Open Access Article
Open Access ArticleSuperoleophobic micro-nanostructure surface formation of PVDF membranes by tannin and a condensed silane coupling agent†
Shuman
Feng‡
a,
Mu
Li‡
b,
Songfeng
Zhang
b,
Yaowen
Zhang
b,
Bing
Wang
a and
Lili
Wu
 *b
*b
aPeople's Hospital of Henan Province, Zhengzhou, Henan 450003, China
bSchool of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China. E-mail: polym_wl@whut.edu.cn
First published on 8th October 2019
Abstract
A tannin-based hybrid coating was coated on the PVDF membrane surface through a simple one-step co-deposition of tannin and KH550. A micro/nano hierarchical structure was formed on the PVDF membrane surface through hydrolysis/condensation of KH550 and Michael addition reaction between oxidized tannin and an amino group revealed by the field-emission scanning electron microscopy, atomic force microscopy and Fourier transform infrared spectroscopy, which established a harsh surface. Abundant hydrophilic groups and high surface roughness endowed the modified membranes with high hydrophilicity and underwater superoleophobicity. The modified PVDF membranes possess excellent oil/water separation and antifouling performance due to the underwater superoleophobicity. Moreover, the modified membrane exhibited outstanding stability.
The oil/water separation process, especially for separating emulsified oil-in-water emulsions, has become an increasingly important part of water treatment as more and more oily wastewater has been produced from industrial processing and oil spills.1–5 Among many conventional methods, polymer-dominated filtration technology has been widely applied to water treatment for its simple operation, low cost and easy control of pores.6–10 Polyvinylidene fluoride (PVDF) is a promising membrane material owing to its excellent mechanical and chemical properties. However, the intrinsic hydrophobicity makes PVDF easily fouled during treatment of aqueous solutions containing organic matters, decreasing its service life, which has limited its application in the field of oil/water separation.11–14 Thus, it is of great importance to improve the hydrophilicity of PVDF porous membranes for highly efficient and eco-friendly separation of oily wastewater.
The concept of bio-adhesion stems from mussel adhesion protein (MAP) with broad adhesion potency. MAP rich in catechol, amino acids and 3,4-dihydroxyphenylalanine (DOPA) allows mussels to adhere to a variety of materials. Dopamine containing amine and catechol groups exhibits a molecular structure similar to that of DOPA, and has become a focus of attention as a novel bioadhesive coating.15–19 Low-cost tannin, which has analogous polyphenols units with dopamine, has also been reported to have excellent interfacial properties and could be coated on a variety of substrates including hydrophobic polymer materials.20–24 In our previous work,25 we demonstrated that the plant tannin layer can strongly construct on the surface of the PVDF membrane and effectively improve the hydrophilicity and filtration performance of PVDF membrane. In this work, in order to further enhance the underwater superoleophobicity of PVDF membrane for oil-in-water emulsion separation, we constructed a tannin-based hybrid coating on the PVDF membrane surface through a simple one step co-deposition of tannin and (3-aminopropyl) triethoxy-silane (KH550) which is a silane coupling agent containing amino group. On the one hand, hybrid coating can coat on the PVDF membrane surface because of the existence of polyphenols tannin, on the other hand, tannin and KH550 can bond together by means of Michael addition reaction between oxidized tannin and amino of KH550, at the same time, hydrolysis and condensation of KH550 can form crosslinking agents, which can greatly enhance the adhesive ability of coating layer.
The modified membranes were fabricated through a simple one step dip-coating method in plant tannin and KH550 hybrid solution in the context of a weak alkaline solution (pH 7.8) and contacting with atmosphere. Fig. 1 shows the co-deposition pathway. The phenolic group of tannin is oxidized to benzoquinone under weakly alkaline conditions and undergoes a Michael addition reaction with the amino group in KH550.26–28 The alkoxy group of the silane coupling agent has a hydrogen bond with the phenolic hydroxyl group of the tannin after hydrolysis, and will condense with itself to form a silicon-containing oligomer. Tannin and KH550 form a strong coating on the membrane surface through this complex cross-linking structure. The silicon-containing oligomer produced by KH550 changed the surface morphology and roughness of the membrane surface, and the wettability of the membrane surface was significantly improved.
