Open Access Article
Open Access ArticleComparative study of mesoporous NixMn6−xCe4 composite oxides for NO catalytic oxidation
Li
Weiman
 abcd,
Liu
Haidi
a,
Zhang
Min
ab and
Chen
Yunfa
abcd,
Liu
Haidi
a,
Zhang
Min
ab and
Chen
Yunfa
 *ad
*ad
aState Key Laboratory of Multi-phase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: chenyf@ipe.ac.cn
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, China
cZhongke Langfang Institute of Process Engineering, Langfang Economic & Technical Development Zone, Fenghua Road No 1, Hebei, China
dCAS Center for Excellence in Urban Atmospheric Environment, Xiamen 361021, China
First published on 1st October 2019
Abstract
In this work, a series of mesoporous NixMn6−xCe ternary oxides were prepared to investigate their NO catalytic oxidation ability. The sample Ni2Mn4Ce4 showed a 95% NO conversion at 210 °C (GHSV, ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1). Characterization results showed the good catalytic performance of Ni2Mn4Ce4 was due to its high specific surface area, more surface oxygen and high valance manganese species, which can be ascribed to the incorporation of three elements. Based on the results of XRD, H2-TPR, O2-TPD and XPS, we confirmed the existence of Ni3+ + Mn3+ → Ni2+ + Mn4+, Ce4+ + Ni2+ → Ce3+ + Ni3+ in Ni2Mn4Ce4, and the oxidation–reduction cycles were proved to be helpful for NO oxidation. The results from an in situ DRIFTS study indicated the presence of bidentate nitrate and monodentate nitrate species on the catalyst's surface. The nitrate species were proved to be intermediates for NO oxidation to NO2. A nitrogen circle mechanism was proposed to explain the possible route for NO oxidation. Nickel introduction was also helpful to improve the SO2 resistance of the NO oxidation reaction. The activity drop of Ni2Mn4Ce4 was 13.15% in the presence of SO2, better than Mn6Ce4 (25.29%).
000 h−1). Characterization results showed the good catalytic performance of Ni2Mn4Ce4 was due to its high specific surface area, more surface oxygen and high valance manganese species, which can be ascribed to the incorporation of three elements. Based on the results of XRD, H2-TPR, O2-TPD and XPS, we confirmed the existence of Ni3+ + Mn3+ → Ni2+ + Mn4+, Ce4+ + Ni2+ → Ce3+ + Ni3+ in Ni2Mn4Ce4, and the oxidation–reduction cycles were proved to be helpful for NO oxidation. The results from an in situ DRIFTS study indicated the presence of bidentate nitrate and monodentate nitrate species on the catalyst's surface. The nitrate species were proved to be intermediates for NO oxidation to NO2. A nitrogen circle mechanism was proposed to explain the possible route for NO oxidation. Nickel introduction was also helpful to improve the SO2 resistance of the NO oxidation reaction. The activity drop of Ni2Mn4Ce4 was 13.15% in the presence of SO2, better than Mn6Ce4 (25.29%).
1 Introduction
Nitrogen monoxide, nitrogen dioxide and nitrous oxide, known as nitrogen oxides (NOx) are important pollutants in causing a series of environmental problems, such as acid rain, photochemical smog, and ozone depletion.1 Many technologies have been developed to deal with NOx, such as NH3-selective catalytic reduction, ozone assisted NO oxidation, and NOx storage reduction.2 The oxidation of NO to NO2 is an important step for almost all the NO abatement technologies; for example, NO oxidation can enhance NH3-SCR to follow a fast SCR mechanism,3 and in the wet scrubbing method, the conversion of NO to NO2 is helpful to increase NOx removal efficiency.4,5 Therefore, much effort has been made to develop catalysts with good NO oxidation ability.Several types of catalysts, including noble metal catalysts,6 supported catalysts,7 and multi-metal oxide catalysts8,9 were reported to have good catalytic activities under different reaction conditions. Multi-metal composites have good catalytic activities in the low and middle temperature range (100–320 °C), which are suitable to be applied in flue gas. Among them, manganese and ceria composite oxides have been reported several times for their excellent redox properties, oxygen storage capacity, and excellent performance in NO oxidation.10 However, the NO oxidation ability of Mn–Ce composites is severely affected by SO2 in real application condition. Researches confirmed that SO2 can react with manganese species to form sulfate salts, leading to irreversible activity decrease.11 It is commonly accepted that in the process of NO catalytic oxidation, NO were firstly adsorbed on the surface of catalysts to form nitrates; and then nitrates were reacted with O2 to form NO2.9,12 On one hand, the introduction of SO2 in the flue gas may influence the formation of different types of nitrates. On the other hand, the type of nitrates formed on the catalysts surface are related to surface oxygen species and manganese valence, which were influenced by morphology, element dopant et al. To get catalysts with excellent catalytic performance and good SO2 resistance, a second/third element dopant is a facile way. Several studies have reported a third dopant to Mn–Ce composites to improve their NO oxidation performance, because the regulated manganese and oxygen species.11b,13 However, few were focused on both catalytic performance and SO2 tolerance. The goal of this study is to improve both NO oxidation ability and SO2 tolerance of classical Mn–Ce composites. Nickel was selected as the third dopant, because introduction of nickel lead to more surface oxygen and regulate metal valence. Moreover, SO2-TPD confirmed that introduction of nickel to Mn–Ce composites was helpful to reduce SO2 adsorption.14 In this study, we fabricated a series of NixMn6−xCe4 ternary oxides to further discuss interaction among three elements. The Ni2Mn4Ce4 catalysts showed best low temperature activity and good SO2 resistance.
2 Experimental sections
2.1 Catalysts preparation
Previous studies15,16 had shown that Mn/Ce composites with a molar ratio equal or slightly more than 1 showed a most active catalytic activity; similar with our previous work, in which the catalyst with a molar ratio of Mn/Ce = 6/4 had the best catalytic performance, because strong interaction between manganese and ceria. As a result, nickel substituted Mn6Ce4 catalysts were prepared, denoted as NixMn6−xCe4, where x represented the relative molar ratio of Ni and Mn. All chemicals used in this study, such as Mn(NO3)2 (50 wt%), Ce(NO3)3·6H2O, Ni(NO3)2·6H2O and NaOH were purchased from Xilong Cop. (China) and used without further purification. The mesoporous composites were prepared through a nano-casting method by employing KIT-6 as templates. KIT-6 silica was prepared as previously reported studies.17 In a typical procedure, 1.0 g KIT-6 was suspended in 80 mL n-hexane and stirred at room temperature for 2 h. Then a mixed solution of Mn(NO3)2, Ni(NO3)2 and Ce(NO3)3 was added slowly with vigorous stirring. After stirred overnight, the mixture was filtered and dried at 80 °C for 24 h. The obtained powder was calcined at 550 °C for 4 h, with a heating rate of 2 °C min−1 in air. Finally, the sample was treated three times with a 2 M NaOH solution, washed to pH ∼ 7 and dried at 80 °C.2.2 Catalytic activity evaluation
The NO catalytic oxidation activity tests of prepared samples were performed in a fix-bed quartz tubular reactor. 100 mg catalysts (40–60 mesh) were placed in the reactor, with a weight hourly space velocity (WHSV) of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1 (GHSV ∼ 85
000 mL g−1 h−1 (GHSV ∼ 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1). The feed gas consisted of 200 ppm NO, 5% O2, 200 ppm SO2 (when used) and balance N2, with a flow rate of 0.1 L min−1. The concentration of NO and NO2 were monitored by a two channel CLD60 (Eco physics) instrument. NO conversion rate was calculated by:
000 h−1). The feed gas consisted of 200 ppm NO, 5% O2, 200 ppm SO2 (when used) and balance N2, with a flow rate of 0.1 L min−1. The concentration of NO and NO2 were monitored by a two channel CLD60 (Eco physics) instrument. NO conversion rate was calculated by:| NO conversion = (NOin − NOout)/NOin × 100% |
2.3 Characterization
XRD patterns were recorded on a PANalytical X'Per PRO X-ray diffraction in 2θ from 5° to 90° with a scanning step of 0.0334°. The specific surface area and pore size distributions of all catalysts were obtained according to the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively, using N2 adsorption–desorption method on an automatic surface analyzer (SSA-7300, BJ-Builder, China) at 77 K. Each sample was pre-degassed at 150 °C for 3 h. H2 temperature-programmed reduction (H2-TPR) was conducted on a Micromeritics Chemisorb 2720 analyzer (Micromeritics, USA) at a heating rate of 10 °C min−1 with 5% H2/Ar gas. The H2 consumption was recorded continuously to investigate reduction abilities. O2-TPD was performed on a BJ-Builder 1200 apparatus; 100 mg 40–60 mesh sample was firstly heated to 500 °C and kept for 30 min in He flow to remove surface adsorptive species. After cooling down to 50 °C, the sample was treated in O2 for 1 h, and then blew with He for 30 min. Finally, the TCD signal was collected to 900 °C at 10 °C min−1 in 30 mL min−1 He flow. Surface species of as-prepared catalysts were determined by X-ray photoelectron spectroscopy (XPS) using a XLESCALAB 250 Xi electron spectrometer (Thermo Scientific, USA) with monochromatic Al Kα radiation (1486.6 eV). A Bruker Vertex 70 spectrometer (Bruker, USA) equipped with diffuse reflectance accessory (PIKE, and MCT/A detector cooled by liquid nitrogen) was used for recording the in situ DRIFT spectra of the samples. The sample was pretreated at 300 °C for 2 h in N2 (50 mL min−1). The reaction system was cooled to 200 °C in N2, and the spectra were collected as background. The feed gas was 250 ppm NO, 5% O2 (when used), and balance N2, with a flow rate of 0.1 L min−1. When the spectra was pre-adsorbed by NO or NO + O2, then purged by N2 for 30 min to remove weakly adsorbed species, and finally recorded by accumulating 32 scans at a resolution of 4 cm−1.3 Results and discussions
3.1 Catalytic performances
The NO–NO2 catalytic activities of as-prepared catalysts were tested and compared from 120 °C to 360 °C, and the results were shown in Fig. 1. NO conversion rate over all catalysts firstly raised to a peak with the temperature increasing; then NO conversion rate decreased as the reaction temperature continuous increasing. Ni1Mn5Ce4 and Ni2Mn4Ce4 reached its highest NO conversion of 90% and 95% at 210 °C, respectively. Mn6Ce4 and Ni3Mn4Ce4 reached their maximum NO conversion of 85% and 81% at 240 °C, respectively. Ni4Mn2Ce4 and Ni1Mn5Ce4 had a similar NO conversion of 77% at 270 °C. Ni6Ce4 performed the worst NO conversion, with its maximum conversion of 60% at 300 °C. The catalytic activity of different samples varied at low temperature, and mainly followed the sequence of Ni2Mn4Ce4 > Ni1Mn5Ce4 > Mn6Ce4 > Ni3Mn3Ce4 > Ni4Mn2Ce4 > Ni5Mn1Ce4 > Ni6Ce4. The sample Ni2Mn4Ce4 can reach its maximum NO conversion of 95% at 210 °C, better than previous studies shown in Table 1.| Catalyst | Preparation method | Reaction conditions | NO conversion | References |
|---|---|---|---|---|
| γ-MnO2 | Hydro-thermal synthesis, powder | 500 ppm NO, 5% O2, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 000 h−1 |
250 °C, 86.4% | 19 |
| MnO2 | Precipitation method, powder | 500 ppm NO, 5% O2, 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1 000 mL g−1 h−1 |
210 °C, 91% | 12a |
| Ce–Co composite oxides | Sol–gel method, powder | 300 ppm NO, 10% O2, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 000 h−1 |
230 °C, 93% | 18 |
| Urchin-like γ-MnO2 | Hydro-thermal synthesis, powder | 500 ppm NO, 10% O2, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 000 h−1 |
280 °C, 88% | 20 |
| Ni2Mn4Ce4 | Nano-casting method, powder | 200 ppm NO, 5% O2, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1 000 mL g−1 h−1 |
210 °C, 95% | This work |
As the conversion of NO to NO2 is an exothermal reaction, and the reaction is thermodynamic limited at higher temperature.12,18 The thermodynamic equilibrium of NO to NO2 under the same condition was shown in dashed line. At high temperature, the catalytic activity was closely related to the equilibrium, which indicated the reaction was thermodynamic control. However, it can be seen that the low temperature catalytic activity was related to the relative ratios of manganese and nickel. To investigate the effect, further characterizations were applied and discussed in the following parts.
3.2 Physicochemical properties
As surface area and pore size of catalysts contributed much to catalytic activity, the N2 adsorption–desorption isotherms and pore size distributions were shown in Fig. 3; the specific area and average pore size were also summarized in Table 2. All the catalysts showed a type IV isotherm with a H3 hysteresis loop, indicating the existence of mesoporous structure. The specific surface area followed a sequence of Ni2Mn4Ce4 > Ni1Mn5Ce4 > Ni3Mn3Ce4 > Ni4Mn2Ce4 > Mn6Ce4 > Ni4Mn2Ce4 > Ni5Mn1Ce4 > Ni6Ce4. It can be seen that the surface area of the catalysts firstly increased with nickel addition, and then decreased, indicating the three elements incorporated with each other. The Ni2Mn4Ce4 catalyst had the largest surface area, which may also have more active sites for NO adsorption and oxidation, consistent with NO catalytic activity. It was interesting that when x < 4, nickel content increase could enlarge surface area; when x > 4, surface area decreased with further nickel addition. The results were consistent with XRD patterns, because when x > 4, the high crystallinity was a reason for surface area decrease. As the samples all presented only CeO2 phase when x < 4, it was more meaningful to discuss these samples. The specific area of samples from Ni1Mn5Ce4 to Ni4Mn2Ce4 were larger than Mn6Ce4, so it is reasonable to attribute the change to the introduction of nickel into Mn–Ce system. The H2-TPR and XPS results in the following parts would also support our hypothesis.
 | ||
| Fig. 3 N2 adsorption–desorption isotherm and pore size distributions of composites, and their specific surface area data. | ||
| Samples | BETa (m2 g−1) | Pore volumeb (mL g−1) | Pore diametera (nm) | Oadsc/(Olatt + Oads) | Ni3+/Ni2+c | Mn3+/Mn4+c | Ce3+/Ce4+c |
|---|---|---|---|---|---|---|---|
| a Calculated by BET method, obtained from N2 adsorption at 77 K. b Determined by adsorption capacity at relative pressure of P/P0 = 0.99. c Calculated from XPS data. | |||||||
| Mn6Ce4 | 152.116 | 0.3955 | 10.70 | 0.81 | — | 0.68 | 0.27 |
| Ni1Mn5Ce4 | 208.031 | 0.3051 | 7.28 | 0.91 | 11.15 | 0.64 | 0.34 |
| Ni2Mn4Ce4 | 221.013 | 0.3867 | 6.24 | 0.98 | 3.46 | 0.42 | 0.28 |
| Ni3Mn3Ce4 | 160.861 | 0.2849 | 8.66 | 0.88 | 3.74 | 0.51 | 0.56 |
| Ni4Mn2Ce4 | 159.982 | 0.3237 | 7.22 | 0.94 | 1.87 | 0.50 | 0.33 |
| Ni5Mn1Ce4 | 122.246 | 0.3285 | 11.20 | 0.90 | 0.57 | 0.81 | 0.45 |
| Ni6Ce4 | 108.082 | 0.5125 | 17.64 | 0.91 | 0.35 | — | 0.41 |
The O2-TPD spectra were shown in Fig. 4(B), and were used to determine the oxygen species in different samples. The O2-desorption spectra were divided into three segments, according to their desorption temperature.27 The peaks < 400 °C (denoted as O2-α) was ascribed to the desorption of weakly adsorbed oxygen species. The peaks between 400 and 600 °C (O2-β) were related to surface non-stoichiometric oxygen desorption.28 And the peaks > 600 °C (O2-γ) represented that the remained lattice oxygen was continuously declined, indicating higher valence of metal ions were reduced to lower valence. As the reaction temperature of NO oxidation in this study was lower than 400 °C, it was more meaningful to discuss O2-α. The peak areas related to O2-α for samples NixMn6−xCe4 (x from 0 to 6) were 6381, 7235, 8793, 6125, 5006, 236, and 6104, respectively. Ni2Mn4Ce4 exhibited the most O2-α species, and the result was consistent with XPS data in the next part.
Fig. 5(B) showed the Mn 2p spectra of NixMn6−xCe4 (x = 0–6), and Mn 2p3/2 can be de-convoluted into two peaks. The peaks at 643.2 eV and 641.4 eV can be assigned to Mn4+ and Mn3+, indicating a mixed valence manganese state.31 As shown in Table 2, the ratio of Mn3+/Mn4+ was associated with Ni/Mn molar ratio. Ni2Mn4Ce4 showed the smallest proportion of Mn3+/Mn4+, indicating more high valance Mn species in it. Furthermore, the catalytic performance sequence is consistent with Mn3+/Mn4+, indicating high valance manganese may be the active sites for NO oxidation.
The Ni 2p XPS spectra was convoluted into three peaks in Fig. 5(C). The peaks can be assigned to the main peak of Ni 2p and two satellite peaks, which were located at 854.4 eV, 856.5 eV and 861.5 eV, respectively. The peak at 854.4 eV (with satellite 861.5 eV) was corresponding to Ni2+ species;32 and the peak at 856.5 eV origins from Ni3+ species.33 The Ni3+/Ni2+ ratios of different samples were summarized in Table 1. When x was less than 3, the Ni3+/Ni2+ firstly decreased and then increased. Ni2Mn4Ce4 had the lowest Ni3+/Ni2+ and Mn3+/Mn4+, indicating the existence of Ni3+ + Mn3+ → Ni2+ + Mn4+. However, when x was more than 3, Ni3+/Ni2+ decreased sharply, indicating nickel species were mainly existed in the form of Ni2+. This was consistent with XRD results, with the appearance of NiO peaks. The results showed that the addition of nickel could regulate the manganese valence, especially when x < 3. When x > 3, nickel oxide was hard to form solid solution with ceria, therefore leading to separate phase of NiO and CeO2.
The Ce 3d spectra can be decomposed into eight peaks, as shown in the assignments of Fig. 5(D). According to literatures, The two bands labeled as v′ and u′ corresponded to Ce3+, and the other six bands can be assigned to Ce4+.34 The Ce3+/Ce4+ ratios were summarized in Table 1. Surprisingly, although molar ratio of cerium didn't change, the Ce3+/Ce4+ ratios varied among different samples. This indicated there was also an interaction between cerium, nickel and manganese. Compared with other samples, Ni2Mn4Ce4 presented lowest Ce3+/Ce4+ ratio. More surface Ce4+ species would facilitate Ni2+ + Ce4+ → Ce3+ + Ni3+, therefore enhancing the reaction of Ni3+ + Mn3+ → Ni2+ + Mn4+.
From the above characterizations, it can be concluded Ni2Mn4Ce4 exhibited biggest specific surface area, high ratio of Mn4+, which can make it more productive in NO catalytic oxidation.
3.3 In situ DRIFTs study
As Ni2Mn4Ce4 presented the best NO catalytic oxidation activity, a series of in situ DRIFTs experiments were investigated in order to study NO and/or O2 adsorption behaviors. | ||
| Fig. 6 In situ DRIFTs spectra of Ni2Mn4Ce4 catalysts exposed to (A) NO + O2/N2 at different temperatures; (B) NO + N2 and NO + O2/N2 at 200 °C. | ||
When 250 ppm NO (balanced with N2) was introduced, the peaks intensity increased with time. The existence of NO2 related peak indicated that NO can react with surface oxygen to form NO2. The peak at 1288 cm−1 can be assigned to monodentate nitrate and it shifted to 1341 cm−1, which can be ascribed to cis-dimer nitroso. When O2 was introduced to the flue gas, the nitrate peaks first increased and then decreased. However, the NO2 peaks increased with time. This indicated nitrate may be an important intermediate to form NO2, and it can be easily desorbed from the catalyst surface. Interestingly, the peaks related to cis-dimer nitroso disappeared after introducing O2, suggesting it was rapidly oxidized by oxygen to form NO2.
3.4 Proposed mechanism of NO catalytic oxidation on Ni2Mn4Ce4
From the results of surface elements distribution, temperature programmed reduction analysis, we obtained that the existence of Ni3+ + Mn3+ → Ni2+ + Mn4+ and Ce4+ + Ni2+ → Ni3+ + Ce3+. Higher valence manganese was the main sites for NO catalytic oxidation. From the in situ DRIFTs, the main intermediates were bidentate nitrate and monodentate nitrate species. Therefore, a mechanism based on the above experimental results was proposed, as shown in Fig. 7. The surface –M–O–M– firstly reacted with oxygen to form –M–O, and then the later species was reacted with gaseous NO to form nitrates intermediates. At proper temperature, the nitrates intermediates were easily decomposed into gaseous NO2, and the metal–oxygen species was returned to original states to form a complete catalytic circle.3.5 SO2 resistance of different catalysts
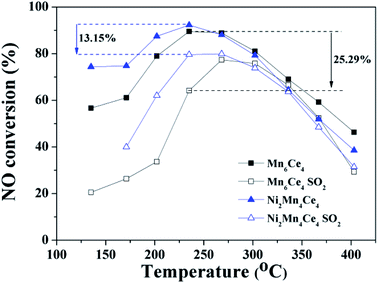
In practical conditions, SO2 that co-existed with NO may influence the catalytic oxidation of NO. Studies of NO oxidation in the existence of SO2 (Ni2Mn4Ce4 and Mn6Ce4) were conducted, and the results were shown in Fig. 8. When SO2 was introduced, the NO conversion rate was decreased for both two catalysts. When the temperature was above ∼270 °C, the activity decrease was not significant; mainly because the reaction is thermodynamic limited at higher temperature. However, at the lower temperature range, the NO conversion rate dropped drastically. For example, the NO conversion rate for Mn6Ce4 and Ni2Mn4Ce4 dropped by 25.29% and 13.15% at 180 °C, respectively. Ni2Mn4Ce4 had a better SO2 resistance compared with its counterpart without nickel, because nickel may decrease SO2 adsorption, similar with our previous study.144 Conclusions
In this paper, a series of mesoporous Ni–Mn–Ce ternary oxides were fabricated for NO catalytic oxidation, and the properties were characterized by various experiments. The Ni2Mn4Ce4 catalyst showed a 95% NO conversion rate at 210 °C. The introduction of nickel into Mn–Ce composites can regulate the ratio of surface oxygen and manganese species, therefore improving the NO catalytic oxidation. Compared with other catalysts, Ni2Mn4Ce4 exhibited a higher specific surface area, lower reduction temperature, more surface Mn4+ species, which led to its higher NO oxidation activity. XPS results can provide proofs for Ni2+ + Ce4+ → Ce3+ + Ni3+, and Ni3+ + Mn3+ → Ni2+ + Mn4+. From the results of in situ DRIFTs, a possible mechanism was proposed, in which nitrates species were main intermediates. The SO2 resistance experiments showed that nickel addition can increase SO2 durability of catalysts, mainly because nickel may decrease SO2 competitive adsorption on catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by the National Key Research and Development Plan (2016YFC0204100, 2016YFC0204103), Control Strategy and Technology Integrated Demonstration of Industrial Source Pollution in Guanzhong area of China (ZDRW-ZS-2017-6-2). The authors also acknowledge the analytical and testing center of Institute of Process Engineering, Chinese Academy of Sciences, for their extensive help in testing of samples.References
- (a) G. Busca, L. Lietti, G. Ramis and F. Berti, Appl. Catal., B, 1998, 18, 1 CrossRef CAS; (b) P. G. Smirniotis, P. M. Sreekanth, D. A. Peña and R. G. Jenkins, Ind. Eng. Chem. Res., 2006, 45, 6436 CrossRef CAS.
- L. J. Alemany, L. Lietti, N. Ferlazzo, P. Forzatti, G. Busca, E. Giamello and F. Bregani, J. Catal., 1995, 155, 117 CrossRef CAS.
- H. Hu, S. X. Cai, H. R. Li, L. Huang, L. Y. Shi and D. S. Zhang, ACS Catal., 2015, 5, 6069 CrossRef CAS.
- S. Adjimi, J. M. García-Vargas, J. A. Díaz, L. Retailleau, S. Gil, M. Pera-Titus, Y. Guo and A. Giroir-Fendler, Appl. Catal., B, 2017, 219, 459 CrossRef CAS.
- C. Ciardelli, I. Nova, E. Tronconi, D. Chatterjee and B. Bandl-Konrad, Chem. Commun., 2004, 2718 RSC.
- J. Li, W. H. Goh, X. Yang and R. T. Yang, Appl. Catal., B, 2009, 90, 360 CrossRef CAS.
- (a) Z. Wu, N. Tang, L. Xiao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2010, 352, 143 CrossRef CAS; (b) F. Meng, S. Zhang, X. Li, Y. Zeng and Q. Zhong, Mol. Catal., 2019, 473, 110393 CrossRef.
- W. Cai, Q. Zhong, J. Ding and Y. Bu, Chem. Eng. J., 2015, 270, 1 CrossRef CAS.
- Z. Hong, Z. Wang and X. Li, Catal. Sci. Technol., 2017, 7, 3440 RSC.
- (a) M. Machida, M. Uto, D. Kurogi and T. Kijima, J. Mater. Chem., 2001, 11, 900 RSC; (b) P. Sudarsanam, B. Hillary, M. H. Amin, S. B. A. Hamid and S. K. Bhargava, Appl. Catal., B, 2016, 185, 213 CrossRef CAS.
- (a) N. Tang, Y. Liu, H. Wang and Z. Wu, J. Phys. Chem. C, 2011, 115, 8214 CrossRef CAS; (b) K. Li, X. Tang, H. Yi, P. Ning, D. Kang and C. Wang, Chem. Eng. J., 2012, 192, 99 CrossRef CAS.
- (a) H. Chen, Y. Wang and Y.-K. Lyu, Mol. Catal., 2018, 454, 21 CrossRef CAS; (b) S. J. Ma, X. W. Wang, T. Chen and Z. H. Yuan, Chem. Eng. J., 2018, 354, 191 CrossRef CAS.
- T. Zhang, H. Li, Z. Yang, F. Cao, L. Li, H. Chen, H. Liu, K. Xiong, J. Wu, Z. Hong and W. Wang, Appl. Catal., B, 2019, 247, 133 CrossRef CAS.
- W. M. Li, H. D. Liu and Y. F. Chen, RSC Adv., 2019, 9, 11912 RSC.
- S. Lai, Y. She, W. Zhan, Y. Guo, Y. Guo, L. Wang and G. Lu, J. Mol. Catal. A: Chem., 2016, 424, 232 CrossRef CAS.
- Z. Liu, Y. Yi, S. Zhang, T. Zhu, J. Zhu and J. Wang, Catal. Today, 2013, 216, 76 CrossRef CAS.
- F. Kleitz, S. Hei Choi and R. Ryoo, Chem. Commun., 2003, 2136 RSC.
- Z. Wang, F. Lin, S. Jiang, K. Qiu, M. Kuang, R. Whiddon and K. Cen, Fuel, 2016, 166, 352 CrossRef CAS.
- F. Gao, X. Tang, H. Yi, C. Chu, N. Li, J. Li and S. Zhao, Chem. Eng. J., 2017, 322, 525 CrossRef CAS.
- B. Zhao, R. Ran, X. Wu and D. Weng, Appl. Catal., A, 2016, 514, 24 CrossRef CAS.
- X. Tang, J. Chen, X. Huang, Y. Xu and W. Shen, Appl. Catal., B, 2008, 81, 115 CrossRef CAS.
- C. Lamonier, A. Ponchel, A. D'Huysser and L. Jalowiecki-Duhamel, Catal. Today, 1999, 50, 247 CrossRef CAS.
- T. Zhu and M. Flytzani-Stephanopoulos, Appl. Catal., A, 2001, 208, 403 CrossRef CAS.
- G. Long, M. Chen, Y. Li, J. Ding, R. Sun, Y. Zhou, X. Huang, G. Han and W. Zhao, Chem. Eng. J., 2019, 360, 964 CrossRef CAS.
- X. Hu, L. Huang, J. Zhang, H. Li, K. Zha, L. Shi and D. Zhang, J. Mater. Chem. A, 2018, 6, 2952 RSC.
- Y. Wan, W. Zhao, Y. Tang, L. Li, H. Wang, Y. Cui, J. Gu, Y. Li and J. Shi, Appl. Catal., B, 2014, 148–149, 114 CrossRef CAS.
- X. Zhou, X. Lai, T. Lin, J. Feng, Z. Hou and Y. Chen, New J. Chem., 2018, 42, 16875 RSC.
- H. Li, W. K. Ho, J.-j. Cao, D. Park, S. C. Lee and Y. Huang, Environ. Sci. Technol., 2019, 53, 10906 CrossRef CAS.
- Y. Xie, J. Wu, G. Jing, H. Zhang, S. Zeng, X. Tian, X. Zou, J. Wen, H. Su, C.-J. Zhong and P. Cui, Appl. Catal., B, 2018, 239, 665 CrossRef CAS.
- W. Tang, M. Yao, Y. Deng, X. Li, N. Han, X. Wu and Y. Chen, Chem. Eng. J., 2016, 306, 709 CrossRef CAS.
- W. Xu, G. Zhang, H. Chen, G. Zhang, Y. Han, Y. Chang and P. Gong, Chin. J. Catal., 2018, 39, 118 CrossRef CAS.
- S. R. Kirumakki, B. G. Shpeizer, G. V. Sagar, K. V. R. Chary and A. Clearfield, J. Catal., 2006, 242, 319 CrossRef CAS.
- J. Liu, X. Li, R. Li, Q. Zhao, J. Ke, H. Xiao, L. Wang, S. Liu, M. Tadé and S. Wang, Appl. Catal., A, 2018, 549, 289 CrossRef CAS.
- (a) C. Tang, J. Li, X. Yao, J. Sun, Y. Cao, L. Zhang, F. Gao, Y. Deng and L. Dong, Appl. Catal., A, 2015, 494, 77 CrossRef CAS; (b) J. Zhang, Y. Cao, C.-A. Wang and R. Ran, ACS Appl. Mater. Interfaces, 2016, 8, 8670 CrossRef CAS.
- (a) G. Qi and R. T. Yang, J. Phys. Chem. B, 2004, 108, 15738 CrossRef CAS; (b) H. Wang, H. Chen, Y. Wang and Y.-K. Lyu, Chem. Eng. J., 2019, 361, 1161 CrossRef CAS; (c) C. C. Dinerman and G. E. Ewing, J. Chem. Phys., 1970, 53, 626 CrossRef CAS.
- G. Zhou, B. Zhong, W. Wang, X. Guan, B. Huang, D. Ye and H. Wu, Catal. Today, 2011, 175, 157 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |