Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deciphering the nutritive and antioxidant properties of Malay cherry (Lepisanthes alata) fruit dominated by ripening effects
Yan Zhang ab,
Shufei Chenb,
Junwei Huo
ab,
Shufei Chenb,
Junwei Huo *a and
Dejian Huang
*a and
Dejian Huang *bc
*bc
aCollege of Horticulture and Landscape Architecture, Northeast Agricultural University, Heilongjiang 150030, China. E-mail: huojunwei@neau.edu.cn
bDepartment of Food Science and Technology, National University of Singapore, 3 Science Drive 3, Singapore 117543, Singapore. E-mail: chmhdj@nus.edu.sg; Fax: +65 6775 7895; Tel: +65 6516 8821
cNational University of Singapore (Suzhou) Research Institute, Suzhou Industrial Park, Jiangsu 215123, China
First published on 21st November 2019
Abstract
In this study, Malay cherry fruit were explored for the changes in their nutritive and phenolic compositions upon ripening (unripe and ripe stages). Nutritive compositions (sugars, proteins, and fats) of the fruit increased, whilst organic acids of the fruit decreased in ripe fruit. Twenty-eight non-anthocyanin phenolics of the fruit were identified by the high-performance liquid chromatography-high resolution-time of flight mass spectrometry (HPLC-HR-TOF/MS2). Among them, quercetin-3-O-rutinoside and quercetin-3-O-glucoside are dominant species in the unripe fruit, and four more phenolics are shown in the ripe fruit. Additionally, seventeen anthocyanins were solely identified in the ripe fruit. This could be the signature phenolic profile of Malay cherry fruit. The total phenolics and total proanthocyanidins of the fruit significantly decreased upon ripening. Consistently, antioxidant capacities of the fruit also decreased upon ripening. Our results suggest unripe fruit are good sources of phenolic antioxidants that are worthwhile for utilisation as functional food sources.
1. Introduction
From ancient to modern times, ripe fruit are more acceptable to human beings than unripe ones. This could be simply because the ripe fruit always give a more enjoyable sensory experience. Knowledge of nutrients (sugars, organic acids, proteins, and fats) and phenolics in ripe and unripe fruit is likely to be ignored despite their large differences. McCune et al. revealed the fact that nutrients and phenolics of fruit are dominated by their ripening stages.1 A growing body of literature has, in fact, demonstrated that ripe sweet cherry,2 red raspberry,3 and blueberry4 possess higher total phenolic contents (TPC) than unripe ones; however, the contrary was observed in the TPC of black raspberry,3 strawberry,3 and blackberry.5. In other words, the changes in the TPC of ripe fruit are unpredictable. Therefore, an individual study is necessary to understand the nutritive and phenolic changes in a specific fruit upon ripening.The ripening of fruit includes three distinct stages: unripe, veraison, and ripe stages. As the nutritive and phenolic compositions of fruit vary largely at the intermediate stage, it is hard to be tracked. Therefore, the two steady stages (unripe and ripe) of fruit are expected to be further studied.6
Fruit are great sources of phenolic antioxidants that are believed to have wide spectrum of health benefits. This is especially so for berries and cherries. The berries are rich in phenolic antioxidants of diverse chemical structures and health promotion effects.7 We have witnessed the market penetration of acai berry from South America to become mainstream health foods because of their high antioxidant contents.8 Similarly, tart cherries are also known to contain high phenolic antioxidants with anti-inflammatory properties that can help reducing pain and accelerating strength recovery after exercise.9 It is well known that the phenolics of cherries support the potential preventive health benefits of cherry intake in relation to cancer, cardiovascular disease, inflammatory disease, diabetes, and Alzheimer's disease.1 There are many more small fruit, cultivated or wild, that receive little or no attention in scientific community and their potential values await to be uncovered as functional food ingredients. This is especially so in tropical region where there are plenty of exotic fruit. One such example is Lepisanthes alata, commonly known as Malay cherry. L. alata is a tropical species of Sapindaceae native to Southeast Asia. The fruit of Malay cherry are sub-globose shaped (2–4 cm) with the colour from green to dark reddish purple as fruit ripen. The pulp-with-peel portions of the ripe fruit are eaten fresh with fairly sweet taste.10 While the Malay cherry has long been popular in Singapore as a landscape tree, it attracted international interest resulting from our first report.11 We are intrigued by this tree because we found that the fruit and leaf proanthocyanidins showed potent inhibitory activity against starch hydrolases.11 According to our preliminary study, Malay cherry fruit are rich in not only proanthocyanidins, but also in other phenolic compounds (polyphenols and simple phenolics). Among these phenolic compounds, a continuously vast interest has been focused on anthocyanins and polyphenols due to their relatively high antioxidant capacities and corresponding health benefits.2,12 However, in terms of Malay cherry fruit, there is virtually no information on the profile of phenolic compounds, not to mention phenolic changes upon ripening. To our best knowledge, this is the first study to evaluate the effect of ripening on the nutritive and antioxidant properties of Malay cherry fruit.
From a food product design standpoint, nutritive and phenolic compositions, which largely determine the functionality of foods, can be modulated by ripening. Therefore, it is a prerequisite to be clear on the nutritive and phenolic compositions of the unripe and ripe fruit of Malay cherry. As well, additional understandings of the changes in total phenolics, total anthocyanins, total proanthocyanidins, and antioxidant capacities of these fruit were of great importance. Therefore, the aim of this study was to investigate the above descriptions. More specifically, unripe and ripe fruit were subjected to assess their antioxidant capacities and consequently identify the major phenolic compounds within the fruit.
2. Materials and methods
2.1 Materials
The Folin–Ciocalteu reagent, gallic acid, potassium chloride, sodium acetate, 4-dimethylaminocinnamaldehyde (DMAC), procyanidin A2, Trolox, 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), fluorescein, potassium dihydrogen phosphate, dibasic potassium phosphate, total dietary fibre assay kit (TDF-100A), sodium phosphate, catalyst mixture (47.7% anhydrous sodium sulphate, 47.7% potassium sulphate, 2.8% titanium dioxide, and 1.8% copper sulphate), methyl red, boric acid, 3,5-dinitrosalicylic acid (DNSA), maltose, glucose, fructose, sucrose, malic acid, succinic acid, citric acid, ammonium acetate, and sodium acetate were purchased from Sigma-Aldrich (St. Louis, MO). Sulphuric acid (98%), hydrochloric acid (37%), phosphoric acid (85%), and analytical grade solvents were purchased from Merck (Darmstadt, Germany). Sodium carbonate was purchased from GCE Chemicals (Malmö, Sweden). Acetic acid (glacial) was purchased from RCI Labscan (Bangkok, Thailand). HPLC grade solvents were purchased from VWR International GmbH (Darmstadt, Germany).The fresh Malay cherry fruit were sampled from 20 multiple trees at unripe (green) and ripe (dark reddish purple) stages on 9 and 23 October in Singapore, respectively. The sampling area is a 1 km radius around 1.449 N 103.820 E in Sembawang district of Singapore. Over 100 fruit were sampled at each stage for further study. After removal of the seeds of fruit, only the pulp-with-peel portions of fruit were used in this study. The phytonutrient contents in the seeds will be studied separately.