The surface morphology of the pristine membrane (TK-0) and modified membranes with different adding contents of KH550 were observed via FESEM and AFM, which can be seen in the Fig. 2a and b. As indicated by the FESEM images in Fig. 2a, some spherical particles have formed on the all modified membranes through co-deposition process, but the size and number of particles are greatly different among various membranes. With increasing the content of KH550, the particle size and number sharply increase. Moreover, it can be seen that the top surface of TKN-0.6 is almost completely coated by the particles, which shows a micro/nano hierarchical structure. These phenomena can be rationalized by the formation of hybrid oligomer. The more the content of KH550 in the co-deposition process, the more and bigger hybrid oligomer will be formed through hydrolysis and condensation reaction of KH550. AFM images are shown in Fig. 2b, the roughness obviously increased with extending the content of KH550. This result corresponds with the FESEM and indicates the formation of micro/nano structure. Interestingly, the roughness of TK-0 are higher than TKN-0.15, it can be explained that the main part of the coating layer on the TKN-0.15 are tannin due to the less adding content of KH550, which can form a homogeneous coating with a few particles. In contrast to polydopamine, our modified membranes show a slight change in color (Fig. S1†), which indicates that this novel process has more widely application.
The deposition ratio (DR) can directly reflect the amount of coating layer which deposited on the membranes. As increasing of the content of KH550, the DR of the modified membranes are increasing linearly, which demonstrates that more hybrid oligomer was formed and anchored on the membrane surface (Fig. S2†). The surface chemical elements and groups are critical for wettability of materials. The XPS spectrum of the membranes were shown in Fig. 2c and Table S1,† and the elemental compositions of different modified membranes were determined. The presence of oxygen, nitrogen, and silicon elements can be confirmed in the XPS spectrum, wherein the oxygen element is derived from tannin and KH550, and the nitrogen and silicon elements are derived only from PVP. Therefore, the Si/O, Si/C and O/C ratio can illustrate the content of tannin and KH550 applied to the surface of the membrane to a certain extent. The atomic ratio about Si/O, Si/C and O/C are all raising with the increase of KH550, this shows that more silicon-containing oligomers are fixed on the membrane surface. These indicate that tannin and KH550 have co-deposited on membrane surface successfully. This result is also confirmed by ATR-FTIR measurement (Fig. S3†). Four notably absorption peaks at 1608 cm−1, 1499 cm−1, 1323 cm−1, and 1098 cm−1 were detected, which assigned to skeletal vibration of aromatic rings, N–H bending vibrations, C–N stretching vibration and Si–O–Si stretching vibration, respectively. These imply that a lot of hydrophilic groups have coated on the hydrophobic PVDF membrane surface, which can significantly improve its hydrophilicity.
As an interfacial issue, membrane surface energy and surface roughness can simultaneously affect the wettability.29–32 According to Wenzel's model, roughness can increase the actual contact area between droplet and substrate, thus improving the hydrophilicity and total surface energy of hydrophilic substrate.33,34 In this work, the hybrid coating layer contains abundant hydrophilic groups, thus, the hydrophilicity of modified PVDF membranes have improved greatly. As shown in Fig. 3a, the water contact angle (WCA) of modified membranes sharply decreased comparing with TK-0, and the minimum WCA value was occurred to TKN-0.3 rather than TKN-0.6, which indicates that this modified process exists a threshold WCA with the increase of KH550. The water droplet permeation of modified membrane can be observed in Fig. 3b, the WCA rapidly decrease to 0° within 5 s, which exhibit much high hydrophilicity. Fig. 3b shows the dynamic underwater oil-adhesion property of TKN-0.6. An extremely low oil-adhesion performance was detected, it can be explained that a water barrier has formed on the modified PVDF membrane surface under water due to the high hydrophilicity, which can greatly reject the oil droplets and effectively decrease fouling. The underwater oil contact angle (OCA) directly revealed the excellent oleophobicity of our modified membranes as shown in Fig. 3c and d. The underwater chloroform contact angle of different membranes are raising with the increase of KH550. The value of TK-0 is 127°, but the modified membranes are all higher than 150°, the value of TKN-0.6 is 163° which show a outstanding oleophobicity. Therefore, underwater different oils (dichloromethane, toluene, petroleum ether and diesel) contact angle of TKN-0.6 were measured to further characterize the oil repelling performance. It can be seen that the OCA of four oils are 161°, 162°, 163° and 156° respectively, indicating a underwater superoleophobic property which is critical to oil/water separation. This underwater superoleophobicity is caused by the cooperation of high hydrophilicity and roughness of tannin and KH550 hybrid coating.
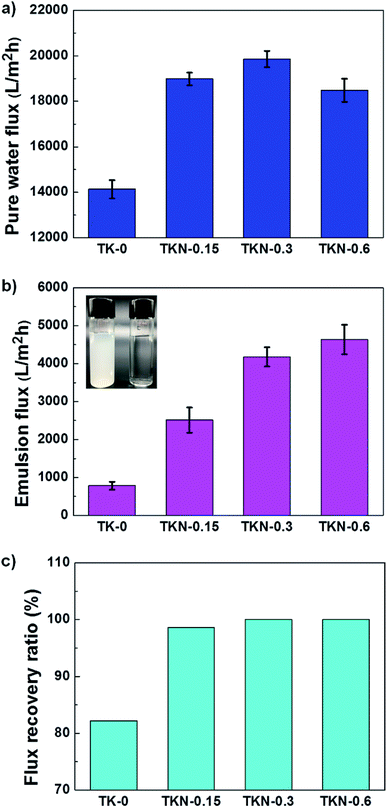
The pure water flux, emulsion filtration flux and flux recovery ratio (FRR) were measured by a vacuum driven filtration system (Fig. S4†) to evaluate filtration performance, as shown in Fig. 4. The pristine membrane and modified membranes were all wetted before measurement to avoid the compaction effect. The pure water flux was shown in Fig. 4a, the flux value of pristine membrane is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 L m−2 h−1, however, the modified membranes are 18
133 L m−2 h−1, however, the modified membranes are 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 L m−2 h−1, 19
982 L m−2 h−1, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 L m−2 h−1 and 18
863 L m−2 h−1 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 L m−2 h−1 respectively, which are much higher than pristine membrane. This result is triggered by the excellent hydrophilicity of coating layer on modified membranes. Meanwhile, the slightly different flux values among the modified membranes can be detected, which result in the blocking of micro/nano particles. The emulsion filtration performance is shown in Fig. 4b, the prepared emulsion had an average particle diameter of 487 nm as measured by a Malvern particle size analyzer. The flux value of pristine membrane is 782 L m−2 h−1, however, all the modified membranes exhibit high emulsion filtration flux, as the flux of 2512 L m−2 h−1, 4178 L m−2 h−1 and 4632 L m−2 h−1 for TKN-0.15, TKN-0.3 and TKN-0.6 respectively were obtained. This indicates the great emulsion separation efficiency of modified membranes. After filtration, the toluene-in-water emulsion is transformed into transparent exhibited by the inset images. The underwater superoleophobic property of modified membranes is the main reason for this outstanding filtration performance. The antifouling property was characterized by flux recovery ratio (FRR) shown in Fig. 4c. It can be seen that the FRR value of modified membranes are all higher than the pristine membrane (82%) and all more than 98%, even reaches 100% for TKN-0.3 and TKN-0.6. This excellent antifouling property should give the credit to the superoleophobic property which is aroused by the high hydrophilicity and roughness of tannin and KH550 hybrid coating. To measure the stability of modified membranes, a water rinsing experiment was taken and underwater chloroform contact angle was measured to evaluate the stability of underwater superoleophobic property of modified membrane (Fig. 5a). It is found that the underwater chloroform contact angle of TKN-0.3 stabilized at 157–160° during seven days rinsing, which disclosed the outstanding stability of hybrid coating coated PVDF membrane. As shown in Fig. 5b, the emulsion flux declines gradually with the increase of time due to the fouling of the membrane. The emulsion flux was almost completely recovered by washing with pure water after 1 hour of emulsion filtration, indicating the stability of the TKN-0.3 membrane.
480 L m−2 h−1 respectively, which are much higher than pristine membrane. This result is triggered by the excellent hydrophilicity of coating layer on modified membranes. Meanwhile, the slightly different flux values among the modified membranes can be detected, which result in the blocking of micro/nano particles. The emulsion filtration performance is shown in Fig. 4b, the prepared emulsion had an average particle diameter of 487 nm as measured by a Malvern particle size analyzer. The flux value of pristine membrane is 782 L m−2 h−1, however, all the modified membranes exhibit high emulsion filtration flux, as the flux of 2512 L m−2 h−1, 4178 L m−2 h−1 and 4632 L m−2 h−1 for TKN-0.15, TKN-0.3 and TKN-0.6 respectively were obtained. This indicates the great emulsion separation efficiency of modified membranes. After filtration, the toluene-in-water emulsion is transformed into transparent exhibited by the inset images. The underwater superoleophobic property of modified membranes is the main reason for this outstanding filtration performance. The antifouling property was characterized by flux recovery ratio (FRR) shown in Fig. 4c. It can be seen that the FRR value of modified membranes are all higher than the pristine membrane (82%) and all more than 98%, even reaches 100% for TKN-0.3 and TKN-0.6. This excellent antifouling property should give the credit to the superoleophobic property which is aroused by the high hydrophilicity and roughness of tannin and KH550 hybrid coating. To measure the stability of modified membranes, a water rinsing experiment was taken and underwater chloroform contact angle was measured to evaluate the stability of underwater superoleophobic property of modified membrane (Fig. 5a). It is found that the underwater chloroform contact angle of TKN-0.3 stabilized at 157–160° during seven days rinsing, which disclosed the outstanding stability of hybrid coating coated PVDF membrane. As shown in Fig. 5b, the emulsion flux declines gradually with the increase of time due to the fouling of the membrane. The emulsion flux was almost completely recovered by washing with pure water after 1 hour of emulsion filtration, indicating the stability of the TKN-0.3 membrane.
 | ||
| Fig. 5 (a) The underwater chloroform contact angle of TKN-0.3 membrane during the pure water rinsing test for 7 days. (b) Emulsion flux and flux recovery over three cycles. | ||
In summary, a hydrophilic and underwater superoleophobic PVDF membrane was fabricated through a simple one step co-deposition method, the low cost tannin and commercial KH550 hybrid coating layer was coated on PVDF membrane surface successfully. A micro/nano hierarchical structure, which can be adjusted by the adding content of KH550, was observed on the modified membrane surface. It can increase the roughness of membrane surface and obtain an excellent hydrophilic and underwater superoleophobic property. The underwater superoleophobicity endows the membrane with a superior antifouling property (underwater oil contact angle reaches 160°, FRR reaches 100%) and high oil/water separation efficiency (the emulsion flux reaches 4600 L m−2 h−1). The great performance indicates that this strategy is promising for practical applications in the field of treating oily wastewater.
Experimental section
The tannin solution was extracted from the mixture solution of tara powder and deionized water (1/10, w/w), which has been stirred for 4 h and left to stand overnight at room temperature. The modified membranes were fabricated through a simple dip-coating method. Firstly, commercial PVDF membranes were pre-wetted by isopropyl alcohol for 0.5 h and then placed in deionized water for at least 1 h to prepare for modification. Secondly, the extracted tannin aqueous solution (100 mL) was adjusted pH to weak alkaline with Tris-buffer (50 mM, pH ≈ 7.8), then certain amounts of KH550 (0.15 g, 0.3 g and 0.6 g) dissolved in 20 mL ethanol was immediately mixed with above-mentioned alkaline tannin solution. Thirdly, the per-wetted PVDF membranes were immersed in the mixture solution for 12 h contacting with atmosphere at room temperature. Finally, the modified membranes were rinsed deeply by deionized water and placed in deionized water or dried in air for further research. The various modified membranes were named TKN-0.15, TKN-0.3 and TKN-0.6 according to the adding content of KH550. For comparison, the pristine membrane was immersed in deionized water for 12 h which was named TK-0. Detailed procedures for a series of characterizations are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Program of Young Scholar sponsored by China Scholarship Council (201306955022) and Students Innovation and Entrepreneurship Training Program sponsored by Wuhan University of Technology (20161049701006).References
- S. Jamaly, A. Giwa and S. W. Hasan, J. Environ. Sci., 2015, 37, 15–30 CrossRef CAS.
- Z. Xue, Y. Cao, N. Liu, L. Feng and L. Jiang, J. Mater. Chem. A, 2014, 2, 2445–2460 RSC.
- Q. Ma, H. Cheng, A. G. Fane, R. Wang and H. Zhang, Small, 2016, 12, 2186–2202 CrossRef CAS.
- Z. Chu, Y. Feng and S. Seeger, Angew. Chem., 2015, 54, 2328–2338 CrossRef CAS.
- Y. Zhu, D. Wang, L. Jiang and J. Jin, NPG Asia Mater., 2014, 6, e101 CrossRef CAS.
- J. Yin and B. Deng, J. Membr. Sci., 2015, 479, 256–275 CrossRef CAS.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946 RSC.
- D. J. Miller, D. R. Dreyer, C. W. Bielawski, D. R. Paul and B. D. Freeman, Angew. Chem., 2017, 56, 4662–4711 CrossRef CAS.
- V. Kochkodan and N. Hilal, Desalination, 2015, 356, 187–207 CrossRef CAS.
- Z. Cui, E. Drioli and Y. M. Lee, Prog. Polym. Sci., 2014, 39, 164–198 CrossRef CAS.
- G.-d. Kang and Y.-m. Cao, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS.
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- J. Ji, F. Liu, N. A. Hashim, M. R. M. Abed and K. Li, React. Funct. Polym., 2015, 86, 134–153 CrossRef CAS.
- Z. Wang, X. Jiang, X. Cheng, C. H. Lau and L. Shao, ACS Appl. Mater. Interfaces, 2015, 7, 9534–9545 CrossRef CAS.
- Y. Xiang, F. Liu and L. Xue, J. Membr. Sci., 2015, 476, 321–329 CrossRef CAS.
- L. Shao, Z. X. Wang, Y. L. Zhang, Z. X. Jiang and Y. Y. Liu, J. Membr. Sci., 2014, 461, 10–21 CrossRef CAS.
- Z.-X. Wang, C.-H. Lau, N.-Q. Zhang, Y.-P. Bai and L. Shao, J. Mater. Chem. A, 2015, 3, 2650–2657 RSC.
- Y. L. Ji, M. B. M. Y. Ang, H. C. Hung, S. H. Huang, Q. F. An, K. R. Lee and J. Y. Lai, J. Membr. Sci., 2018, 557, 58–66 CrossRef CAS.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. Lau and P. B. Messersmith, Angew. Chem., 2013, 52, 10766–10770 CrossRef CAS.
- D. G. Barrett, T. S. Sileika and P. B. Messersmith, Chem. Commun., 2014, 50, 7265–7268 RSC.
- L. Pan, H. Wang, C. Wu, C. Liao and L. Li, ACS Appl. Mater. Interfaces, 2015, 7, 16003–16010 CrossRef CAS.
- X. Zhang, P.-F. Ren, H.-C. Yang, L.-S. Wan and Z.-K. Xu, Appl. Surf. Sci., 2016, 360, 291–297 CrossRef CAS.
- X. Zhang, Y. Lv, H. C. Yang, Y. Du and Z. K. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 32512–32519 CrossRef CAS.
- S. Zhang, L. Wu, F. Deng, D. Zhao, C. Zhang and C. Zhang, RSC Adv., 2016, 6, 71287–71294 RSC.
- H. C. Yang, K. J. Liao, H. Huang, Q. Y. Wu, L. S. Wan and Z. K. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 RSC.
- X. Zhang, P. F. Ren, H. C. Yang, L. S. Wan and Z. K. Xu, Appl. Surf. Sci., 2016, 360, 291–297 CrossRef.
- S. Ryu, Y. Lee, J. W. Hwang, S. Hong, C. Kim, T. G. Park, H. Lee and S. H. Hong, Adv. Mater., 2011, 23, 1971–1975 CrossRef CAS PubMed.
- B. Su, Y. Tian and L. Jiang, J. Am. Chem. Soc., 2016, 138, 1727–1748 CrossRef CAS.
- R. Du, X. Gao, Q. Feng, Q. Zhao, P. Li, S. Deng, L. Shi and J. Zhang, Adv. Mater., 2016, 28, 936–942 CrossRef CAS.
- Y. Dong, J. Li, L. Shi, X. Wang, Z. Guo and W. Liu, Chem. Commun., 2014, 50, 5586 RSC.
- M. Liu, Y. Zheng, J. Zhai and L. Jiang, Acc. Chem. Res., 2010, 43, 368 CrossRef CAS.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- J. Jiang, L. Zhu, L. Zhu, B. Zhu and Y. Xu, Langmuir, 2011, 27, 14180–14187 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05381f |
| ‡ These authors contributed to the work equally and should be regarded as co-first authors. |
| This journal is © The Royal Society of Chemistry 2019 |