicylidene-based macrocyclic ligand has been synthesized as described earlier.10 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) were obtained from Avanti polar Lipids (Alabaster, AL). Spectroscopic grade DMSO was purchased from Spectrochem (India), and was used for the preparation of probe stock solution. All other chemicals used in the work were of the highest available purity. Water was purified through Millipore (Bedford, MA) Milli-Q water purification system.Sample preparation
We have used small unilamellar vesicles (SUVs) of POPC and mixture of POPC/POPG of different mole fractions as the membrane systems for measurements. The concentrations of lipid and probe were kept constant at 500 μM and 5 μM, respectively in all experiments. The lipid was dissolved in chloroform and air dried to make a thin film. The film was kept in vacuum desiccator for complete removal of chloroform. The DMSO solution of the probe was added to the dried lipid and mixed well with the lipid before buffer was added to the lipid. The mixture of the lipid and probe mixture was hydrated (swelled) by adding 10 mM phosphate buffer of pH 7.4. The sample was vortexed for 1 h for uniform dispersion of lipids. The vesicles have been prepared by sonication method and the average hydrodynamic radii of the vesicles are 40–50 nm with a polydispersity index less than 0.2.11,12 Background samples were prepared the same way except that the probe was omitted.Steady state fluorescence measurements
Steady state fluorescence measurements were performed with Hitachi (Japan), model F-7000 spectrofluorometer, using quartz cuvette with 1 cm pathlength. Excitation and emission slits with a nominal band pass of 5 nm were used for all measurements. Fluorescence measurements were performed keeping excitation wavelength at 340 nm.Results and discussion
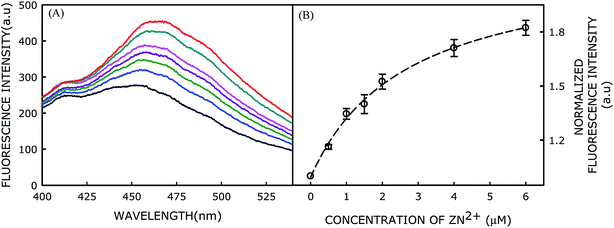
Experimentally, 5 μM probe was reconstituted in 500 μM POPC and its fluorescence spectrum was recorded after excitation at 340 nm. Fluorescence spectrum of the membrane-incorporated probe is shown in Fig. 2A. Although the probe does not show much fluorescence in homogeneous solution (like DMSO) in absence of Zn2+ ion, it has decent fluorescence intensity in membrane environment. This could be attributed to the rotational deactivation pathways of the probe being restricted in the membrane environment.13 The relatively broad fluorescence spectrum of the probe in the membrane system could be owing to emissions from multiple solvated states. The heterogeneity in the solvated states might be originated from the rotation of various single bonds present in the probe molecule. Addition of Zn2+ ions to the probe-incorporated membrane in buffer at pH 7.4 further enhances the fluorescence intensity by ∼2-fold with a red shift of approximately 15 nm (Fig. 2A). This change in fluorescence intensity upon Zn2+ addition is highly consistent and reproducible. The observed red-shift in emission maximum could be attributed to the ground state complex formation between the probe and Zn2+ ion, which has already been confirmed by UV-visible spectroscopic measurements.10The enhancement of fluorescence intensity with significant red shift in emission maximum clearly indicates complex formation between the probe and Zn2+ ion. Fluorescence intensity of the probe as a function of Zn2+ ion concentration has been shown in Fig. 2B and the data were analyzed using classical Langmuir model for adsorption of ligand to multiple, equivalent, and non-interacting surface sites, as described earlier, to obtain the dissociation constant (Kd) utilizing the following equation:14
The dissociation constant (Kd) of Zn2+ with respect to the probe in POPC membranes was found to be 2.68 μM, which clearly implies that its affinity for Zn2+ is approximately 3-fold higher in lipid membranes compared to that in DMSO solution. Moreover, the Zn2+ ion-dependent change in fluorescence intensity of the probe suggests the interfacial localization of the probe as the ions do not permeate in the hydrophobic region of the membrane. In membranes, the sensing activity occurs in a 2-dimensional surface rather than in a 3-dimensional solution (DMSO or any homogeneous solution). Thus, the effective concentrations of both probe and Zn2+ ion increase in 2-dimensional surface with increasing probability of productive collisions between a probe molecule and Zn2+ ion,15 and thereby enhancing the probability of probe-ion interaction. Therefore, the higher affinity could be due to the complex formation between the probe and Zn2+ at the membrane surface.
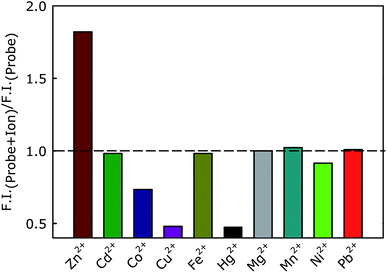
We have further evaluated the selectivity of the lipid-incorporated probe toward Zn2+ ion by measuring the fluorescence intensity in presence of other metal ions (6 μM). Interestingly, we have observed an enhancement of fluorescence intensity only in presence of Zn2+ ion (Fig. 3).
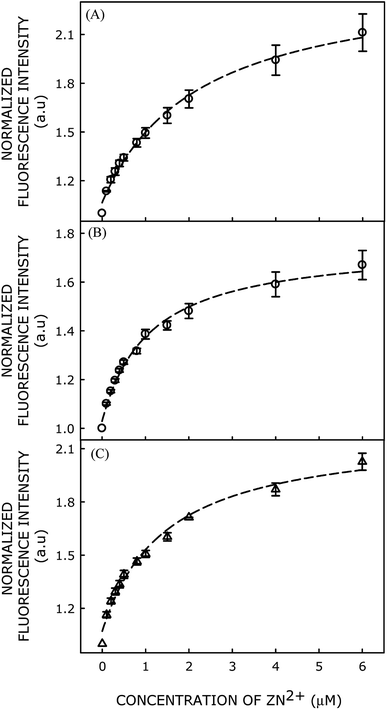
Moreover, we have modulated the membrane surface charge by adding various proportions of negatively charged lipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) to POPC, and evaluated Zn2+ affinity of the probe in different POPC/POPG lipid membranes. Fluorescence intensities of the probe in presence of different concentrations of Zn2+ ions in POPC/POPG (90/10 mol%), POPC/POPG (80/20 mol%), and POPC/POPG (70/30 mol%) membranes are shown in Fig. 4(A–C).
Dissociation constant of Zn2+ binding to the probe in various membranes has been calculated using classical Langmuir model for adsorption of ligand to multiple, equivalent, and non-interacting surface sites, and subsequently plotted as a function of mol% of POPG in Fig. 5. Interestingly, the Kd value decreases (i.e., affinity for Zn2+ increases) with increasing mol fraction of negatively charged POPG in the membrane (Fig. 4), eventually saturating beyond 20 mol% of POPG. Our results clearly demonstrate that binding affinity of the probe toward Zn2+ ion increases significantly with increasing negative charge on the membrane surface. This implies that the negative charge of the membrane augments Zn2+ ion concentration near the membrane surface, which increases affinity of the probe for binding with Zn2+ ion.
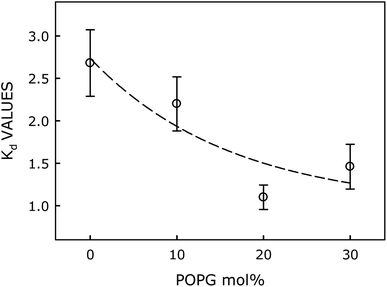 | ||
| Fig. 5 Plot of Kd as a function of mol% of the negatively charged lipid (POPG). Lines joining data points are provided as viewing guides. | ||
Taken together, our results suggest that membrane encapsulation of the macrocyclic probe (insoluble in aqueous medium) enables it to sense nanomolar concentration (detection limit is in nanomolar) of Zn2+ in phosphate buffer at pH 7.4 (schematic representation in Fig. 6). Moreover, Zn2+ sensitivity of the probe can be fine-tuned by manipulating the proportion of negative charge on the membrane surface. Successful application of the membrane-incorporated probe as an ion sensor in aqueous solution opens up the possibility to utilize an organic solvent-soluble probe in aqueous medium, without undergoing any synthetic modification.
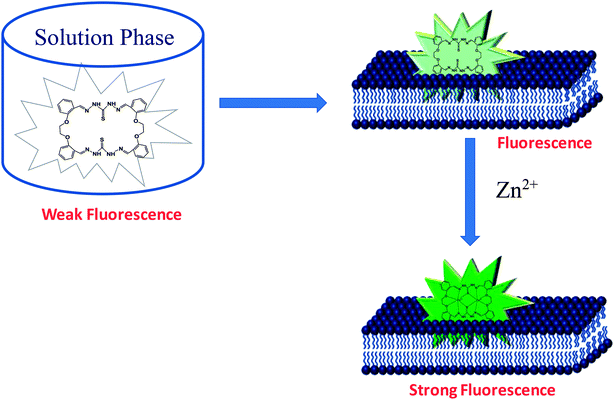 | ||
| Fig. 6 Schematic representation of membrane incorporation of the ion sensor for ion detection in aqueous solution. | ||
Conclusion
In conclusion, this study proposes lipid encapsulation of a thiocarbonohydrazone-locked salicylidene-based macrocyclic probe as a strategy to selectively detect Zn2+ ions in aqueous medium with approximately 3-fold higher efficiency. Further, introduction of negative surface charge on the membrane enhances its ion-sensing efficiency dramatically. Our findings can open up novel possibilities of ion sensing in aqueous medium by using hydrophobic probes, without undergoing any synthetic modifications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by research grants from the SERB-DST, New Delhi (File No. ECR/2015/000195) to H. C. and S&T Department, Govt. of Odisha and GNM Foundation for Prof. GN Mahapatra Endowment Chair award to S. N. S. University Grants Commission (UGC) is acknowledged by H. C. for a UGC-Assistant Professor position. G. P. P. acknowledges SERB-DST, New Delhi for Research Fellowship. G. M. and S. K. P. thank the UGC for BSR fellowship. We acknowledge DST and UGC, New Delhi for providing instrument facility to the School of Chemistry, Sambalpur University under the FIST and DRS programs, respectively.References
- I. J. Allan, B. Vrana, R. Greenwood, G. A. Mills, B. Roig and C. Gonzalez, Talanta, 2006, 69, 302–322 CrossRef CAS.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. H. Weiss, S. L. Sensi and J. Y. Koh, Trends Pharmacol. Sci., 2000, 21, 395–401 CrossRef CAS.
- M. P. Cuajungco and G. J. Lees, Neurobiol. Dis., 1997, 4, 137–169 CrossRef CAS.
- A. I. Bush, W. H. Pettingell Jr, M. D. Paradis and R. E. Tanzi, J. Biol. Chem., 1994, 269, 12152–12158 CAS.
- D. Noy, I. Solomonov, O. Sinkevich, T. Arad, K. Kjaer and I. Sagi, J. Am. Chem. Soc., 2008, 130, 1376–1383 CrossRef CAS.
- H. Chakraborty, S. Haldar, P. L. Chong, M. Kombrabail, G. Krishnamoorthy and A. Chattopadhyay, Langmuir, 2015, 31, 11591–11597 CrossRef CAS.
- J. Seelig, Q. Rev. Biophys., 1977, 10, 353–418 CrossRef CAS PubMed.
- C. D. Stubbs, C. Ho and S. J. Slater, J. Fluoresc., 1995, 5, 19–28 CrossRef CAS.
- S. K. Padhan, J. Palei, P. Rana, N. Murmu and S. N. Sahu, Spectrochim. Acta, Part A, 2019, 208, 271–284 CrossRef CAS.
- G. P. Pattnaik and H. Chakraborty, Chem. Phys. Lipids, 2018, 217, 35–42 CrossRef CAS.
- H. Chakraborty, P. K. Tarafdar, M. J. Bruno, T. Sengupta and B. R. Lentz, Biophys. J., 2012, 102, 2751–2760 CrossRef CAS PubMed.
- M. Shinitzky and Y. Barenholz, Biochim. Biophys. Acta, 1978, 515, 367–394 CrossRef CAS.
- V. Koppaka and B. R. Lentz, Biophys. J., 1996, 70, 2930–2937 CrossRef CAS.
- P. G. Saffman and M. Delbrück, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 3111–3113 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |