Open Access Article
Open Access ArticleZein film functionalized with gold nanoparticles and the factors affecting its mechanical properties†
Mohammed Ajmal Puthiyaveetil Yoosafa,
Anjana Jayaprakasha,
Somnath Ghosha,
Vivek Sheel Jaswalb,
Kuldeep Singhb,
Soumit Mandala,
M. Shahid d,
Munendra Yadave,
Subhojit Das*c and
Pankaj Kumar
d,
Munendra Yadave,
Subhojit Das*c and
Pankaj Kumar *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research (IISER), Tirupati 517507, India. E-mail: pankaj@iisertirupati.ac.in; Tel: +91-0877-2500-240
bDepartment of Chemistry, Maharishi Markandeshwar University, Mullana 133203, India
cDepartment of Chemistry, National Institute of Technology, Agartala–799046, India. E-mail: subhojit.chem@gmail.com
dDepartment of Chemistry, Aligarh Muslim University, Aligarh–202002, India
eDepartment of Chemistry, University of Texas at El Paso, El Paso, Texas 79968, USA
First published on 13th August 2019
Abstract
In this article, we report a simple method to synthesize biodegradable zein films functionalized with gold nanoparticles (AuNPs) with significantly improved mechanical properties, as an environmentally benign substitute to biologically hazardous polymers. Zein-coated AuNPs were synthesized using the zein protein as a reducing agent and characterized with IR, UV, CD, ζ-potential, and TEM measurements. The zein protein interaction with the negatively charged surface of AuNPs provides excellent strength to the zein thin film. For the first time, FT-IR spectral studies suggested the strong interaction between AuNPs and zein protein, which was further supported by the higher binding constant (Kb) value. The films were characterized for mechanical properties with spectroscopic and physical experimental investigations. The surface morphology of AuNP-doped zein film was explored by AFM and SEM, which suggested that the AuNPs prevent the buckling of zein film and increase the strength as well as flexibility of the film.
During the last few years, the use of polyethylene has become a major environmental threat due to its non-degradable nature.1,2 It has been estimated that ∼8.3 billion metric tons of plastic has been produced since its invention, out of which 40% has been used for packaging.3 Owing to its non-degradable nature, ∼79% of the total amount of plastic produced remains as waste.4,5 To overcome this, we need an alternative to plastics which should be bio-degradable and economical. Scientists have developed a number of biologically driven thin films using cellulose, lactic acid, and starch, however, these face issues related to tensile strength, hydrophobicity, and flexibility.6–9 Thin films derived from naturally occurring zein, a predominant corn protein, can serve as a cost-effective substitute due to its intrinsic hydrophobic nature.10 Zein is a mixture of proteins, mainly consisting of alpha-zein, a 19 kDa protein which is specifically rich in glutamine, proline and alanine amino acids.11 The natural hydrophobicity of zein arises due to its secondary structure where nine anti-parallel helices arranged in a distorted cylinder which is stabilized by hydrogen bonds.12 Some major physical properties of the film like its moisture barrier property, tensile strength, flexibility, and gas permeability need to be regulated in order to transform zein into an alternative to polythene. The inherent brittleness of zein restricts its application as a structural material.13 This can be improved by treating it with plasticizers, such as polyols, oligosaccharides, lipids, and fatty acids prior to casting in order to make zein films more useful.14,15 Addition of plasticizers increase the strength and flexibility of the zein films, however, excess will make them too weak and sticky to be handled.16
Several studies have been conducted with the aim to improve the functional properties of zein films using various physical and chemical modification techniques.17–19 Incorporation of nanoparticles (NPs) into the thin films of zein protein can be used as a strategy to impart the desired tensile strength and flexibility to the films by enhancing fibrillation.20–22 Nevertheless, the mechanism for protein–NP interaction leading to improved mechanical properties is underexplored and still is a topic of great interest due to its wide range of applications.23,24
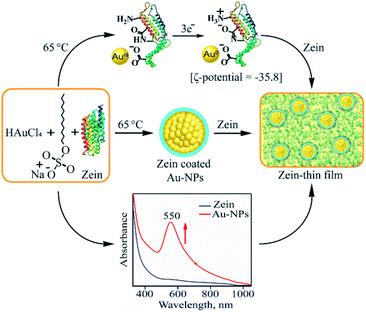
Here, we report the synthesis and characterization of zein protein film doped with gold-nanoparticles (AuNPs) having enhanced mechanical properties as an alternative to the synthetic polymer. In this regards, first, the AuNPs functionalized with zein protein were synthesized (vide infra) and characterized.25–27 Binding constant (Kb) and Fourier Transform Infrared (FT-IR) spectral measurement suggest a strong interaction between the AuNPs and zein protein. These interactions provide the ground to develop a method to prepare AuNP doped zein film where the reaction conditions were most optimum and economic (i.e., NTP). AFM, SEM and spectral characterization of zein films suggested that the strong binding of AuNPs with zein enhances the fibrillation and therefore declines the buckling or otherwise provides extraordinary strength to the film. Physical parameters such as tensile strength and strain also demonstrate explicitly that the AuNP doped zein film is an ideal substitute for the biologically hazardous polymer. The present study, for the first time, explains how the AuNP interaction with zein provides enhanced strength and strain to the zein film.
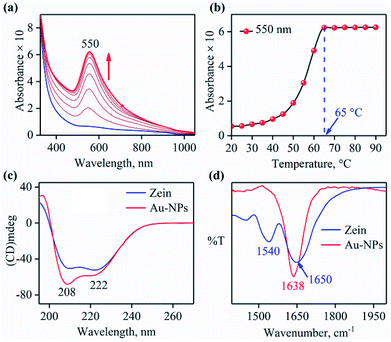
AuNPs, doping agent for the zein thin film, was prepared by heating an aqueous solution of HAuCl4 with zein protein in the presence of SDS (ESI,† ES).26 The solution color changed from yellowish to pink, indicating the conversion of tri-cationic gold (Au3+) to the AuNPs. UV-visible spectral measurement of the above reaction showed the formation of a band at 550 nm (Fig. 1a). Surface Plasmon Resonance (SPR) absorption band at 550 nm is a characteristic feature for AuNPs. The reaction followed the first order rate kinetics, and the pseudo-first order rate constant determined to be 1.25 × 10−3 s−1 (ESI, Fig. S1a†). To determine the best suitable temperature for uniform AuNP formation with the highest yield, we followed the formation of NPs by UV-visible spectral measurements as a function of temperature (20–90 °C) and found it to be 65 °C (Fig. 1b; ESI, ES, and Fig. S1b†). Also, we determined the optimal zein amount for AuNP synthesis by following the 550 nm peak in UV-visible spectral measurements and observed to be 0.15% with 0.20 mM HAuCl4 (ESI, ES, and Fig. S2†) (Scheme 1).
To gain insight into the zein coated AuNP formation and their interaction with zein protein, which provides strength to the zein film, circular dichroism (CD) and FT-IR spectroscopic measurements were carried out. The CD spectra of zein exhibited a band at 222 nm due to peptide n–π* transition, characteristic of typical alpha-helical structure and the band at 208 nm represents exciton splitting of lowest peptide π–π* transition (Fig. 1c).28–30 The change in CD spectra of zein (red) is attributed to the unfolding of zein or immobilization of zein on AuNPs, reflecting the surface functionalization of the AuNPs. The unfolding of zein in aqueous HAuCl4 solution in the presence of surfactant leads to an exposure of cysteine which initiate the reduction of Au3+ to Au0.31–33 This result can be correlated to the change in the secondary structure of zein protein when functionalized on AuNPs.34 To elaborate further, the CD measurement of zein by varying the temperature from 20 °C to 90 °C suggested that zein denatured in the range of 65 °C to 80 °C (Fig. S3a†). Interestingly, the CD spectrum of a mixture of zein plus AuNPs (45 °C), as otherwise in case of AuNP doped zein film, exactly matches with the CD spectrum of only zein at 80 °C (Fig. S3b†). This result suggests that the presence of Au3+/AuNPs enhances the zein unfolding/denaturation and hence explain the clear temperature-dependent transitions of AuNP formation as or else shown in the UV-visible titration curve (Fig. 1b). The FT-IR spectrum of zein exhibited a characteristic peak at 1650 cm−1,35 corresponding to an amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of peptide chain shifted to 1638 cm−1 in zein-AuNPs due to the interaction of amide functional groups with Au0 of AuNPs (Fig. 1d; ESI, Fig. S4†).36 Disappearance of the peak at 1540 cm−1 (N–H), suggests the removal of H+ from N–H and therefore create a negative charge on amide N-atom. The distribution of charge on –N–C
O stretching of peptide chain shifted to 1638 cm−1 in zein-AuNPs due to the interaction of amide functional groups with Au0 of AuNPs (Fig. 1d; ESI, Fig. S4†).36 Disappearance of the peak at 1540 cm−1 (N–H), suggests the removal of H+ from N–H and therefore create a negative charge on amide N-atom. The distribution of charge on –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety is responsible for shifting/decreasing of C
O moiety is responsible for shifting/decreasing of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak to 1636 cm−1. This was additionally supported from the ζ-potential measurement, which suggested a negative charge on the surface of AuNPs (−35.8 mV) (ESI Fig. S5†), which was further confirmed by gel electrophoresis (ESI Fig. S6†).37 Furthermore, Transmission Electron Microscopy (TEM) images (Fig. S7a†) of AuNPs prepared by zein (0.15% w/v) with HAuCl4 (0.20 mM) showed that the particles were mostly spherical (Fig. S7a†) with an size around ∼27 ± 5 nm. TEM images of AuNPs of the same sample show clearly that the zein adheres to the surface of AuNPs (Fig. S7b†).
O peak to 1636 cm−1. This was additionally supported from the ζ-potential measurement, which suggested a negative charge on the surface of AuNPs (−35.8 mV) (ESI Fig. S5†), which was further confirmed by gel electrophoresis (ESI Fig. S6†).37 Furthermore, Transmission Electron Microscopy (TEM) images (Fig. S7a†) of AuNPs prepared by zein (0.15% w/v) with HAuCl4 (0.20 mM) showed that the particles were mostly spherical (Fig. S7a†) with an size around ∼27 ± 5 nm. TEM images of AuNPs of the same sample show clearly that the zein adheres to the surface of AuNPs (Fig. S7b†).
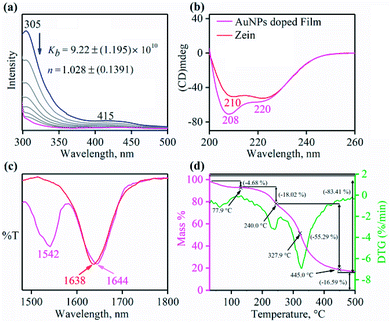
We then attempted to prepare and characterize the biodegradable AuNP functionalized zein-protein film. AuNP doped zein protein film was prepared by mixing the solution of zein (0.75 g, 7.5% w/v) in aqueous ethanol (80% v/v), glycerol (0.25 g, 2.5% w/v) as plasticizer and a freshly prepared AuNP suspension (15% v/v, vide supra) (ESI,† ES). Different experiments were performed to evaluate various physical parameters of the AuNP doped zein film. In this regards, first, we calculated the binding constant (Kb) for AuNPs with zein protein to understand the strength of the interaction between the two components. We have calculated the Kb by the fluorescence spectroscopic measurements as zein protein is known to show fluorescence due to the presence of tyrosine (5.25%, major component), rather tryptophan (0.16%, minor component).38,39 The binding constant was found to be 9.22 ± 1.195 × 1010 M−1, using Stern–Volmer equation, suggesting the reasonably good binding of AuNPs with zein protein (Fig. 2a; ESI, ES and Fig. S8, ESI†).40,41 The Hill coefficient (n) determined to be 1.028 (±0.1391), which is due to the enhanced degree of cooperativity in binding of zein with AuNP surface, suggesting a strong interaction between the immobilized AuNPs' surface ligand and zein.42 Interaction of zein with AuNPs and change in the conformation of protein was confirmed using CD.43 The spectra of zein protein with or without AuNPs are different from each other and showed two negative peaks at about ∼207–210 nm and 220 nm with a positive one around 192 nm, suggesting a typical protein with α + β structure, in which intensity of α-helix is more than β-sheet structure (Fig. 2b; ESI, ES, ESI†).44–46 This indicates a major conformational change arising from the interaction of AuNPs with zein. Also, we recorded FT-IR spectrum of zein-AuNP thin film showing a broad peak at 1644 cm−1,23 an additive spectrum of amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of zein peptide chain and the AuNP caped zein ligand [(1650 cm−1 + 1638 cm−1)/2 = 1644 cm−1], clearly indicating a very strong binding between zein and AuNP caped zein ligand (Fig. 2c; ESI, Fig. S9†). This strong interaction contributes to the mechanical strength of the zein-AuNP thin film, which was further confirmed by the estimation of the tensile strength (TS) and strain at failure (E) (ESI,† ES, and Fig. 4a and b).47,48
O stretching of zein peptide chain and the AuNP caped zein ligand [(1650 cm−1 + 1638 cm−1)/2 = 1644 cm−1], clearly indicating a very strong binding between zein and AuNP caped zein ligand (Fig. 2c; ESI, Fig. S9†). This strong interaction contributes to the mechanical strength of the zein-AuNP thin film, which was further confirmed by the estimation of the tensile strength (TS) and strain at failure (E) (ESI,† ES, and Fig. 4a and b).47,48
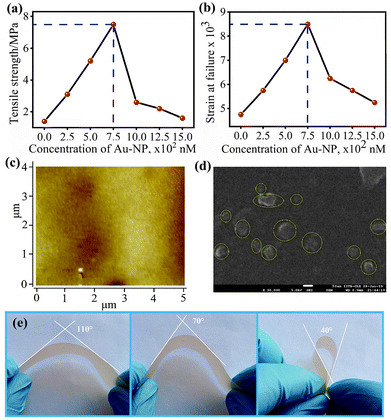
The morphology of films in the presence and absence of AuNPs were probed with the help of Optical Microscope, Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM). Optical microscope and AFM images confirmed the change in the morphology of these films (Fig. 3a and ESI, Fig. S10†). The RMS roughness (Sq), amplitude (A), and wavelength (λ) of the buckled zein film were determined by the optical microscope and found to be 120.5 nm, 42 μm, 137 μm (Fig. 3b), respectively. Optical microscope and AFM images of the AuNP doped film showed that there is almost no buckling (Sq = 1.6 nm, Fig. 4c). However, the AFM resolution was not enough to observe small size AuNPs (Fig. 3a); yet the clear difference in surface morphology was observed for films with or without AuNPs (Fig. 4c, ESI, and Fig. S11†). The internal compressive stress causes buckling formation due to the difference in Young's modulus at the adjacent region.49,50 Doping of AuNPs enhances the effective modulus and decreases critical stress, which regulates the disparities in the tensile stress hence eliminate buckling.51 Surface topography of the film is a key factor in controlling the extent and alignment of the buckling. Alteration of surface topography as shown in the AFM image induced by NPs diminishes the buckling which in turn increase the mechanical strength as well as the smoothness of the film surface (Fig. 4c).51 Additionally, SEM imaging was used to determine the surface as well as the distribution of AuNPs in the protein film. AuNPs embedded in the zein film appeared to be bigger than their independent size determined by TEM (Fig. 4d). This result also correlates with the shifting as well as the broadening of the SPR band in UV-visible spectra of the film from 550 to 560 nm (ESI, Fig. S11†).
These results are in good agreement with our observation (vide supra) of higher tensile strength for the films incorporated with AuNPs. Also, we have determined the thermal stability of AuNPs functionalized zein thin film from the TG-DSC experiment by following the temperature range from 27 °C to 500 °C. TG-DSC studies showed that the film could be easily used over a wide range of temperature (0 to 200 °C). However, the protein starts decomposing and completely burns out close to 328 °C (Fig. 2d). The thin film prepared from zein plus AuNPs showed the essential physical parameters such as flexibility, tensile strength, and strain required for a wide range of applications. The AuNPs functionalized film showed great flexibility, the bending at different angles suggesting high pliability, which is essential to adopt various from without breaking (Fig. 4e). Also, we have calculated the parameters (TS and E) by varying the ratio of AuNPs to zein. The titration of AuNPs with zein indicates the most suitable ratio for zein and AuNPs is 15% v/v to 7.5 × 10−2 nM (TS = 7.5 MPa, S = 8.5 × 10−3), respectively. However, an increase in the amount of AuNPs to zein significantly decreases both TS and E, suggesting that an optimum amount of AuNPs required to improve the mechanical properties of zein films (ESI, Fig. S10†). Additionally, Young's modulus and hardness of AuNPs doped zein films were obtained by fitting the Derjaguin–Müller–Toporov (DMT) model to the section of the force–distance curve where zein and the tip are in contact, and by measuring the adhesion forces between the tip and the zein film.52,53 Force–distance curves were obtained and was compared with standard (polystyrene). Young's Modulus was found to be 3.6 MPa (ESI ES and Fig. S12†).
In summary, we have demonstrated the synthesis of a novel zein thin film functionalized with AuNPs with increased mechanical properties. The characterization of AuNPs, as well as their interaction with zein protein, were studied using a combination of spectroscopic and microscopic techniques. We have also confirmed by FT-IR spectral data, for the first time, that the zein protein strongly binds on AuNPs' surface and also establishes that the free zein protein has a strong affinity towards the AuNPs. Also, physical parameters (tensile strength, strain, and Young's modulus) supports that the AuNPs' interactions provide the strength to zein film, which is further explained by the prevention of zein film buckling by the AuNPs interaction with zein protein. We believe that this is because of the decrease in compressive stress due to the uniform distribution of the AuNPs. We envisage that the further development of such proteins films with cheap, readily available metal oxides NPs will allow us to develop the materials for various industrial applications to a large extent.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by Grants-in-Aid (Grant No. EEQ/2016/000466) from the Science and Engineering Research Board-Department of Science and Technology (SERB-DST). We thank Dr Jatish Kumar for the interpretation of AFM images.Notes and references
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- C. M. Free, O. P. Jensen, S. A. Mason, M. Eriksen, N. J. Williamson and B. Boldgiv, Mar. Pollut. Bull., 2014, 85, 156–163 CrossRef CAS PubMed.
- K. Kaiser, M. Schmid and M. Schlummer, Recycling, 2017, 3, 1–26 CrossRef.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- C. G. Avio, S. Gorbi and F. Regoli, Mar. Environ. Res., 2017, 128, 2–11 CrossRef CAS PubMed.
- Y. Y. Zhong, Y. B. Li, W. X. Liang, L. S. Liu, S. L. Li, J. Q. Xue and D. W. Guo, J. Food Process Eng., 2018, 41, e12645 CrossRef.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- Natural fibers, biopolymers, and biocomposites, ed. A. K. Mohanty, M. Misra and L. T. Drzal, Taylor & Francis, 2005 Search PubMed.
- D. Garlotta, J. Polym. Environ., 2001, 9, 63–84 CrossRef CAS.
- B. P. Mooney, Biochem. J., 2009, 418, 219–232 CrossRef CAS PubMed.
- F. A. Momany, D. J. Sessa, J. W. Lawton, G. W. Selling, S. A. Hamaker and J. L. Willett, J. Agric. Food Chem., 2006, 54, 543–547 CrossRef CAS PubMed.
- P. Argos, K. Pedersen, M. D. Marks and B. A. Larkins, J. Biol. Chem., 1982, 257, 9984–9990 CAS.
- T. P. Aydt, C. L. Weller and R. R. Testinlton, Trans. ASAE, 1991, 34, 207–211 CAS.
- F. X. Budi Santosa and G. W. Padua, J. Agric. Food Chem., 1999, 47, 2070–2074 CrossRef CAS PubMed.
- H.-M. Lai and G. W. Padua, Cereal Chem., 1997, 74, 771–775 CrossRef CAS.
- J. W. Lawton, Cereal Chem., 2004, 81, 1–5 CrossRef CAS.
- N. Parris and D. R. Coffin, J. Agric. Food Chem., 1997, 45, 1596–1599 CrossRef CAS.
- H.-M. Lai, G. W. Padua and L. S. Wei, Cereal Chem., 1997, 74, 83–90 CrossRef CAS.
- H. J. Park, J. M. Bunn, C. L. Weller, P. J. Vergano and R. F. Testin, Trans. ASAE, 1994, 37, 1281–1285 CAS.
- N. Vahedikia, F. Garavand, B. Tajeddin, I. Cacciotti, S. M. Jafari, T. Omidi and Z. Zahedi, Colloids Surf., B, 2019, 177, 25–32 CrossRef CAS PubMed.
- J. O. Morales, A. C. Ross and J. T. McConville, J. Pharm. Pharmacol., 2013, 65, 827–838 CrossRef CAS PubMed.
- V. L. Colvin and K. M. Kulinowski, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8679–8680 CrossRef CAS PubMed.
- G. Labib, Expert Opin. Drug Delivery, 2018, 15, 65–75 CrossRef CAS PubMed.
- K. G. DeFrates, R. Moore, J. Borgesi, G. Lin, T. Mulderig, V. Beachley and X. Hu, Nanomaterials, 2018, 8, 457–483 CrossRef PubMed.
- D. Qin, L. Zhang, X. Du, Y. Wang and Q. Zhang, J. Nanopart. Res., 2016, 18, 254 CrossRef.
- A. Mahal, P. Khullar, H. Kumar, G. Kaur, N. Singh, M. Jelokhani-Niaraki and M. S. Bakshi, ACS Sustainable Chem. Eng., 2013, 1, 627–639 CrossRef CAS.
- K. K. Li, S. W. Yin, X. Q. Yang, C. H. Tang and Z. H. Wei, J. Agric. Food Chem., 2012, 60, 11592–11600 CrossRef CAS PubMed.
- V. I. Dodero, Z. B. Quirolo and M. A. Sequeira, Front. Biosci., Landmark Ed., 2011, 16, 61–73 CrossRef.
- I. B. Grishina and R. W. Woody, Faraday Discuss., 1994, 99, 245–262 RSC.
- S. W. Provencher and J. Glockner, Biochemistry, 1981, 20, 33–37 CrossRef CAS PubMed.
- D. Zhang, O. Neumann, H. Wang, V. M. Yuwono, A. Barhoumi, M. Perham, J. D. Hartgerink, P. Wittung-Stafshede and N. J. Halas, Nano Lett., 2009, 9, 666–671 CrossRef CAS PubMed.
- N. Biswas, A. J. Waring, F. J. Walther and R. A. Dluhy, Biochim. Biophys. Acta, 2007, 1768, 1070–1082 CrossRef CAS PubMed.
- K. Moriyama, K. Hirao and K. Takeda, Colloid Polym. Sci., 2000, 278, 979–985 CrossRef.
- Z. J. Deng, M. Liang, M. Monteiro, I. Toth and R. F. Minchin, Nat. Nanotechnol., 2011, 6, 39–44 CrossRef CAS PubMed.
- R. G. Aswathy, S. Balasubramanian, B. Dhandayudhapani, F. Takahiro, Y. Yasuhiko, M. Toru and D. S. Kumar, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2012, 3, 025006 Search PubMed.
- A. A. Kamnev, Infrared spectroscopy in studying biofunctionalised gold nanoparticles, in Nanomaterials Imaging Techniques, Surface Studies, and Applications, Springer, 2013, pp. 35–50 Search PubMed.
- M. Hanauer, S. Pierrat, I. Zins, A. Lotz and C. Sonnichsen, Nano Lett., 2007, 7, 2881–2885 CrossRef CAS PubMed.
- Principles of Fluorescence Spectroscopy, ed. J. R. Lakowicz, 2006 Search PubMed.
- R. Shukla and M. Cheryan, Ind. Crops Prod., 2001, 13, 171–192 CrossRef CAS.
- S. P. Boulos, T. A. Davis, J. A. Yang, S. E. Lohse, A. M. Alkilany, L. A. Holland and C. J. Murphy, Langmuir, 2013, 29, 14984–14996 CrossRef CAS PubMed.
- C. Wang, Q. H. Wu, C. R. Li, Z. Wang, J. J. Ma, X. H. Zang and N. X. Qin, Anal. Sci., 2007, 23, 429–433 CrossRef PubMed.
- J. N. Weiss, FASEB J., 1997, 11, 835–841 CrossRef CAS PubMed.
- P. Satzer, F. Svec, G. Sekot and A. Jungbauer, Eng. Life Sci., 2016, 16, 238–246 CrossRef CAS PubMed.
- T. C. Bicudo, R. C. Bicudo, L. A. Forato, L. M. Beltramini, L. A. R. Batista, R. B. Filho and L. A. Colnago, Biopolymers, 2008, 89, 175–178 CrossRef CAS PubMed.
- S. Venyaminov and K. S. Vassilenko, Anal. Biochem., 1994, 222, 176–184 CrossRef CAS PubMed.
- P. Manavalan and W. C. Johnson Jr, Nature, 1983, 305, 831–832 CrossRef CAS.
- R. Kumar, Ind. Eng. Chem. Res., 2010, 49, 3479–3484 CrossRef CAS.
- Q. Wang and G. W. Padua, J. Agric. Food Chem., 2005, 53, 3444–3448 CrossRef CAS PubMed.
- P. J. Yoo, K. Y. Suh, S. Y. Park and H. H. Lee, Adv. Mater., 2002, 14, 1383–1387 CrossRef CAS.
- G. M. Whitesides, N. Bowden, S. Brittain, A. G. Evans and J. W. Hutchinson, Nature, 1998, 393, 146–149 CrossRef.
- T. R. Hendricks and I. Lee, Nano Lett., 2007, 7, 372–379 CrossRef CAS PubMed.
- M. Lorenzoni, L. Evangelio, S. Verhaeghe, C. Nicolet, C. Navarro and F. Pérez-Murano, Langmuir, 2015, 31, 11630–11638 CrossRef CAS PubMed.
- O. Krivosheeva, M. Sababi, A. Dedinaite and P. M. Claesson, Langmuir, 2013, 29, 9551–9561 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra04527a |
| This journal is © The Royal Society of Chemistry 2019 |