2.2 Physicochemical analysis
The sugar contents and compositions of unripe and ripe fruit were evaluated as follows. The defatted and lyophilised fruit powder (1.0 g) was ultrasonicated with aqueous ethanol (10.0 mL, 80%, v/v) for 30 min. The slurry was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074 g for 10 min at 4 °C to get the supernatant followed by evaporation at 40 °C. The concentrated sugar extracts were diluted with deionised water in the ratio of 1
074 g for 10 min at 4 °C to get the supernatant followed by evaporation at 40 °C. The concentrated sugar extracts were diluted with deionised water in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to get the ethanol–free sugar extracts (4 mL) for the following measurement. The total soluble solids (TSS) of the sugar extracts were measured using a digital RX-5000α refractometer (ATAGO, Tokyo, Japan) and calibrated using deionised water at 20 °C. The TSS was expressed as percent sucrose. The reducing sugar contents of the sugar extracts were measured using the DNSA assay and expressed as milligram maltose equivalent per gram of dry weight of fruit, according to our previous study.14 In brief, 100 μL of DNSA reagent was mixed with 100 μL of sugar extracts or maltose standard solutions (0 to 1.5 g L−1) in a 96-well microplate and boiled for 5 min. 100 μL of the cooling mixture was transferred to another 96-well microplate for measuring the absorbance at 540 nm with a Synergy HT microplate reader (Biotek Instruments Inc., Winooski, VT). The sugar compositions of the sugar extracts were analysed by a ultrafast liquid chromatograph Prominence system coupled with a LTII evaporative light scattering detector (Shimadzu, Kyoto, Japan), according to a literature method.15 Serial diluted sugar extracts (10 μL) or standards (fructose, glucose, and sucrose ranging from 0.1 to 10.0 g L−1) were filtered through a regenerated cellulose filter (0.45 μm) and injected into a Zorbax carbohydrate column (4.6 × 150 mm, 5 μm) with a guard column made by the same materials (Agilent, Palo Alto, CA). Mobile phase was acetonitrile (80%, v/v) at a flow rate of 1.4 mL min−1 for 23 min. The column oven temperature was set at 40 °C. Detector was set at 40 °C, gain 5, and pressure of 350 kPa. Sugars in the sample were identified by matching the retention times of standards and their concentrations were calculated by peak areas of the standard curves of the respective sugars. The standard curves were plotted with R2 greater than 0.99. The total sugar contents of fruit were calculated as combined values of fructose, glucose, and sucrose.
1 to get the ethanol–free sugar extracts (4 mL) for the following measurement. The total soluble solids (TSS) of the sugar extracts were measured using a digital RX-5000α refractometer (ATAGO, Tokyo, Japan) and calibrated using deionised water at 20 °C. The TSS was expressed as percent sucrose. The reducing sugar contents of the sugar extracts were measured using the DNSA assay and expressed as milligram maltose equivalent per gram of dry weight of fruit, according to our previous study.14 In brief, 100 μL of DNSA reagent was mixed with 100 μL of sugar extracts or maltose standard solutions (0 to 1.5 g L−1) in a 96-well microplate and boiled for 5 min. 100 μL of the cooling mixture was transferred to another 96-well microplate for measuring the absorbance at 540 nm with a Synergy HT microplate reader (Biotek Instruments Inc., Winooski, VT). The sugar compositions of the sugar extracts were analysed by a ultrafast liquid chromatograph Prominence system coupled with a LTII evaporative light scattering detector (Shimadzu, Kyoto, Japan), according to a literature method.15 Serial diluted sugar extracts (10 μL) or standards (fructose, glucose, and sucrose ranging from 0.1 to 10.0 g L−1) were filtered through a regenerated cellulose filter (0.45 μm) and injected into a Zorbax carbohydrate column (4.6 × 150 mm, 5 μm) with a guard column made by the same materials (Agilent, Palo Alto, CA). Mobile phase was acetonitrile (80%, v/v) at a flow rate of 1.4 mL min−1 for 23 min. The column oven temperature was set at 40 °C. Detector was set at 40 °C, gain 5, and pressure of 350 kPa. Sugars in the sample were identified by matching the retention times of standards and their concentrations were calculated by peak areas of the standard curves of the respective sugars. The standard curves were plotted with R2 greater than 0.99. The total sugar contents of fruit were calculated as combined values of fructose, glucose, and sucrose.
The organic acids of unripe and ripe fruit were quantified as follows. The fruit powder (1.0 g) was ultrasonicated with deionised water (10.0 mL) for 30 min. The slurry was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074 g for 10 min at 4 °C to get the supernatant. The supernatant was filtered and diluted with deionised water up to 25 mL in a volumetric flask. Organic acids of the solution were identified and quantified by ultrafast liquid chromatograph Prominence system coupled with a photodiode array detector (PDA, Shimadzu, Kyoto, Japan), according to a literature method.15 The solution (10 μL) was injected into a Supelcogel C-610H ion exchange column (7.8 mm × 300 mm, Supelco, Inc., Bellefonte, PA). The mobile phase was 0.10% H2SO4 at an isocratic flow rate of 0.40 mL min−1 for 50 min at 40 °C. Standards were prepared with serial concentrations of malic, succinic, and citric acids at 0.02–10.00 g L−1. The absorbance was monitored at 210 nm. The concentrations of respective standards were calculated from calibration curves. All the curves had good linearity fit (R2 > 0.99). A FE20 K pH meter (Mettler Toledo, Switzerland) was used for pH measurements of unripe and ripe fruit.
074 g for 10 min at 4 °C to get the supernatant. The supernatant was filtered and diluted with deionised water up to 25 mL in a volumetric flask. Organic acids of the solution were identified and quantified by ultrafast liquid chromatograph Prominence system coupled with a photodiode array detector (PDA, Shimadzu, Kyoto, Japan), according to a literature method.15 The solution (10 μL) was injected into a Supelcogel C-610H ion exchange column (7.8 mm × 300 mm, Supelco, Inc., Bellefonte, PA). The mobile phase was 0.10% H2SO4 at an isocratic flow rate of 0.40 mL min−1 for 50 min at 40 °C. Standards were prepared with serial concentrations of malic, succinic, and citric acids at 0.02–10.00 g L−1. The absorbance was monitored at 210 nm. The concentrations of respective standards were calculated from calibration curves. All the curves had good linearity fit (R2 > 0.99). A FE20 K pH meter (Mettler Toledo, Switzerland) was used for pH measurements of unripe and ripe fruit.
2.3 Extraction and purification of phenolic compounds
The unripe and ripe fruit powders (20.0 g) were separately extracted with methanol (80%, v/v, 2 × 100 mL) for 2 h by shaking on a vortex shaker. Each slurry was centrifuged and then each supernatant was evaporated at 40 °C to obtain crude extracts for solid-phase extraction (SPE).The phenolic compounds were purified by SPE according to a literature method.16 The C18 Sep-Pak cartridges (Waters, Wexford, Ireland) were preconditioned sequentially with ethyl acetate (10 mL), methanol (10 mL), and 0.01 M HCl (15 mL). The crude extract (1 mL, approximately 0.5 g L−1) was loaded on the C18 cartridge that was eluted with 0.01 M HCl (15 mL). The adsorbed non-anthocyanin phenolics were eluted with ethyl acetate (40 mL). The adsorbed anthocyanins were then eluted with acidic methanol (0.1% HCl in methanol, v/v) until the eluent turned colourless. All eluents were separately evaporated at 40 °C and filtered for characterisation and antioxidant capacity.
2.4 Characterisation of phenolic compounds using HPLC-PDA and HPLC-HR-TOF/MS2
Samples (20 μL) were injected into a 2695 high-performance liquid chromatography (HPLC) coupled with a 2669 PDA detector (Waters, Milford, MA) and a HPLC coupled with micrOTOF-Q II high resolution time of flight mass spectrometry (HR-TOF/MS2, Bruker, Billerica, MA) with a reversed-phase C18 Sunfire column (250 mm × 4.6 mm i.d., 5 μm, Waters, Wexford, Ireland).For the analysis of non-anthocyanin phenolics, the mixture were eluted with the ternary mobile phases consisting of A (50 mM aqueous ammonia acetate, pH 3.6), B (20% A in acetonitrile, v/v, pH 3.6), and C (200 mM acetate acid, pH 2.6). Elution programme started with 14% B and 86% C, changing to 16.5% B and 83.5% C at 12.5 min, 25% B and 75% C at 17.5 min, 80% B and 20% C at 40 min, and washing with 100% A for another 20 min. The flow rate and temperature were set at 1.0 mL min−1 and 25 °C. Anthocyanins were eluted with the binary mobile phases consisting of A (acetonitrile) and B (acidic water). Mobile phase B was prepared with 10% acetic acid and 5% acetonitrile, by volume. Elution programme started with 100% B for 5 min, decreasing to 80% B at 20 min, 60% B at 25 min, ramping up to 100% B at 30 min, and holding for 5 min at a flow rate of 1.0 mL min−1 at 25 °C.
HR-TOF/MS2 analyses were performed using a TOF mass spectrometer via electrospray ionisation (ESI) interface and controlled by Compass Data Analysis software. Mass spectra were acquired in negative mode for non-anthocyanin phenolics and in positive mode for anthocyanins with the range of m/z 50–1500. MS calibration standard was performed using sodium acetate. The MS2 collision gas was nitrogen. The negative ion ESI parameters were capillary voltage 3500 V, dry gas temperature 200 °C, dry gas flow 7.0 L min−1, and nebuliser 3.0 bar. The positive ion ESI parameters were capillary voltage 4500 V, dry gas temperature 200 °C, dry gas flow 7.0 L min−1, and nebuliser 3.0 bar.
2.5 Quantification of total phenolic, anthocyanin, and proanthocyanidin contents
The unripe and ripe fruit powder (1.0 g) was separately extracted with methanol (80%, v/v, 2 × 5 mL) for 2 h and centrifuged to get the supernatant. The supernatant was filtered and diluted with 80% methanol up to 25 mL in a volumetric flask. The solution was used for further analysis. The total phenolic content (TPC) of fruit was measured using the Folin–Ciocalteu assay with slight modifications.17 The solutions with serious dilutions (20 μL) were mixed with deionised water (90 μL) and Folin–Ciocalteu reagent (10 μL) in a 96-well plate and incubated for 5 min at room temperature in the dark. The Na2CO3 solution (80 μL, 75 g L−1) was added to the mixture and incubated for another 2 h. The absorbance was captured at 765 nm using the microplate reader. A calibration curve of gallic acid was constructed yielding a linear correlation (y = 4.8595x + 0.0047) with a high R2 value of 0.99. The TPC of fruit was expressed as milligram gallic acid equivalent per gram of dry weight of fruit.The total anthocyanin content (TAC) of fruit was separately measured according to the pH differential method with slight modifications of a reported method.18 Potassium chloride buffer (0.025 M, pH 1.0) and sodium acetate buffer (0.4 M, pH 4.5) were prepared for dilution. Two diluted extracts (20 μL) were mixed with corresponding buffer (180 μL) in a 96-well plate and the absorbance of each well was read at 510 nm and 700 nm using the microplate reader. Absorbance was calculated with the aid of eqn (1) below. The TAC of fruit was calculated using following eqn (2) and expressed as milligram cyanidin-3-O-glucoside equivalent per gram of dry weight of fruit.
| A = (A510 nm − A700 nm)pH1.0 − (A510 nm − A700 nm)pH4.5 | (1) |
| Cyanidin-3-O-glucoside equivalent (mg g−1 of dry weight of fruit) = (A × MW × DF × 1000)/(ε × 1) | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900).
900).
The total proanthocyanidin content (TPAC) of fruit was measured using the DMAC assay with slight modifications.19 The extracts were serial diluted with a mixture [80% of ethanol (91%): 20% of deionised water, v/v] and pipetted (70 μL) into a 96-well plate to mix with fresh DMAC solution (210 μL, 0.1% DMAC in acidified ethanol, w/v). The acidified ethanol was prepared by mixing 75% of ethanol (91%), 12.5% of deionised water, and 12.5% of HCl (36%), by volume. The absorbance was read for 30 min using the microplate reader at 640 nm at 25 °C. The maximum absorbance was calculated using a pre-determined procyanidin A2 calibration curve (y = 24.303x + 0.0151) with R2 value of 0.99, where y represents the absorbance value and x represents the procyanidin A2 concentration (g L−1). The results were expressed as milligram procyanidin A2 equivalent per gram of dry weight of fruit.
2.6 Antioxidant capacity analysis
Antioxidant capacity was measured according to a reported ORAC assay.20 The ORAC assay was carried out on a Synergy HT microplate fluorescence reader. The potassium phosphate buffer (75 mM, pH 7.4) was prepared with KH2PO4 and K2HPO4. 20 μL of the serial-diluted sample or Trolox was mixed with 160 μL of fluorescein solution (8.16 × 10−5 mM in buffer) in a 96-well plate. 20 μL of AAPH solution (153 mM in buffer) was automatically added into the plate to quench the fluorescence. The fluorescence was measured every minute for 2 h at 37 °C. The excitation wavelengths vary from 465 to 505 nm, and emission wavelengths vary from 505 to 555 nm.2.7 Statistical analysis
Statistical analyses were carried out with an independent sample t-test and one-way analysis of variance (ANOVA) using IBM SPSS Statistics V.22.0 (IBM Corporation, Armonk, NY). All analyses were performed in triplicate and results were expressed as mean ± standard deviation. The significance level was set at 0.05.3. Results and discussion
3.1 Physicochemical analysis
The physicochemical parameters of unripe and ripe fruit are listed in the Table 1. Among the nutritive compositions of fruit, the moisture contents (79.5% and 80.7%) accounted for the largest portion of the unripe and ripe fruit, which were no significant difference (p > 0.05 by t-test) as fruit ripen. Among the other compositions, the total dietary fibre was responsible for the majority of dried fruit, which was 59% of unripe fruit and 58% of ripe fruit. The total dietary fibre of Malay cherry fruit was higher than most of the fruit. For example, it was around 5 times higher than that of ripe apples.21 Dietary fibre is a necessary nutrient in a healthy diet, because it can ease constipation, reduce harmful substance levels (e.g. cholesterol and heavy metals), and prevent large intestine cancer, obesity, diabetes, and coronary heart diseases.22 Although there was no significant difference in the total dietary fibre, it is worth mentioning that the soluble dietary fibre significantly (p < 0.05) increased from 13.5% to 16.2% as fruit ripen. However, the insoluble dietary fibre significantly decreased from 45.7% to 41.4% as fruit ripen, which may due to the partial degradation of cellulose in the plant cell wall. The cellulose, a main insoluble fibre in fruit, could be digested by cellulase into monosaccharides, thereby softening the fruit during ripening.23 There was a rise in the TSS of fruit from 12.76% to 18.71% of ripen fruit. As a result, the total sugar significantly increased as fruit ripen. The total protein and total fat slightly increased during fruit ripening. Total ash contents did not experience a significant change as fruit ripen. The total carbohydrate can be estimated as the summation of total sugar, total dietary fibre, and total starch (starch was not detectable in the fruit).| Physicochemical parameters | Unripe fruit | Ripe fruit |
|---|---|---|
| a Means of parameters within rows followed by different letters are significantly different (p < 0.05) according to the independent sample t-test. Values are means ± standard deviations (n = 3). Values are expressed on dry weight of fruit, except for the values of moisture on fresh fruit weight. | ||
| Nutritive compositions (%) | ||
| Moisture | 79.5 ± 0.7a | 80.7 ± 0.3a |
| Total ash | 4.6 ± 0.1a | 4.1 ± 0.3a |
| Total protein | 3.30 ± 0.05b | 3.56 ± 0.05a |
| Total fat | 2.25 ± 0.08b | 2.8 ± 0.1a |
| Total sugar | 5.91 ± 0.05b | 7.3 ± 0.2a |
| Insoluble dietary fibre | 45.7 ± 0.5a | 41.4 ± 0.5b |
| Soluble dietary fibre | 13.5 ± 0.6b | 16.2 ± 0.6a |
| Total dietary fibre | 59 ± 1a | 58 ± 1a |
| Total soluble solids (%) | 12.76 ± 0.01b | 18.71 ± 0.01a |
| Reducing sugar (mg maltose equivalent per g dry weight of fruit) | 166 ± 2b | 184 ± 1a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Sugar (mg g−1 dry weight of fruit) | ||
| Fructose | 22.7 ± 0.2b | 29.6 ± 0.7a |
| Glucose | 17.8 ± 0.7b | 23.4 ± 0.3a |
| Sucrose | 18.6 ± 0.3a | 20 ± 1a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Organic acid (mg g−1 dry weight of fruit) | ||
| Malic acid | 23.1 ± 0.8a | 18.3 ± 0.8b |
| Succinic acid | 11.5 ± 0.6a | 5.7 ± 0.6b |
| Citric acid | 9.0 ± 0.3a | 2.2 ± 0.2b |
| pH | 5.58 ± 0.01b | 5.71 ± 0.04a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Colour | ||
| L* | 60 ± 1a | 24.4 ± 0.5b |
| a* | −7.8 ± 0.2b | 10 ± 2a |
| b* | 30 ± 1a | 2.4 ± 0.3b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Dimension (cm) | ||
| Length | 2.6 ± 0.3a | 2.8 ± 0.2a |
| Diameter | 2.2 ± 0.3b | 3.0 ± 0.3a |
| Weight (g) | 7.5 ± 0.6b | 16 ± 3a |
The main sugars found in the Malay cherry fruit were fructose, glucose, and sucrose. The fructose level was always higher than glucose and sucrose in response to various stresses.24 As fruit ripen, the fructose and glucose significantly increased from 22.7 and 17.8 to 29.6 and 23.4 mg g−1 dry weight of fruit, while the sucrose content slightly increased from 18.6 to 20 mg g−1 dry weight of fruit. Therefore, the total sugar and reducing sugar (fructose and glucose) of fruit experienced significant increases as fruit ripen. Similarly, an increase in sugars (fructose, glucose, total sugar, and reducing sugar) as sweet cherries ripen.25 The principle organic acid was malic acid in Malay cherry fruit, while the second and the third major acids were succinic and citric acids. This finding was agreement with the organic acids in sweet cherries.26 The three organic acids were high in unripe fruit and decrease in ripe fruit. Malay cherry fruit reflected a significant increase in pH as fruit ripen. In conclusion, the major compositions changes in ripening fruit are a significant increase in reducing sugar and a significant fall in organic acids.
The green colour of unripe fruit was as follows: L* = 60, a* = −7.8, b* = 30. The dark reddish-purple colour of ripe fruit (L* = 24.4, a* = 10, b* = 2.4) was from anthocyanins, which are pigments in the plants. Moreover, during ripening, the fruit dimensions increased from 2.6 to 2.8 cm in length and from 2.2 to 3.0 cm in diameter; the fruit weight increased from 7.5 to 16 g.
3.2 Structural characterisation of anthocyanins
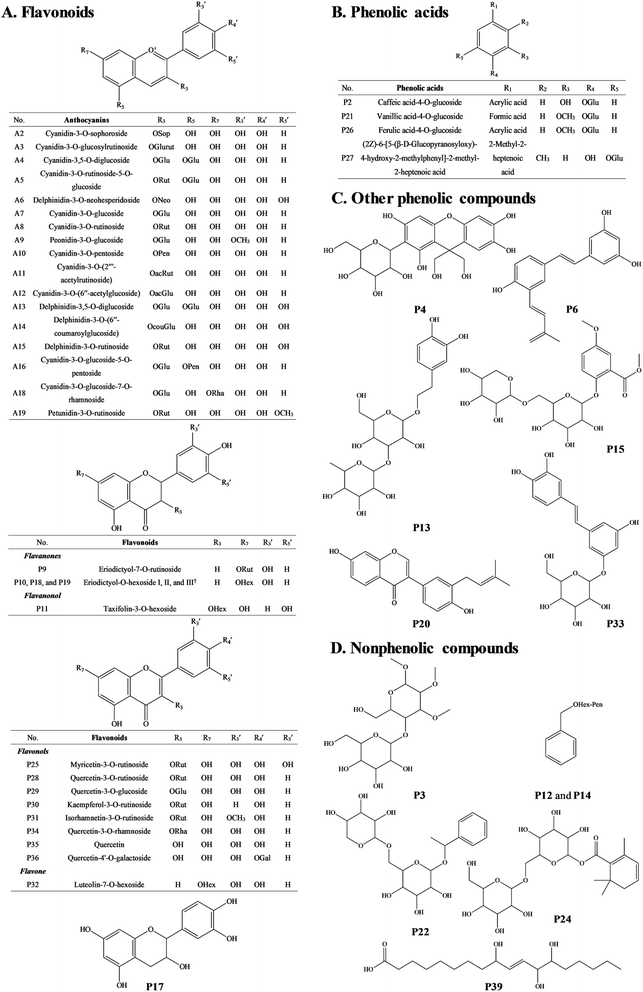
The anthocyanin pigments found only in the ripe fruit were characterised by HPLC-HR-TOF/MS2 spectroscopy (Fig. 1A and B) and the corresponding MS profile data are summarised in Table 2. The chemical structures of anthocyanins are shown in the Fig. 3.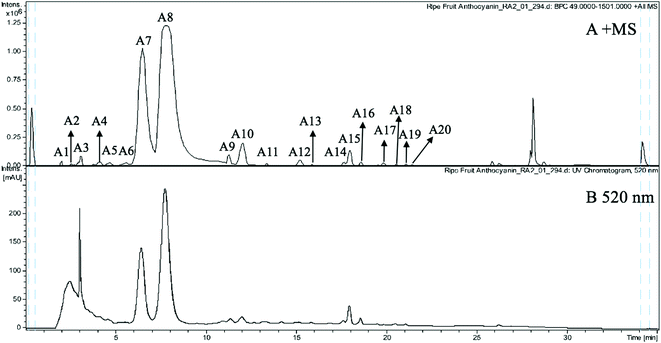 | ||
| Fig. 1 (A) MS spectrogram (in positive ion mode) and (B) HPLC chromatogram (UV 520 nm) of anthocyanins from ripe Malay cherry fruit. | ||
| Anthocyanins | RT (min) | Tentative assignment | Chemical formula | MW | MS (m/z) [M]+ | MS2 (m/z) | Error (ppm) | Exact mass (m/z) | HPLC-PDA λmax (nm) | Ripe fruit | Unripe fruit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a +, detected; −, not detected. | |||||||||||
| A1 | 2.0 | Unknown | C5HN4O12+ | 308 | 308.9582 | 297.8974 | 1.1 | 308.9585 | 525, 278 | + | − |
| A2 | 2.5 | Cyanidin-3-O-sophoroside | C27H31O16+ | 611 | 611.1577 | 287.0565 | 4.9 | 611.1607 | 526, 278 | + | − |
| A3 | 3.0 | Cyanidin-3-O-glucosylrutinoside | C33H41O20+ | 757 | 757.2188 | 611.4959, 287.0565 | −0.4 | 757.2186 | 516, 279 | + | − |
| A4 | 4.0 | Cyanidin-3,5-O-diglucoside | C27H31O16+ | 611 | 611.1607 | 287.0555, 449.1098 | −0.1 | 611.1607 | 508, 278 | + | − |
| A5 | 4.6 | Cyanidin-3-O-rutinoside-5-O-glucoside | C33H41O20+ | 757 | 757.2179 | 595.1640, 449.1074, 287.0563 | 0.9 | 757.2186 | 516, 278 | + | − |
| A6 | 5.5 | Delphinidin-3-O-neohesperidoside | C27H31O16+ | 611 | 611.1597 | 303.0508 | 1.6 | 611.1607 | 517, 278 | + | − |
| A7 | 6.5 | Cyanidin-3-O-glucoside | C21H21O11+ | 449 | 449.1087 | 287.0570 | −2.0 | 449.1078 | 515, 328, 280 | + | − |
| A8 | 7.8 | Cyanidin-3-O-rutinoside | C27H31O15+ | 595 | 595.1657 | 287.0568, 449.1083 | 0.0 | 595.1657 | 514, 329, 280 | + | − |
| A9 | 11.2 | Peonidin-3-O-glucoside | C22H23O11+ | 463 | 463.1232 | 301.0722 | 0.7 | 463.1235 | 519, 279 | + | − |
| A10 | 12.0 | Cyanidin-3-O-pentoside | C20H19O10+ | 419 | 419.0965 | 287.0559 | 1.8 | 419.0973 | 517, 279 | + | − |
| A11 | 13.3 | Cyanidin-3-O-(2′′′-acetylrutinoside) | C29H33O16+ | 637 | 637.1783 | 287.0559 | −3.0 | 637.1763 | 525, 276 | + | − |
| A12 | 15.2 | Cyanidin-3-O-(6′′-acetylglucoside) | C23H23O12+ | 491 | 491.1180 | 287.0550 | 0.7 | 491.1184 | 525, 275 | + | − |
| A13 | 15.8 | Delphinidin-3,5-O-diglucoside | C27H31O17+ | 627 | 627.1558 | 303.0504, 465.0924 | −0.4 | 627.1556 | 519, 279 | + | − |
| A14 | 17.6 | Delphinidin-3-O-(6′′-coumaroylglucoside) | C27H31O16+ | 611 | 611.1606 | 303.0501 | 0.1 | 611.1607 | 525, 281 | + | − |
| A15 | 18.0 | Delphinidin-3-O-rutinoside | C27H31O16+ | 611 | 611.1596 | 303.0501 | 1.7 | 611.1607 | 525, 281 | + | − |
| A16 | 18.6 | Cyanidin-3-O-glucoside-5-O-pentoside | C26H29O15+ | 581 | 581.1375 | 449.1254, 419.1015, 287.0796 | > 5.0 | 581.1501 | 525, 281 | + | − |
| A17 | 19.8 | Unknown | C18H30NO10+ | 420 | 420.1866 | 240.1006 | −0.4 | 420.1864 | — | + | − |
| A18 | 20.5 | Cyanidin-3-O-glucoside-7-O-rhamnoside | C27H31O15+ | 595 | 595.1677 | 287.0552 | −3.4 | 595.1657 | — | + | − |
| A19 | 21.0 | Petunidin-3-O-rutinoside | C28H33O16+ | 625 | 625.1747 | 317.0668 | 2.5 | 625.1763 | — | + | − |
| A20 | 21.4 | Unknown | C21H31O9+ | 427 | 427.1971 | 240.1016 | −2.0 | 427.1963 | — | + | − |
| Non-anthocyanin phenolics | RT (min) | Tentative assignment | Chemical formula | MW | MS (m/z) [M − H]− | MS2 (m/z) | Error (ppm) | Exact mass (m/z) | HPLC-PDA λmax (nm) | Ripe fruit | Unripe fruit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 1.7 | Unknown | C16H32O2 | 256 | 255.2316 | — | 5.4 | 255.2330 | — | + | + |
| P2 | 2.5 | Caffeic acid-4-O-glucoside | C15H18O9 | 342 | 341.1064 | — | >10 | 341.0878 | 244 | + | + |
| P3 | 3.4 | Methyl 4-O-galactopyranosyl-2,3-di-O-methyl-galactopyranoside | C15H28O11 | 384 | 383.1547 | — | 3.2 | 383.1559 | 232 | + | + |
| P4 | 3.6 | Mangiferdiol | C21H24O12 | 468 | 467.1218 | 287.0584 | −4.8 | 467.1195 | 233 | + | + |
| P5 | 4.5 | Unknown | C30H30O3 | 438 | 437.2123 | 218.1074 | −0.1 | 437.2122 | 233 | + | + |
| P6 | 5.2 | 3-Isopentadienyl-3′,4,5′-trihydroxystilbene | C19H18O3 | 294 | 293.1205 | 113.3011 | −7.4 | 293.1183 | 273 | + | − |
| P7 | 5.9 | (Epi)gallocatechin-(epi)catechin | C30H26O13 | 594 | 593.1327 | 407.0740, 289.0685 | −4.5 | 593.1301 | 286 | + | − |
| P8 | 6.5 | Unknown | C17H32O12 | 428 | 427.1822 | 367.1615 | −0.2 | 427.1821 | 278 | + | + |
| P9 | 7.0 | Eriodictyol-7-O-rutinoside | C27H32O15 | 596 | 595.1675 | 287.0575 | −1.0 | 595.1668 | 281 | + | − |
| P10 | 7.6 | Eriodictyol-O-hexoside I | C21H22O11 | 450 | 449.1088 | 287.6681 | 0.4 | 449.1089 | 283 | + | − |
| P11 | 8.8 | Taxifolin-3-O-hexoside | C21H22O12 | 466 | 465.1047 | 303.0512 | −1.8 | 465.1038 | 286 | + | + |
| P12 | 9.4 | Benzyl alcohol-hexoside-pentoside I | C18H26O10 | 402 | 401.1462 | 269.0992 | −2.2 | 401.1453 | 291 | + | − |
| P13 | 10.0 | Verbasoside | C20H30O12 | 462 | 461.1680 | 269.0987 | −3.3 | 461.1664 | 285 | + | − |
| P14 | 10.5 | Benzyl alcohol-hexoside-pentoside II | C18H26O10 | 402 | 401.1448 | 269.1046 | 1.3 | 401.1453 | 273 | + | + |
| P15 | 10.7 | Primulaverin | C20H28O13 | 476 | 475.1439 | — | 3.9 | 475.1457 | 284 | − | + |
| P16 | 11.6 | Procyanidin dimer | C30H26O12 | 578 | 577.1356 | 407.0767, 289.0683 | −0.7 | 577.1351 | 280 | + | + |
| P17 | 14.6 | (Epi)catechin | C15H14O6 | 290 | 289.0711 | 221.0824 | 2.2 | 289.0718 | 279 | + | + |
| P18 | 15.2 | Eriodictyol-O-hexoside II | C21H22O11 | 450 | 449.1087 | 287.0550 | 0.6 | 449.1089 | 287 | + | + |
| P19 | 15.5 | Eriodictyol-O-hexoside III | C21H22O11 | 450 | 449.1090 | 287.0582 | −0.2 | 449.1089 | 287 | − | + |
| P20 | 16.3 | Neobavaisoflavone | C20H18O4 | 322 | 321.1151 | — | −5.7 | 321.1132 | 281 | + | − |
| P21 | 16.6 | Vanillic acid-4-O-glucoside | C14H18O9 | 330 | 329.0863 | 209.0509 | 4.6 | 329.0878 | 281 | + | + |
| P22 | 18.2 | Primeveroside | C19H28O10 | 416 | 415.1627 | — | −4.1 | 415.1610 | 285 | + | − |
| P23 | 18.6 | (Epi)catechin-(epi)gallocatechin | C30H26O13 | 594 | 593.1296 | 407.0779, 289.0730 | 0.9 | 593.1301 | 286 | + | + |
| P24 | 19.6 | Jasminoside R | C22H34O12 | 490 | 489.1987 | — | −2.0 | 489.1978 | 283 | + | + |
| P25 | 20.6 | Myricetin-3-O-rutinoside | C27H30O17 | 626 | 625.1412 | 317.0226 | −0.2 | 625.1410 | 272 | + | + |
| P26 | 21.3 | Ferulic acid-4-O-glucoside | C16H20O9 | 356 | 355.1024 | 193.3516 | 2.9 | 355.1035 | 283 | + | + |
| P27 | 22.3 | (2Z)-6-[5-(β-D-Glucopyranosyloxy)-4-hydroxy-2-methylphenyl]-2-methyl-2-heptenoic acid | C21H30O9 | 426 | 425.1813 | 219.1380 | 0.9 | 425.1817 | 275 | + | + |
| P28 | 22.7 | Quercetin-3-O-rutinoside | C27H30O16 | 610 | 609.1457 | 301.0333 | 0.6 | 609.1461 | 257, 354 | + | + |
| P29 | 23.4 | Quercetin-3-O-glucoside | C21H20O12 | 464 | 463.0889 | 301.0321 | −1.6 | 463.0882 | 257, 291, 354 | + | + |
| P30 | 24.2 | Kaempferol-3-O-rutinoside | C27H30O15 | 594 | 593.1514 | 285.0375 | −0.3 | 593.1512 | 267, 286, 344 | + | + |
| P31 | 24.4 | Isorhamnetin-3-O-rutinoside | C28H32O16 | 624 | 623.1618 | 315.0423 | 0.0 | 623.1618 | 267, 354 | − | + |
| P32 | 25.0 | Luteolin-7-O-hexoside | C21H20O11 | 448 | 447.0921 | 285.0367 | 2.7 | 447.0933 | 293 | + | + |
| P33 | 26.1 | Astringin | C20H22O9 | 406 | 405.1197 | — | −1.4 | 405.1191 | 283, 373 | + | + |
| P34 | 26.6 | Quercetin-3-O-rhamnoside | C21H20O11 | 448 | 447.0921 | 301.0332 | 2.8 | 447.0933 | 280 | + | + |
| P35 | 27.2 | Quercetin | C15H10O7 | 302 | 301.0361 | — | −2.4 | 301.0354 | 281 | + | + |
| P36 | 28.3 | Quercetin-4′-O-galactoside | C20H18O12 | 450 | 449.0759 | 363.0729 | −7.5 | 449.0725 | 271, 375 | + | − |
| P37 | 30.7 | Unknown | C12H24O4 | 232 | 231.1604 | — | −0.9 | 231.1602 | 292 | + | − |
| P38 | 31.2 | Unknown | C18H32O5 | 328 | 327.2168 | — | 2.9 | 327.2177 | 273 | + | + |
| P39 | 32.5 | Pinellic acid | C18H34O5 | 330 | 329.2325 | 209.1199 | 2.6 | 329.2333 | 283 | + | + |
Peak A2 produced a cationic m/z 611 [M]+ and a fragment m/z 287 [M − 324]+ (due to the loss of a sophoroside moiety), which was tentatively identified as cyanidin-3-O-sophoroside. The m/z 287 is typical for the cyanidin moiety. Peak A4 also gave a cationic m/z 611 [M]+ but the fragments were m/z 287 [M − 324]+ and m/z 449 [M − 162]+ (due to the loss of a glucoside moiety), and thus it was tentatively identified as cyanidin-3,5-O-diglucoside.27 Peaks A6, A14, and A15 shared the same cationic m/z 611 [M]+ and the same fragment m/z 303 [M − 308]+. The m/z 303 is typical for the delphinidin moiety. The 308 Da is related to different glycoside moieties. Therefore, peaks A6, A14, and A15 were tentatively identified as delphinidin-3-O-neohesperidoside, delphinidin-3-O-(6′′-coumaroylglucoside), and delphinidin-3-O-rutinoside, respectively.28
Peaks A3 and A5 gave the same cationic m/z 757 [M]+. The major fragments of peak A3 were m/z 611 [M − 146]+ (due to the loss of a rhamnoside moiety) and m/z 287 [M − 146−162 − 162]+. The peak A3 was thus tentatively identified to the fragment profile of cyanidin-3-O-glucosylrutinoside. The major fragments of peak A5 were m/z 595 [M − 162]+, m/z 449 [M − 162 − 146]+, and m/z 287 [M − 162 − 146 − 162]+. Therefore, the peak A5 was tentatively identified as cyanidin-3-O-rutinoside-5-O-glucoside. The difference between the two peaks was the loss of glycoside moieties in different sequences.
Peaks A8 and A18 had cationic m/z 595 [M]+. Peak A8 fragmented into m/z 449 [M − 146]+ and m/z 287 [M − 146 − 162]+, which was consistent with and tentatively identified as cyanidin-3-O-rutinoside.27 The peak A18 has fragmentation pattern cyanidin-3-O-rutinoside with one fragment at m/z 287, and thus it was tentatively assigned as cyanidin-3-O-glucoside-7-O-rhamnoside.
Peaks A7 (m/z 449), A10 (m/z 419), A11 (m/z 637), and A12 (m/z 491) yielded the same fragment m/z 287, which equates with cyanidin moiety and derives after the removal of glucoside moiety (162 Da), pentoside moiety (132 Da), acetylrutinoside moiety (350 Da), and acetylglucoside moiety (204 Da) from the [M]+. Peaks A7, A10, A11, and A12 were thus tentatively assigned as cyanidin-3-O-glucoside, cyanidin-3-O-pentoside, cyanidin-3-O-(2′′′-acetylrutinoside), and cyanidin-3-O-(6′′-acetylglucoside), respectively.
Peak A9 yielded m/z 463 [M]+ which fragmented into m/z 301 and a glucosyl moiety. It is consistent with peonidin. Therefore, peak A9 was tentatively assigned as peonidin-3-O-glucoside.
Peak A13 shown a m/z 627 [M]+ and was tentatively assigned as delphinidin-3,5-O-diglucoside. The fragment m/z 303 corresponds to the delphinidin aglycone and the lost neutral fragment of 324 Da corresponds to two glucoside (162 Da) moieties.
Peak A19 was tentatively assigned as petunidin-3-O-rutinoside, because it produced m/z 625 [M]+ and yielded a fragment at m/z 317 [M − 308]+. The 308 Da corresponds to the rutinoside moiety. Peak A16 was tentatively assigned as cyanidin-3-O-glucoside-5-O-pentoside because it had a molecular cation at m/z 581, which fragmented into m/z 449 [M − 132]+ and 419 [M − 162]+.
The indicator of fruit ripening is reddish purple colour development, which results from the accumulation of anthocyanins. In sweet cherries, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside were found as major anthocyanins and exerted high antioxidant capacities, while tart cherries were rich in cyanidin-3-O-glucosylrutinoside and cyanidin-3-O-rutinoside.29 However, more varieties of anthocyanins were found in Malay cherry fruit. The subtle structural variations of anthocyanins may dramatically vary their bioactivity. Therefore, it is of great interest to study the health promoting functions of the anthocyanins from Malay cherry fruit.
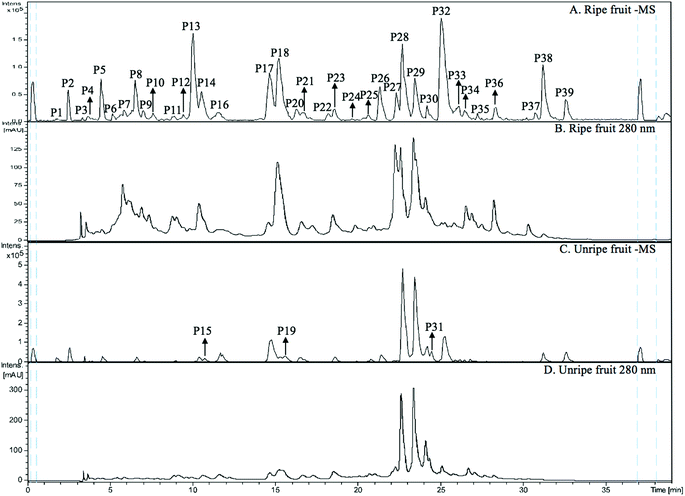
3.3 Structural characterisation of non-anthocyanin phenolics
The MS spectra and HPLC chromatogram of non-anthocyanin fraction from unripe and ripe Malay cherry fruit are shown in Fig. 2, and the corresponding MS profile data are summarised in Table 2. The chemical structures of identified compounds in Malay cherry fruit are shown in Fig. 3. Among the identified compounds, majority of them are flavonoids along with some nonphenolic compounds and unknown structures.Peak P32 was tentatively identified as luteolin-7-O-hexoside (Flavone) while peak P17 with a characteristic ion [M − H]− at m/z 289 was corresponded to catechin or epicatechin (Flavanol). Its fragment m/z 221 [M − H − 68]− was due to a loss of C3O2.
3.4 Quantification of total phenolic, anthocyanin, and proanthocyanidin contents
Table 3 shows the TPC, TAC, and TPAC of unripe and ripe fruit. The TPC of ripe fruit (36.5 ± 0.3 mg gallic acid equivalent per g of dry weight of fruit) showed significantly (p < 0.05 by t-test) lower than that of unripe fruit (92 ± 3 mg gallic acid equivalent per g of dry weight of fruit). A significant fall also can be found in the TPAC as fruit ripen. The polyphenols, especially proanthocyanidins, are responsible for the bitter and astringent tastes.39 As fruit ripen, the decrease in bitterness and astringency in fruit can explain the decrease in TPC and TPAC.40 In addition, the higher TPC and TPAC in unripe fruit can be attributed to the higher rates of metabolite biosynthesis and protection against invasive actions of external organisms (i.e. diseases and insect pests) and adverse environmental conditions (i.e. UV light) at the earlier age of fruit.41 Similarly, the decreases in TPC and TPAC as fruit ripen were reported in dates, apples,42 and grapes.43 However, the TPC of Malay cherry fruit was higher than most of other fruit, such as tart cherries, sweet cherries, and blueberries, and thus Malay cherry fruit can be served as rich sources of phenolic compounds in a healthy diet.44 Anthocyanins are synthesised as fruit ripen, resulting in the development of a reddish purple colour. Consistent with the visual colour change, TAC increased markedly from unripe (not detected) to ripe stages (1.32 ± 0.02 mg cyanidin-3-O-glucoside equivalent per g of dry weight of fruit).| Maturity | SPE fraction | ORACb (μmol Trolox equivalent per g dry weight of fraction) | TPCc (mg gallic acid equivalent per g of dry weight of fruit) | TAC (mg cyanidin-3-O-glucoside![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) equivalent per g of dry weight of fruit) equivalent per g of dry weight of fruit) |
TPACc (mg procyanidin A2 equivalent per g of dry weight of fruit) |
|---|---|---|---|---|---|
| a –, not detected; values are means ± standard deviations (n = 3).b Means values with different letters in the column are significantly different by analysis of variance Tukey test in one-way independent groups design (p < 0.05).c Means values with different letters in the same column are significantly different by independent sample t-test (p < 0.05). | |||||
| Unripe fruit | Crude extracts | 487.17 ± 28.06d | 92 ± 3a | — | 18 ± 1a |
| Water fraction | 44 ± 5d | ||||
| Non-anthocyanin phenolics | 4584 ± 481a | ||||
| Anthocyanins | 168 ± 17d | ||||
| Ripe fruit | Crude extracts | 204 ± 4d | 36.5 ± 0.3b | 1.32 ± 0.02 | 6.4 ± 0.1b |
| Water fraction | 30 ± 3d | ||||
| Non-anthocyanin phenolics | 2254 ± 57b | ||||
| Anthocyanins | 1031 ± 81c | ||||
3.5 Antioxidant capacity
The results for antioxidant capacities of unripe and ripe fruit are shown in Table 3. The crude extracts of unripe and ripe fruit were purified using SPE and obtained three fractions, which were water fraction, non-anthocyanin phenolics, and anthocyanins in sequence. The non-anthocyanin phenolics of unripe fruit had the significantly (p < 0.05 by ANOVA) highest antioxidant capacities at ORAC value of 4584 μmol Trolox equivalent per g dry weight of fraction, followed by the non-anthocyanin phenolics of ripe fruit (2254 μmol Trolox equivalent per g of dry weight of fruit). The anthocyanins of ripe fruit had medium antioxidant capacity (1031 μmol Trolox equivalent per g of dry weight of fruit). The crude extracts, water fraction, and anthocyanins of unripe fruit had low antioxidant capacities. Therefore, it shows that the non-anthocyanin phenolics were the main antioxidants in Malay cherry fruit, and the non-anthocyanin phenolics of unripe fruit showed significantly higher antioxidant capacity than ripe fruit. The above findings implied that the TPC of Malay cherry fruit generally decreased as fruit ripen. In many cases, antioxidant capacity, as well as TPC of berries, such as blueberry45 and strawberry,46 tended to decrease as fruit ripen.4. Conclusions
From our results, nutritive compositions (sugars, proteins, and fats) of Malay cherry fruit increased, whilst organic acids of fruit decreased upon ripening. The antioxidant capacities of fruit decreased, as the polyphenols of fruit decreased upon ripening. It is apparent that Malay cherry fruit has high fibre contents and is rich in polyphenolic antioxidants of diverse structures motifs that are known to have health promotion activity. Therefore, Malay cherry fruit are a good addition to the family of cherries and berries that have been considered as superfoods for human health. The ripening of Malay cherry fruit would be of particular interest to the food industry, as different agricultural practices could obviously affect the levels of beneficial effects that could be obtained from consuming their polyphenolic extracts of different ripening stages. It warrants further study on cellular and animal models, to establish scientific evidence of the bioactivity of the polyphenolic compounds extracted from Malay cherry fruit, particularly the unripe fruit.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledged the financial support received from the National Natural Science Foundation of China (31801553), China Postdoctoral Science Foundation (2018M631903), Heilongjiang Provincial Postdoctoral Science Foundation (LBH-Z18033), and Program for Heilongjiang Innovative Talents in University (UNPYSCT-2018165). The authors gratefully acknowledged the financial support received from the National University of Singapore, National University of Singapore (Suzhou) Research Institute, and China Scholarship Council (CSC).References
- L. M. McCune, C. Kubota, N. R. Stendell-Hollis and C. A. Thomson, Crit. Rev. Food Sci. Nutr., 2010, 51, 1–12 CrossRef.
- M. Serrano, H. M. Díaz-Mula, P. J. Zapata, S. Castillo, F. Guillén, D. Martínez-Romero, J. M. Valverde and D. Valero, J. Agric. Food Chem., 2009, 57, 3240–3246 CrossRef CAS PubMed.
- S. Y. Wang and H. S. Lin, J. Agric. Food Chem., 2000, 48, 140–146 CrossRef CAS PubMed.
- R. L. Prior, G. Cao, A. Martin, E. Sofic, J. McEwen, C. O'Brien, N. Lischner, M. Ehlenfeldt, W. Kalt, G. Krewer and C. M. Mainland, J. Agric. Food Chem., 1998, 46, 2686–2693 CrossRef CAS.
- Ó. Acosta-Montoya, F. Vaillant, S. Cozzano, C. Mertz, A. M. Pérez and M. V. Castro, Food Chem., 2010, 119, 1497–1501 CrossRef.
- C. J. Brady, Annu. Rev. Plant Physiol., 1987, 38, 155–178 CrossRef CAS.
- S. V. Joseph, I. Edirisinghe and B. M. Burton-Freeman, Crit. Rev. Food Sci. Nutr., 2016, 56, 419–444 CrossRef CAS PubMed.
- A. G. V. Costa, D. F. Garcia-Diaz, P. Jimenez and P. I. Silva, J. Funct. Foods, 2013, 5, 539–549 CrossRef CAS.
- K. C. Vitale, S. Hueglin and E. Broad, Current Sports Medicine Reports, 2017, 16, 230–239 CrossRef PubMed.
- T. K. Lim, Edible medicinal and non-medicinal plants, Springer, Netherlands, 2013, pp. 39–41 Search PubMed.
- Y. Zhang, A. I. C. Wong, J. Wu, N. B. A. Karim and D. Huang, J. Funct. Foods, 2016, 25, 568–578 CrossRef CAS.
- N. Balasundram, K. Sundram and S. Samman, Food Chem., 2006, 99, 191–203 CrossRef CAS.
- AOAC, Official methods of analysis of AOAC international, Association of Official Analytical Chemists, Gaithersberg, 2000 Search PubMed.
- Y. Zhang, X. Sui and D. Huang, Food Chem., 2017, 232, 571–578 CrossRef CAS PubMed.
- M. W. Cheong, S. Q. Liu, W. Zhou, P. Curran and B. Yu, Food Chem., 2012, 135, 2505–2513 CrossRef CAS PubMed.
- D. O. Kim and C. Y. Lee, Current protocols in food analytical chemistry, John Wiley & Sons Inc., New Jersey, 2002, pp. I1.2.1–I1.2.12 Search PubMed.
- A. L. Waterhouse, Current protocols in food analytical chemistry, John Wiley & Sons Inc, New Jersey, 2002, pp. I1.1.1–I1.1.8 Search PubMed.
- M. M. Giusti and R. E. Wrolstad, Current protocols in food analytical chemistry, John Wiley & Sons Inc., New Jersey, 2001, pp. F1.2.1–F1.2.13 Search PubMed.
- R. L. Prior, E. Fan, H. Ji, A. Howell, C. Nio, M. J. Payne and J. Reed, J. Sci. Food Agric., 2010, 90, 1473–1478 CrossRef CAS PubMed.
- D. Huang, B. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS PubMed.
- B. W. Li, K. W. Andrews and P. R. Pehrsson, J. Food Compos. Anal., 2002, 15, 715–723 CrossRef CAS.
- U. Telrandhe, R. Kurmi, V. Uplanchiwar, M. Mansoori, V. Raj and K. Jain, Int. J. Pharm. Pharm. Sci., 2012, 2, 179–195 Search PubMed.
- T. H. Emaga, C. Robert, S. N. Ronkart, B. Wathelet and M. Paquot, Bioresour. Technol., 2008, 99, 4346–4354 CrossRef PubMed.
- P. K. Gill, A. D. Sharma, P. Singh and S. S. Bhullar, Plant Growth Regul., 2003, 40, 157–162 CrossRef CAS.
- M. Serrano, F. Guillén, D. Martínez-Romero, S. Castillo and D. Valero, J. Agric. Food Chem., 2005, 53, 2741–2745 CrossRef CAS.
- A. A. Hayaloglu and N. Demir, J. Food Sci., 2015, 80, C564–C570 CrossRef CAS PubMed.
- U. A. Fischer, R. Carle and D. R. Kammerer, Food Chem., 2011, 127, 807–821 CrossRef CAS PubMed.
- X. Wu and R. L. Prior, J. Agric. Food Chem., 2005, 53, 2589–2599 CrossRef CAS PubMed.
- F. Blando, C. Gerardi and I. Nicoletti, BioMed Res. Int., 2004, 2004, 253–258 Search PubMed.
- A. Brito, J. E. Ramirez, C. Areche, B. Sepúlveda and M. J. Simirgiotis, Molecules, 2014, 19, 17400–17421 CrossRef PubMed.
- A. Vallverdú-Queralt, J. F. R. De Alvarenga, R. Estruch and R. M. Lamuela-Raventos, Food Chem., 2013, 141, 3365–3372 CrossRef PubMed.
- L. Bavaresco, M. De Rosso, M. Gardiman, G. Morreale and R. Flamini, BIO web of conferences, Bento Gonçalves: EDP Sciences, 2016, p. 01022 Search PubMed.
- M. Chen, F. Song, M. Guo, Z. Liu and S. Liu, Rapid Commun. Mass Spectrom., 2002, 16, 264–271 CrossRef CAS PubMed.
- L. M. De Souza, T. R. Cipriani, M. Iacomini, P. A. J. Gorin and G. L. Sassaki, J. Pharm. Biomed. Anal., 2008, 47, 59–67 CrossRef CAS PubMed.
- C. Jo, K. Y. Yoon, E. J. Jang and T. H. Kim, Biosci., Biotechnol., Biochem., 2016, 80, 2022–2024 CrossRef CAS PubMed.
- A. Limmongkon, P. Nopprang, P. Chaikeandee, T. Somboon, P. Wongshaya and V. Pilaisangsuree, Food Chem., 2018, 239, 569–578 CrossRef CAS PubMed.
- M. Victoria, G. Roldan, B. Engel, C. Ric, H. De Vos, P. Vereijken, L. Astola, M. Groenenboom, H. Van De Geest and A. Bovy, Metabolomics, 2014, 10, 958 CrossRef.
- K. X. Bastos, C. N. Dias, Y. M. Nascimento, M. S. Da Silva, S. M. Z. Langassner, L. A. Wessjohann and J. F. Tavares, Molecules, 2017, 22, 143 CrossRef PubMed.
- H. Peleg, K. Gacon, P. Schlich and A. C. Noble, J. Sci. Food Agric., 1999, 79, 1123–1128 CrossRef CAS.
- H. A. Bashir and A. B. A. Abu-Goukh, Food Chem., 2003, 80, 557–563 CrossRef CAS.
- T. Acamovic and J. D. Brooker, Proc. Nutr. Soc., 2005, 64, 403–412 CrossRef CAS PubMed.
- S. Burda, W. Oleszek and C. Y. Lee, J. Agric. Food Chem., 1990, 38, 945–948 CrossRef CAS.
- K. A. Bindon and J. A. Kennedy, J. Agric. Food Chem., 2011, 59, 2696–2707 CrossRef CAS PubMed.
- D. Marinova, F. Ribarova and M. Atanassova, J. Univ. Chem. Technol. Metall., 2005, 40, 255–260 CAS.
- A. D. R. Castrejón, I. Eichholz, S. Rohn, L. W. Kroh and S. Huyskens-Keil, Food Chem., 2008, 109, 564–572 CrossRef.
- I. Oliveira, P. Baptista, R. Malheiro, S. Casal, A. Bento and J. A. Pereira, Food Res. Int., 2011, 44, 1401–1407 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |