Open Access Article
Open Access ArticleLigand geometry controlling Zn-MOF partial structures for their catalytic performance in Knoevenagel condensation†
Zimo He‡
 b,
Xi Zhao‡a,
Xinbo Pana,
Yuanyuan Lia,
XiaoXiao Wanga,
Haitao Xu
b,
Xi Zhao‡a,
Xinbo Pana,
Yuanyuan Lia,
XiaoXiao Wanga,
Haitao Xu *a and
Zhenliang Xu
*a and
Zhenliang Xu a
a
aState Key Laboratory of Chemical Engineering, Membrane Science and Engineering R&D Lab, Chemical Engineering Research Center, East China University of Science and Technology (ECUST), 130 Meilong Road, Shanghai 200237, China. E-mail: xuhaitao@ecust.edu.cn
bSchool of Chemistry and Molecular Engineering, East China University of Science and Technology (ECUST), 130 Meilong Road, Shanghai 200237, China
First published on 13th August 2019
Abstract
A series of novel Zn-MOFs {1Zn: [Zn(NIA)2(3-bpdh)2]; 2Zn: [Zn(NPA)2(4-bpdh)2H2O]; 3Zn: [Zn2(CHDA)4(3-bpd)2]} were constructed by dicarboxylic acid and N,N′-bis(pyridine-yl-ethylidene)hydrazine. Ligand geometry revealed 1D to 3D Zn-MOF crystal topologies, whose combined-mode could be affected by the conditions. All these conditions affected the micro-nano crystal morphologies, namely 1Zn micro-sheets or nanospheres, 2Zn micro-clusters or micro-stick, and 3Zn micro-clusters or hollowspheres that were obtained. The catalysts exhibited 100% selectivity for Knoevenagel condensation reactions, among which the benzaldehyde conversion rate of the 3Zn hollowspheres was the highest, reaching a peak of 90%.
Metal–organic frameworks (MOFs) are a kind of rigid skeletal crystal formed via the coordination of metal ions with organic ligands, characterized by a large specific surface area, high porosity and structural diversity.1–3 The abundance of metal atoms and organic ligands led to diverse and intriguing assemblies.4 Since the pioneering work of Yaghi et al.5–7, more than 20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MOFs have been reported and have shown remarkable prospects in many applications, such as adsorption,8–11 electrochemistry,12–14 catalysis15–17 and luminescence,18–20 among which the research on catalysis is one of the rapidly growing applications. The inherent properties of MOFs make them excellent catalysts: these properties include highly dense active sites and even dispersion in the skeletons of MOFs, while their porosity facilitates the transport and diffusion of reactants, facilitating easy contact with those active sites.21
000 MOFs have been reported and have shown remarkable prospects in many applications, such as adsorption,8–11 electrochemistry,12–14 catalysis15–17 and luminescence,18–20 among which the research on catalysis is one of the rapidly growing applications. The inherent properties of MOFs make them excellent catalysts: these properties include highly dense active sites and even dispersion in the skeletons of MOFs, while their porosity facilitates the transport and diffusion of reactants, facilitating easy contact with those active sites.21
The Knoevenagel condensation is a classic reaction, which provides an effective way to form C–C double bonds by the reaction of carbonyl compounds, such as aldehydes or ketones, with compounds containing active methylene groups. It has been widely used in the synthesis of many compounds and is a reaction with an important application value in organic synthesis.22–26 In recent years, many catalysts for such reactions have emerged,27–29 among which MOFs or MOF-derived solid bases have shown remarkable catalytic performance.30–33
In this study, we prepared three new MOFs with one-dimensional, two-dimensional and three-dimensional structures. First, by combining zinc 5-nitroisophthalic acid (NIA) with N,N′-bis(1-pyridine-3-yl-ethylidene)hydrazine (3-bpdh) (1Zn); second, by combining zinc 3-nitrophthalic acid (NPA) with N,N′-bis(1-pyridine-4-yl-ethylidene)hydrazine (4-bpdh) (2Zn); and third, by combining zinc 1,4-cyclohexanedicarboxylic acid (CHDA) with N,N′-bis(1-pyridine-3-yl-ethylidene) (3-bpd) (3Zn). By changing the reaction conditions, 1Zn nanospheres, 2Zn microsticks and 3Zn hollowspheres were obtained. We also investigated the catalytic performance of the six catalysts for Knoevenagel condensation and analyzed the possible catalytic mechanism.
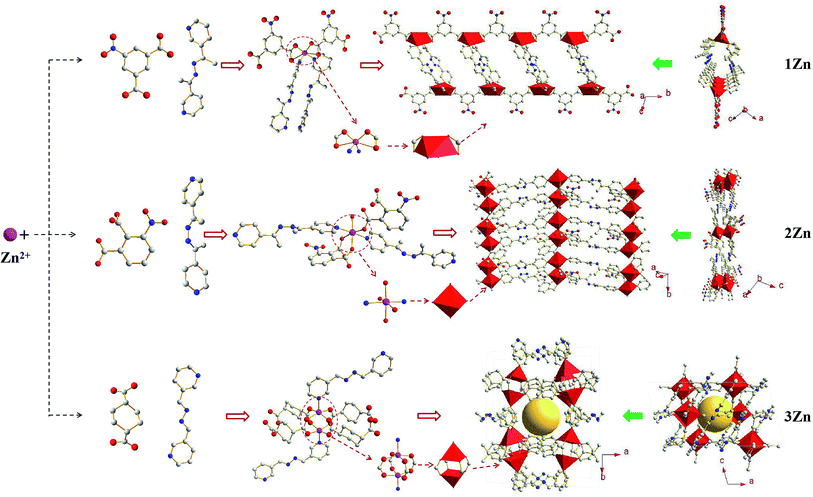
As shown in Fig. 1, the zinc atoms in all three crystals are present in a hexacoordinate environment; however, the coordination direction can be influenced by different ligands coordinated with zinc atoms, so as to obtain three kinds of MOFs with one-dimensional, two-dimensional and three-dimensional structures. 1Zn crystallized in a triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) [a = 8.585(5) Å, b = 10.181(6) Å, c = 14.860(12) Å, α = 104.46(9)°, β = 101.00(9)°, γ = 102.35(6)°, V = 1186.92(140) Å3, Z = 2]. The Zn(II) center was bonded to four O atoms from the carboxylate groups of two 5-nitrophthalic acid ligands and two N atoms from the 3-bpdh ligand, forming ZnO4N2 metal nodes. Two 3-bpdh ligands linked two ZnO4N2 metal nodes to form ring structures, and each ring was connected by 5-nitrophthalic acid molecules. The single coordination direction determines the one-dimensional (1D) tube structure of the crystal. 2Zn crystallized in a monoclinic space group P121/c1(14) [a = 14.74(3) Å, b = 8.444(15) Å, c = 19.15(3) Å, β = 106.96(17)°, V = 2279.87(700) Å3, Z = 4]. The Zn(II) center was bonded to three O atoms from the carboxylate groups of two 3-nitrophthalic acid ligands, two N atoms from the 4-bpdh ligand, and one O atom from H2O, forming ZnO4N2 metal nodes. In this metal node, the carboxyl group had two coordination modes: the O atoms from No.1 and No.2 carboxyls in one 3-nitrophthalic acid chelated the Zn(II) center to form 7-member ring, while the O atom from the No.1 carboxyl in the other 3-nitrophthalic acid bridged this Zn(II) center to other ZnO4N2 metal nodes. The 4-bpdh ligand and 3-nitrophthalic acid ligands formed a double chain with a ZnO4N2 metal node as the center. The coordination method of 2Zn is limited to a two-dimensional (2D) plane, resulting in its 2D structure. 3Zn crystallized in a monoclinic space group C12/c1(15) [a = 17.23(3) Å, b = 19.96(3) Å, c = 9.432(15) Å, β = 105.71(18)°, V = 3122.62(800) Å3, Z = 8]. Two Zn(II) centers were bonded to eight O atoms from the carboxylate groups of four 1,4-cyclohexanedicarboxylic acid (CHDA) ligands and two N atoms from the 3-bpd ligand, forming Zn2O4N2 metal nodes. Four CHDA ligands formed a cross double chain with two zinc atoms as the center, and the 3-bpd ligand linked the double chains to yield a three-dimensional (3D) framework. More detailed information about the crystal structure is contained in the ESI.†
(2) [a = 8.585(5) Å, b = 10.181(6) Å, c = 14.860(12) Å, α = 104.46(9)°, β = 101.00(9)°, γ = 102.35(6)°, V = 1186.92(140) Å3, Z = 2]. The Zn(II) center was bonded to four O atoms from the carboxylate groups of two 5-nitrophthalic acid ligands and two N atoms from the 3-bpdh ligand, forming ZnO4N2 metal nodes. Two 3-bpdh ligands linked two ZnO4N2 metal nodes to form ring structures, and each ring was connected by 5-nitrophthalic acid molecules. The single coordination direction determines the one-dimensional (1D) tube structure of the crystal. 2Zn crystallized in a monoclinic space group P121/c1(14) [a = 14.74(3) Å, b = 8.444(15) Å, c = 19.15(3) Å, β = 106.96(17)°, V = 2279.87(700) Å3, Z = 4]. The Zn(II) center was bonded to three O atoms from the carboxylate groups of two 3-nitrophthalic acid ligands, two N atoms from the 4-bpdh ligand, and one O atom from H2O, forming ZnO4N2 metal nodes. In this metal node, the carboxyl group had two coordination modes: the O atoms from No.1 and No.2 carboxyls in one 3-nitrophthalic acid chelated the Zn(II) center to form 7-member ring, while the O atom from the No.1 carboxyl in the other 3-nitrophthalic acid bridged this Zn(II) center to other ZnO4N2 metal nodes. The 4-bpdh ligand and 3-nitrophthalic acid ligands formed a double chain with a ZnO4N2 metal node as the center. The coordination method of 2Zn is limited to a two-dimensional (2D) plane, resulting in its 2D structure. 3Zn crystallized in a monoclinic space group C12/c1(15) [a = 17.23(3) Å, b = 19.96(3) Å, c = 9.432(15) Å, β = 105.71(18)°, V = 3122.62(800) Å3, Z = 8]. Two Zn(II) centers were bonded to eight O atoms from the carboxylate groups of four 1,4-cyclohexanedicarboxylic acid (CHDA) ligands and two N atoms from the 3-bpd ligand, forming Zn2O4N2 metal nodes. Four CHDA ligands formed a cross double chain with two zinc atoms as the center, and the 3-bpd ligand linked the double chains to yield a three-dimensional (3D) framework. More detailed information about the crystal structure is contained in the ESI.†
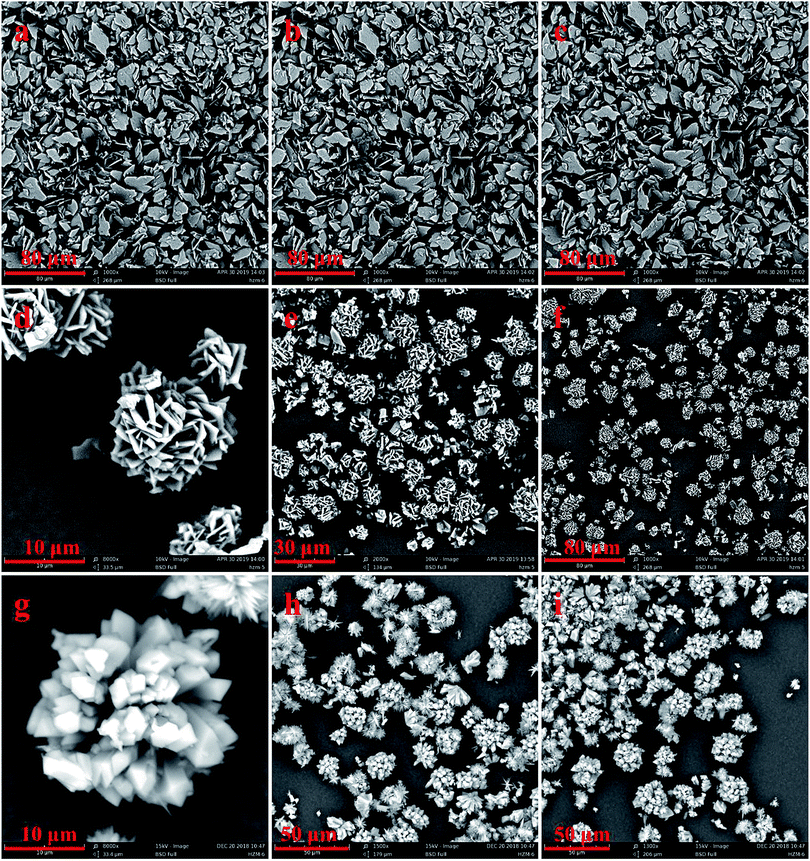
These three kinds of microcrystals were prepared in methanol and water at room temperature. As shown in Fig. 2, the three kinds of MOFs have different morphologies. Under SEM, 1Zn presents a sheet structure, while 2Zn and 3Zn show flower-like morphologies, which are formed by the secondary assembly of tiny crystals. They are about 10 to 20 microns in diameter, and 3Zn is slightly larger than 2Zn. The PXRD patterns of the three crystals coincide with the simulated curves of their single crystal, which proves the existence of the respective crystal phases (Fig. S1–S3†).
On this basis, we used different conditions to change the morphologies of these three crystals, which exhibited three improved morphologies (Fig. 3). More conditions were attempted, and the results are shown in the ESI.† A 1Zn nanosphere was synthesized in a DMF system with the introduction of PVP by a solvent thermal method. PVP is an amphiphilic surfactant, which can adsorb on the surface of nanocrystals and change the growth direction and speed of the crystals, thus affecting the final morphology and particle size of the product.34 With the increase in the PVP dosage, the size of the 1Zn nanosphere was smaller and more uniform (Fig. S10†). At 0.1 g PVP dosage, nanospheres with particle sizes of about 300 nm were obtained. A 2Zn microstick was also synthesized by the solvent thermal method in MeOH solvent. Interestingly, we added sodium acetate as a regulator to the DMF system and obtained 3Zn hollowspheres with the assistance of ultrasound. When the dosage of sodium acetate was 0.05 mmol, a dandelion-like 3Zn spherical crystal reassembled by needle crystals was obtained. On continuously increasing the amount of sodium acetate to 0.1 mmol, the spherical crystals became hollow. However, when sodium acetate was added to 0.2 mmol, the morphology of the product appeared chaotic (Fig. S11†). As a base, sodium acetate can accelerate the deprotonation process of carboxylic acid ligands, thus accelerating the growth rate of the crystals. Moreover, there was competitive coordination between COO− and the carboxylic acid ligands, so the introduction of sodium acetate can affect the growth process of the crystals.35
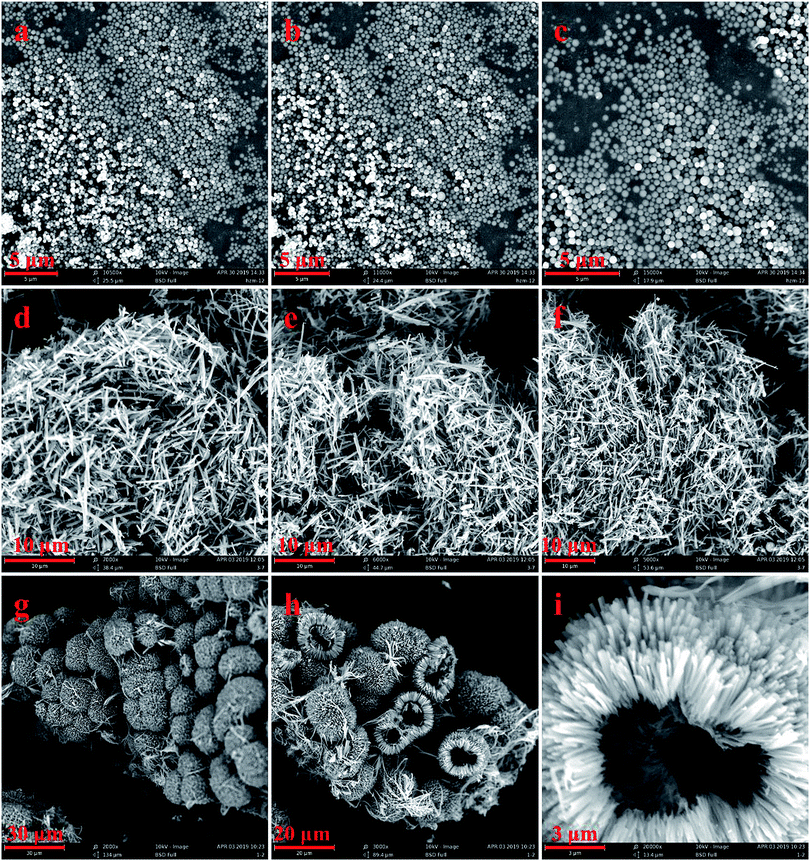 | ||
| Fig. 3 SEM images of (a–c) 1Zn (DMF, 100 °C, 0.1 g PVP); (d–f) 2Zn (MeOH, 120 °C); (g–i) 3Zn (0.1 mmol NaAC, 0.5 h of ultrasonic treatment, standing at room temperature for 24 h). | ||
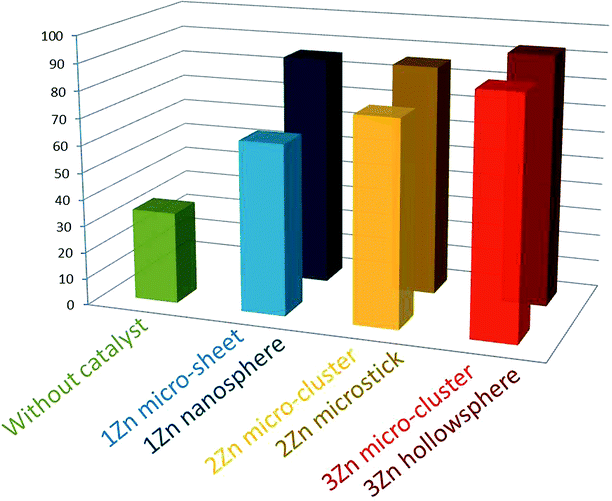
The catalytic properties of the catalysts for the Knoevenagel condensation reactions were investigated. As shown in Fig. 4, when the 1Zn and 2Zn microcrystals were used as catalysts, the conversions are 64% and 76% after 4 hours reaction, respectively, but 3Zn could reach more than 88%. After controlling the morphology, the order of catalytic performance was still 1Zn < 2Zn < 3Zn, which may be related to the structure of the MOFs. 3Zn with its 3D coordination structure may be more conducive to providing a smooth path for the rapid transfer of electrons.
Moreover, after adjusting the morphology, the catalytic effects of 1Zn, 2Zn, 3Zn were improved. The conversion of benzaldehyde was increased to 86% for the 1Zn nanosphere, 87% for the 2Zn microstick and 90% for the 3Zn hollowsphere (Fig. S13†). The catalytic activity of all the Zn-MOFs may be related to the size, dispersion and accessible surface areas of the catalyst.36 For contrast, we studied the Knoevenagel condensation reaction with n-heptanal, keeping other conditions unchanged. The results are shown in the ESI (Fig. S14†). It was found that the results obtained with benzaldehyde or heptanal were similar in general; after adjusting the morphology, the conversion of heptanal catalyzed by the 1Zn nanosphere, the 2Zn microstick and the 3Zn hollowsphere were still higher than those of their microcrystals. The catalytic efficiency of the 1Zn nanosphere reached 90%, while that of the 3Zn hollowsphere was 85%, close to that of the 3Zn micro-cluster. On this basis, we performed the BET analysis of the 3Zn micro-cluster and 3Zn hollowsphere, and the results presented in the ESI (Fig. S12†) show that the median pore width of the 3Zn micro-cluster and 3Zn hollowsphere are similar. The BET surface area of the micro-cluster is larger than that of the hollowsphere, but the volume of the pores is smaller than that of the hollowsphere. So, we suppose that because of the similar pore width, the key to improving the conversion of benzaldehyde is the pore volume. However, in comparison, heptanal is bulky and cannot enter the pore as easily as benzaldehyde, so the catalytic efficiency of the 3Zn hollowsphere is close to that of the 3Zn micro-cluster.31,37,38 The XRD patterns of all the catalysts after catalysis were consistent with those before catalysis (Fig. S4–S9†), indicating that the structure did not change.
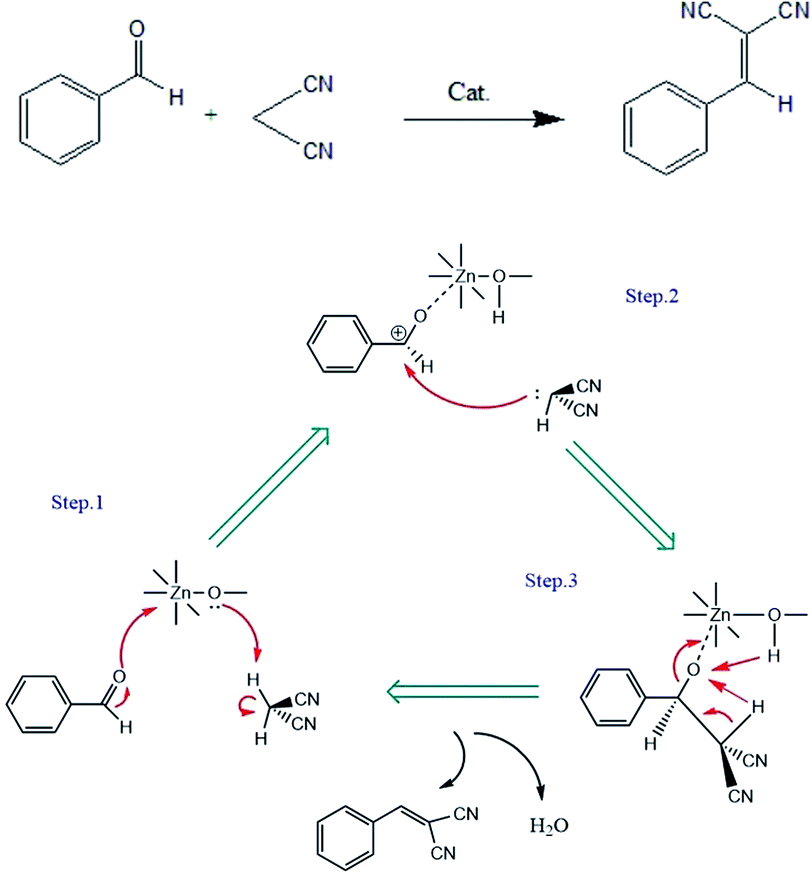
There are two main mechanisms of Knoevenagel condensation catalyzed by MOFs: basic catalysis and bifunctional acid–base catalysis.39 In our study, the three Zn-MOFs seemed to prefer the acid-catalyzed mechanism since they did not have a strong basic group, such as the amino group; although the ligand contains two N atoms, these two N atoms already have three bonds, the electron cloud is dispersed, and the –NO2 and –COOH groups on the organic carboxylic acid ligands also weaken the basicity. Based on other studies,36,40,41 we proposed a possible bifunctional acid–base catalytic mechanism. The Zn(II) in the Zn-MOF has empty atomic orbitals, which can be used as the Lewis acid sites. The carbonyl oxygen carries a pair of lone pair electrons that coordinate with Zn(II), so the positive charge on the O atom can promote further polarization of the carbonyl group. The positive electricity of the carbonyl C atom can thereby be enhanced. At the same time, the hydroxyl O atom coordinated with the Zn(II) ion acted as the center of the Lewis basic sites, which promoted the active methylene of malonitrile to remove protons and formed a carbon anion near benzaldehyde. Then, malonitrile attacked the carbonyl C atom of benzaldehyde, and the intermediate became the product and regenerated the catalyst after rearrangement and dehydration (Fig. 5).
Conclusions
In summary, three kinds of novel Zn-MOFs were synthesized, and their coordination directions were affected by different ligands, resulting in the formation of 1D, 2D and 3D structures. By changing the reaction conditions, the crystal reassembly was affected, and its morphology could be regulated. Finally, 1Zn micro-sheet, 1Zn nanospheres, 2Zn micro-flower, 2Zn micro-stick, 3Zn micro-flower, and 3Zn hollowspheres were obtained. The catalytic properties of all of these catalysts for the Knoevenagel condensation reaction were explored. The selectivity of all catalysts was 100%, among which the benzaldehyde conversion rate of 3Zn hollowspheres was the highest, reaching a peak of 90%. We believe that the work in this paper, especially the control of the crystal growth direction and reassembly mode, can provide a reference for the design of crystals with special morphologies to meet different needs.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21371058) and University Student Research Plan (USRP) of ECUST.Notes and references
- M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404–14405 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- H. Zeng, M. Xie, Y. L. Huang, Y. F. Zhao, X. J. Xie, J. P. Bai, M. Y. Wan, R. Krishna, W. G. Lu and D. Li, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS PubMed.
- M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- O. M. Yaghi and H. Li, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef CAS.
- H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571–8572 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. Xamena and J. Gascon, Nat. Mater., 2015, 14, 48–55 CrossRef CAS PubMed.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356, 430–432 CrossRef CAS PubMed.
- W. Xia, A. Mahmood, R. Q. Zou and Q. Xu, Energy Environ. Sci., 2015, 8, 1837–1866 RSC.
- S. L. Zhao, Y. Wang, J. C. Dong, C. T. He, H. J. Yin, P. F. An, K. Zhao, X. F. Zhang, C. Gao, L. J. Zhang, J. W. Lv, J. X. Wang, J. Q. Zhang, A. M. Khattak, N. A. Khan, Z. X. Wei, J. Zhang, S. Q. Liu, H. J. Zhao and Z. Y. Tang, Nat. Energy, 2016, 1, 1–10 Search PubMed.
- D. Sheberla, J. C. Bachman, J. S. Elias, C. J. Sun, Y. Shao-Horn and M. Dinca, Nat. Mater., 2017, 16, 220–224 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- H. Q. Xu, J. H. Hu, D. K. Wang, Z. H. Li, Q. Zhang, Y. Luo, S. H. Yu and H. L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440–13443 CrossRef CAS PubMed.
- M. T. Zhao, K. Yuan, Y. Wang, G. D. Li, J. Guo, L. Gu, W. P. Hu, H. J. Zhao and Z. Y. Tang, Nature, 2016, 539, 76–80 CrossRef CAS PubMed.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- Y. J. Cui, R. J. Song, J. C. Yu, M. Liu, Z. Q. Wang, C. D. Wu, Y. Yang, Z. Y. Wang, B. L. Chen and G. D. Qian, Adv. Mater., 2015, 27, 1420–1425 CrossRef CAS PubMed.
- S. Y. Wu, Y. N. Lin, J. W. Liu, W. Shi, G. M. Yang and P. Cheng, Adv. Funct. Mater., 2018, 28, 10 Search PubMed.
- Q. H. Yang, Q. Xu and H. L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- Y. Hayashi, Y. Miyamoto and M. Shoji, Tetrahedron Lett., 2002, 43, 4079–4082 CrossRef CAS.
- P. B. Mills, E. Struys, C. Jakobs, B. Plecko, P. Baxter, M. Baumgartner, M. Willemsen, H. Omran, U. Tacke, B. Uhlenberg, B. Weschke and P. T. Clayton, Nat. Med., 2006, 12, 307–309 CrossRef CAS PubMed.
- Y. J. Bian, Y. Qin, L. W. Xiao and J. T. Li, Chin. J. Org. Chem., 2006, 26, 1165–1172 CAS.
- G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Tetrahedron, 2003, 59, 3753–3760 CrossRef CAS.
- P. Lidstrom, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225–9283 CrossRef CAS.
- A. Lee, A. Michrowska, S. Sulzer-Mosse and B. List, Angew Chem. Int. Ed. Engl., 2011, 50, 1707–1710 CrossRef CAS PubMed.
- X. Y. Li, B. N. Lin, H. B. Li, Q. Yu, Y. Ge, X. Jin, X. H. Liu, Y. H. Zhou and J. P. Xiao, Appl. Catal. B-Environ., 2018, 239, 254–259 CrossRef CAS.
- N. Yao, J. Tan, Y. Liu and Y. L. Hu, Synlett, 2019, 30, 699–702 CrossRef CAS.
- L. Zhu, X. Q. Liu, H. L. Jiang and L. B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- J. Gascon, U. Aktay, M. D. Hernandez-Alonso, G. P. M. van Klink and F. Kapteijn, J. Catal., 2009, 261, 75–87 CrossRef CAS.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- U. P. N. Tran, K. K. A. Le and N. T. S. Phan, ACS Catal., 2011, 1, 120–127 CrossRef CAS.
- X. Cai, J. Lin and M. Pang, Cryst. Growth Des., 2016, 16, 3565–3568 CrossRef CAS.
- H. Guo, Y. Zhu, S. Wang, S. Su, L. Zhou and H. Zhang, Chem. Mater., 2012, 24, 444–450 CrossRef CAS.
- J. J. Wang, X. X. Wang, H. T. Xu, X. Zhao, Z. Z. Zheng and Z. L. Xu, ChemPlusChem, 2017, 82, 1182–1187 CrossRef CAS.
- S. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 2607–2614 CrossRef CAS PubMed.
- Y. K. Hwang, D. Y. Hong, J. S. Chang, S. H. Jhung, Y. K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Ferey, Angew. Chem. Int. Ed., 2008, 47, 4144–4148 CrossRef CAS PubMed.
- Y. Yang, H. F. Yao, F. G. Xi and E. Q. Gao, J. Mol. Catal. A: Chem., 2014, 390, 198–205 CrossRef CAS.
- V. N. Panchenko, M. M. Matrosova, J. Jeon, J. W. Jun, M. N. Timofeeva and S. H. Jhung, J. Catal., 2014, 316, 251–259 CrossRef CAS.
- Y. Y. Li, Y. Q. Su, J. Xu, Z. L. Xu and H. T. Xu, Bull. Chem. Soc. Jpn., 2017, 90, 1152–1156 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1916894–1916896. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra04499j |
| ‡ Zimo He and Xi Zhao contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2019 |