Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Structural characterization of ginseng cyclopeptides and detection of capability to induce apoptosis in gastrointestinal cancer cells
Zhuo Liua,
Junhao Fua,
Shengwei Xiaoc and
Dongxin Wang *b
*b
aSino Japanese Friendship Hospital of Jilin University, Changchun 130033, China
bJilin Cancer Hospital, Changchun 130021, China. E-mail: wdxjluniversity@126.com; Fax: +86 0755 866719335; Tel: +86 0755 86671933
cShenzhen Hospital of Southern Medical University, Shenzhen, 5180200, China
First published on 20th September 2019
Abstract
Gastrointestinal tumors are the most frequently diagnosed malignancy and the second highest contributor to cancer mortality. Cyclopeptides are rarely isolated from ginseng because they are often present at low concentrations in a complex matrix. In the current study, seven novel ginseng cyclopeptides (GCPs) were isolated and their anti-tumor potency was explored. Anti-proliferative test results show that the (GCP-1)∼[cyclo-(L-Trp-L-Glu-L-Phe-L-Thr)] peptide display the best anti-proliferative activity in gastric cancer SGC-7901 cells in vitro, with an IC50 value of 37.8 ± 3.13 μM. Flow cytometry analysis shows that GCP-1 (7.56–189 μM) clearly induce early apoptosis and mitochondrial membrane potential collapse, and block the cells at the G0/G1 phase. A further study revealed that GCP-1 induces apoptosis by activating the caspases, suppressing the thioredoxin (Trx) system and subsequently activating a number of Trx-dependent pathways, including those involving apoptotic signal-regulating kinase 1 (ASK1) and mitogen-activated protein kinases (MAPKs). The cyclopeptides in ginseng are an important resource for the research and development of anti-neoplastic drugs.
1 Introduction
In recent years, both the global incidence of cancer and mortality from cancer have increased rapidly, and malignant tumors are still a worldwide problem. With the continuous improvement of living standards, digestive cancers now account for a considerable proportion of malignant tumors.1 It is reported that the four cancers with the highest mortality rates include three digestive cancers: colorectal cancer, gastric cancer and liver cancer.2 At present, the main treatment for cancer is surgical treatment. However, it is difficult to completely cure tumors in the middle and late stages by surgical resection. Chemotherapy and radiotherapy are also important treatment methods, but their effect is poor in some digestive tract tumors such as pancreatic cancer. Furthermore, side effects, poor selectivity and drug resistance limit their clinical application.3 Therefore, the discovery of highly effective and low toxicity anti-neoplastic agents is urgently needed.The caspases are a family of cysteine proteases that play a pivotal role in the initiation and completion of apoptosis, being the executor of apoptosis. The activation of the caspases is able to cause apoptosis in tumor cells. The activation of the caspases can be achieved by activating the mitogen-activated protein kinase (MAPK) pathway which includes the ERK, JNK and p38 families.4 Stimulation of the MAPK pathway, and so phosphorylation, can affect the expression of transcription factors such as Bcl-2 and p53, and activate the caspase family, leading to the initiation of apoptosis.5 As reported, the thioredoxin (Trx)/thioredoxin reductase (TrxR) system is highly expressed in many primary tumors, such as hepatocellular carcinoma.6 As an important regulator of tumor progression, TrxR inhibits tumor apoptosis by inhibiting the MAPK pathway. Therefore, preventing Trx from binding to apoptotic signal-related kinase 1 (ASK1) so that it activates the MAPK-related pathway can induce apoptosis of cancer cells.7
Ginseng has been used as a tonic for more than 2000 years, and is one of the most commonly used and precious traditional Chinese medicines in China. Ginseng is widely used in the treatment of cardiovascular and digestive tract diseases and tumors. The ginsenosides are the main active ingredients in ginseng, and they play an anti-tumor role by inducing apoptosis, inhibiting angiogenesis, enhancing immunity and up-regulating the oncogene p53.8,9 As reported, a series of chemicals have been separated and identified from ginseng, including ginsenosides, triterpenes and steroids. For example, both the ginsenoside monomer Rd and ginsenoside Rg1 display excellent anti-tumor potencies in vivo and in vitro.10,11 However, no studies have focused predominantly on investigating the anti-tumor potency of ginseng cyclopeptides (GCPs). Hence, we isolated GCPs, and explored their anti-tumor potency and underlying mechanism of action.
2 Materials and methods
2.1 Material and cell culture
Ginseng was acquired from the South China Agricultural University (Guangzhou, China) and identified by Professor Wang (South China Agricultural University, Guangzhou, China). A voucher specimen (No. Herb-201902334) was deposited in the School of Pharmaceutical Sciences, South China Agricultural University. Both the cancer and normal cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The reagents propidium iodide (PI) and JC-1 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Radio-immunoprecipitation assay buffer (RIPA) buffer and RNase were purchased from Beyotime (Shanghai, China). All of the antibodies were purchased from Cell Signaling Technology (CST, Beverly, MA, USA). Fetal bovine serum (FBS) was purchased from Invitrogen (Thermo Fisher Scientific, USA). All of the other chemicals were purchased from Sigma or Adamas and were used without any further purification. The human gastric cancer cell lines BGC-823 and SGC-7901, the human esophageal cancer cell lines TE-1 and Eca109, and the human colorectal cancer cell line Caco-2 were cultured in specific media supplemented with 10% FBS and antibiotics (100 U mL−1 streptomycin and 100 U mL−1 penicillin) at 37 °C in 5% CO2.2.2 Isolation and identification of the GCPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v) and then stirred uninterrupted for 5 h. After that, the sediment was collected, freeze-dried and stored at −20 °C.
5 (w/v) and then stirred uninterrupted for 5 h. After that, the sediment was collected, freeze-dried and stored at −20 °C.The defatted precipitate (88.3 g) was dissolved (5%, w/v) in 0.20 M phosphate buffer solution (PBS, pH 7.2). After centrifugation (9000×g, 30 min), the supernatant was collected, freeze-dried and stored at −20 °C as the total ginseng protein.
Hydrophobic chromatography: the GCP-A was dissolved in 1.40 M (NH4)2SO4 prepared with 30 mM PBS (pH 7.2) and loaded onto a Phenyl Sepharose CL-4B hydrophobic chromatography column (2.5 cm × 150 cm). A stepwise elution was carried out with decreasing concentrations of (NH4)2SO4 (1.40, 0.70 and 0 M) dissolved in 30 mM PBS (pH 7.2) at a flow rate of 2.0 mL min−1. Each fraction of 80 mL was collected and the absorbance was monitored at 280 nm. Ten fractions (GCP-A-1∼GCP-A-10) were collected and the anti-proliferative activity of each was detected. The fraction having the best anti-cancer activity was collected and prepared for anion-exchange chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100
0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v). The peptide was purified using a gradient elution from 20% to 80% of solvent B for 30 min at a flow rate of 1.0 mL min−1. The detection wavelength was set at 280 nm and the column temperature was 16 °C.
0.1, v/v). The peptide was purified using a gradient elution from 20% to 80% of solvent B for 30 min at a flow rate of 1.0 mL min−1. The detection wavelength was set at 280 nm and the column temperature was 16 °C.HPLC-ESI-MS was carried out on a SCIEX X500R Q-TOF mass spectrometer (Framingham, U.S.A.) equipped with an ESI source. The mass range was set at m/z 100–1500. The Q-TOF MS data were acquired in positive mode and the conditions of MS analysis were as follows: CAD gas flow-rate, 7 L min−1; drying gas temperature, 550 °C; ion spray voltage, 5500 V; declustering potential, 80 V. The data file was generated using the SCIEX OS 1.0 software.
2.3 Cell proliferation analysis
The GCPs were dissolved in PBS as 10.0 mM stock solutions and then diluted in cell culture medium to different concentrations. The cell cytotoxicity was detected using the MTT method.12 The cancer cells were seeded on 96-well plates at a density of 5 × 104 cells per well and cultured overnight. Then, the cells were treated with different concentrations of GCPs for 24 h. After that, 10 μL per well of MTT was added and incubated for 4 h. The absorbance of the converted dye in living cells was measured at a wavelength of 570 nm, and the IC50 values for the different treatment conditions were calculated using Origin 8 software (OriginLab, Northampton, MA, USA).2.4 Apoptosis and cell cycle assays
After being treated with GCP-1 (0, 7.56, 37.8 or 189 μM; samples denoted Control, GCP-1-L, GCP-1-M and GCP-1-H, respectively) for 24 h, the SGC-7901 cells were collected and washed with cold PBS. Subsequently, the cells were suspended in 200 μL of staining buffer and stained with 10 μL of Annexin V-FITC. Cell apoptosis was detected using flow cytometry.After the GCP-1 treatments as described above, the SGC-7901 cells were harvested, fixed in 70% ethanol and stored at −20 °C overnight. After that, the cells were washed with PBS. The cell cycle distribution was detected by PI staining and analyzed using flow cytometry (BD FACSCalibur; Franklin Lakes, CA, USA). All of the tests were repeated at least 3 times.
2.5 Intracellular ROS and mitochondrial membrane potential (Δψm) assays
2,7-Dichlorodi-hydrofluorescein diacetate (DCFH-DA) can pass through the cell membrane to produce DCFH by hydrolysis of the intracellular esterase. Reactive oxygen species (ROS) can oxidize DCFH and produce fluorescent DCF.13 Therefore, the detection of DCF fluorescence can reveal the intracellular ROS level. Briefly, 1 mL samples of 1 × 106 SGC-7901 cells were incubated with GCP-1 (0, 7.56, 37.8 or 189 μM) at 37 °C for 48 h, or GCP-1 (37.8 μM) for 0, 2, 4, 8, 12, 24, 48 or 60 h. After that, the cells were washed three times with serum-free medium, and incubated in 200 μL of serum-free medium containing DCFH-DA (25 μM) for 30 min at room temperature. After that, the cells were collected, washed three times with serum-free medium, and assayed by flow cytometry (BD FACSCalibur, Franklin Lakes, CA, USA).The Δψm decline of SGC-7901 cells treated with GCP-1 was detected by JC-1 staining. Briefly, 2 mL samples of 1 × 106 cells per mL were treated with GCP-1 (0, 7.56, 37.8 or 189 μM) at 37 °C for 48 h, or GCP-1 (37.8 μM) for 0, 2, 4, 8, 12, 24, 48 or 60 h. After that, the Δψm decline was detected by JC-1 staining according to the manufacturer's protocol (Beyotime, China) using flow cytometry (BD FACSCalibur, Franklin Lakes, CA, USA).
2.6 Western blotting
After the GCP-1 treatments (0, 7.56, 37.8 and 189 μM) for 24 h, the SGC-7901 cells were collected and lysed with ice-cold RIPA lysis buffer and centrifuged at 1.20 × 104 rpm for 10 min at 4 °C. The total protein content was then measured using the Bradford method. Equal amounts of total protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins in the SDS-PAGE gel were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore). The membrane was blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) at 37 °C for 2 h, incubated with the primary antibodies and β-actin overnight at 4 °C, and then incubated with horseradish peroxidase labeled secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) at 37 °C for 1 h. After washing with TBST, protein bands were visualized using enhanced chemiluminescence detection reagents (Bio-Rad, USA).14 β-Actin was used as the internal control.
5000) at 37 °C for 1 h. After washing with TBST, protein bands were visualized using enhanced chemiluminescence detection reagents (Bio-Rad, USA).14 β-Actin was used as the internal control.
2.7 Statistical analysis
All of the data are expressed as the mean ± standard deviation (SD) of three independent experiments. Statistical significance was assessed using the Student's t-test (for comparisons of two treatment groups) or one-way ANOVA (for comparisons of three or more groups). A p-value <0.05 was considered to be statistically significant.3 Results and discussion
3.1 Isolation and identification of GCPs
Four novel and twelve known GCPs were isolated (Fig. 1). The purity of the GCPs was detected by HPLC (>97.5%). The GCPs were dissolved in PBS, each at a concentration of 10.0 mM as a stock solution, and stored at −20 °C.3.2 Inhibitory effect of GCPs on the growth of cancer and normal cell lines
Gastric cancer, esophageal cancer and colorectal cancer are very common types of cancer, and so the human gastric cancer cell lines BGC-823 and SGC-7901, the human esophageal cancer cells lines TE-1 and Eca109, and the human colorectal cancer cell line Caco-2 were chosen for the current study and acquired from the Cell Bank of the Chinese Academy of Sciences.As shown in Table 1, most of the GCPs displayed anti-proliferative activity in the experimental cancer cell lines. Interestingly, GCP-1∼4 displayed significant anti-proliferative activity in all five cancer cell lines, and GCP-1 showed the best anti-proliferative activity in SGC-7901 cells (IC50 value of 37.8 ± 3.13 μM). Therefore, a further study was carried out to check the anti-tumor potency of GCP-1 in SGC-7901 cells.
| GCPs | Amino acid sequences | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| BGC-823 | SGC-7901 | TE-1 | Eca109 | Caco-2 | ||
| a IC50 values are shown as mean ± SD, from at least three independent experiments. | ||||||
| GCP-1 | Cyclo-(Trp-Glu-Phe-Thr) | 71.1 ± 6.42 | 37.8 ± 3.13 | 62.8 ± 5.92 | 76.3 ± 6.27 | 81.6 ± 7.25 |
| GCP-2 | Cyclo-(Val-Phe-Leu-Glu) | 76.2 ± 6.91 | 86.3 ± 7.42 | 97.5 ± 9.13 | > 200 | > 200 |
| GCP-3 | Cyclo-(Ser-His-Leu-Tyr) | >200 | 109 ± 9.20 | >200 | 106 ± 9.19 | 102 ± 9.65 |
| GCP-4 | Cyclo-(Val-Ile-Pro-Phe) | 99.6 ± 9.18 | 104 ± 9.30 | 107 ± 10.9 | >200 | >200 |
| GCP-5 | Cyclo-(Pro-Val) | >200 | >200 | >200 | >200 | >200 |
| GCP-6 | Cyclo-(Pro-Thr) | 145 ± 14.9 | 178 ± 16.3 | 142 ± 13.1 | 113 ± 10.4 | 135 ± 12.3 |
| GCP-7 | Cyclo-(Pro-Ile) | >200 | >200 | >200 | >200 | >200 |
| GCP-8 | Cyclo-(Pro-Tyr) | 118 ± 12.9 | >200 | 197 ± 18.0 | >200 | >200 |
| GCP-9 | Cyclo-(Pro-Leu) | 120 ± 12.5 | 153 ± 12.5 | 182 ± 16.3 | 181 ± 15.3 | 151 ± 13.6 |
| GCP-10 | Cyclo-(Tyr-Phe) | >200 | 122 ± 11.3 | 107 ± 9.96 | 116 ± 9.21 | 115 ± 9.22 |
| GCP-11 | Cyclo-(Tyr-Leu) | 104 ± 9.17 | >200 | >200 | >200 | 186 ± 19.7 |
| GCP-12 | Cyclo-(Tyr-Val) | 113 ± 9.95 | 126 ± 12.7 | 157 ± 13.5 | 178 ± 11.9 | 155 ± 12.2 |
| GCP-13 | Cyclo-(Leu-Phe) | 108 ± 9.11 | 153 ± 11.0 | 151 ± 13.4 | 183 ± 19.3 | 156 ± 13.4 |
| GCP-14 | Cyclo-(Tyr-Ile) | 127 ± 13.2 | 146 ± 16.1 | >200 | 134 ± 15.7 | 147 ± 13.6 |
| GCP-15 | Cyclo-(Pro-Phe) | 191 ± 18.3 | 163 ± 14.4 | 185 ± 18.0 | 172 ± 12.0 | >200 |
| GCP-16 | Cyclo-(Ala-Phe) | >200 | 158 ± 9.15 | >200 | 154 ± 9.30 | >200 |
GCP-1 shows promising anti-cancer potency. Therefore, the cytotoxicity in normal cell lines from healthy tissue was evaluated using the same testing method as that applied to the cancer cells. By comparing the IC50 values after the same incubation time, it was clear that the cytotoxicity against human liver HL-7702 cells and normal gastric epithelial RGM-1 cells was much lower than that against SGC-7901 cells. The IC50 value was 7.63 ± 0.62 mM (24 h) in human liver HL-7702 cells while the IC50 value was 6.22 ± 0.48 mM (24 h) in normal gastric epithelial RGM-1 cells. Definitely, GCP-1 showed significantly attenuated cytotoxicity in normal cells. We speculated that the GCP-1 could have potential and should be further evaluated.
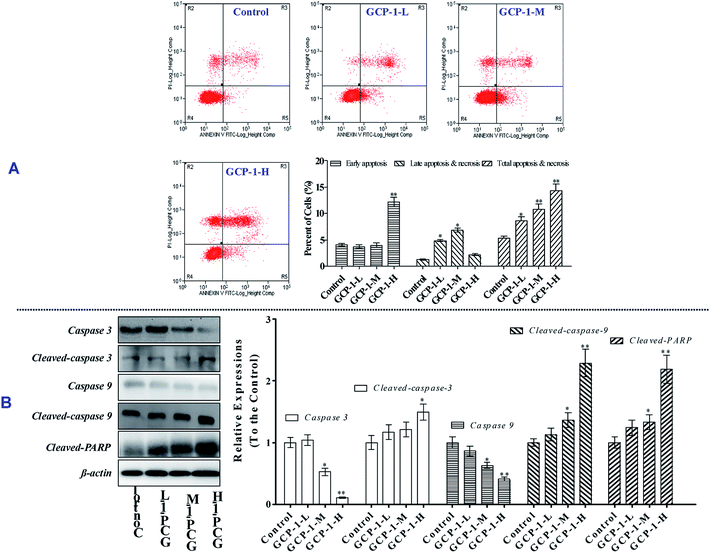
3.3 GCP-1 induces cell apoptosis and caspase activation
The MTT results revealed that GCP-1 inhibited the proliferation of SGC-7901 cells. Apoptosis is one of the critical molecular mechanisms in drug-induced cell death which plays a major negative regulatory role in the process of tumorigenesis.15 Flow cytometric analysis is a common method used to investigate whether or not drugs can induce the apoptosis of cancer cells. Consequently, flow cytometry was used to explore whether or not the anti-proliferative activity of GCP-1 was due to the induction of apoptosis.As shown in Fig. 2A, compared with the control group, GCP-1 increases apoptosis by 3.22%, 5.39% and 9.03%, for GCP-1-L, GCP-1-M and GCP-1-H, respectively. The results suggest that the anti-proliferative activity of GCP-1 in SGC-7901 cells is associated with cell apoptosis, and they provide visual evidence of the anti-cancer potency of GCP-1.
As we know, the caspases are a family of cysteine proteases that play a pivotal role in apoptosis. Caspase 9 is an initiator; Caspase 3 is involved in the apoptotic process, in which it is responsible for chromatin condensation and DNA fragmentation. On the other hand, poly(ADP-ribose) polymerase (PARP) is one of a family of proteins involved in a number of cellular processes involving mainly DNA repair and programmed cell death. When PARP is cleaved by enzymes such as the caspases or cathepsins, typically the function of PARP is inactivated.16
In order to disclose whether or not GCP-1 induces cell apoptosis by activating the caspases, we used western blotting to examine the expression levels of Caspase 3 and Caspase 9, cleaved Caspase 3 and Caspase 9 and cleaved PARP. We found that GCP-1 suppressed the expression levels of Caspase 3 and Caspase 9, while it increased the expression levels of cleaved Caspases 3 and 9, and cleaved PARP (Fig. 2B). The initiator Caspase 9 goes on to cleave Procaspase 3 and Procaspase 7. When PARP is cleaved by enzymes such as the caspases or cathepsins, typically the function of PARP is inactivated. Therefore, these data support the hypothesis that GCP-1 induces cell apoptosis via the modulation of Caspase 3, Caspase 9 and PARP, which closely participate in programmed cell death.
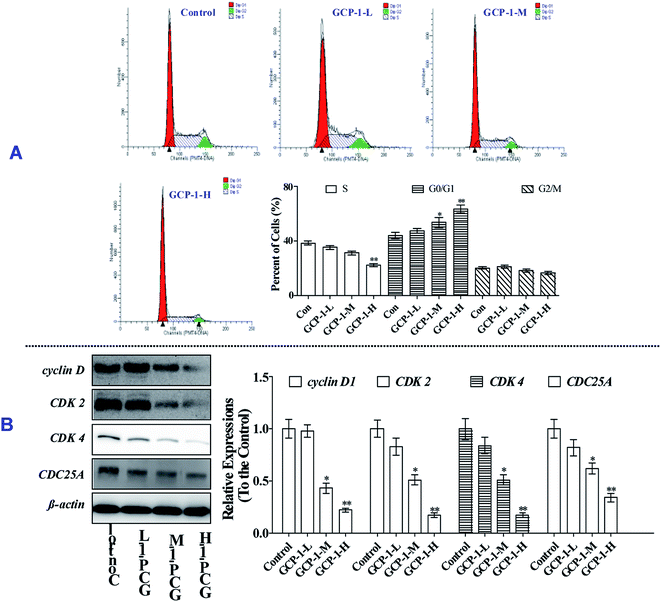
3.4 GCP-1 induces cell cycle arrest by regulating cell cycle regulatory proteins
Mitosis is the main mode of cell proliferation which goes through three stages of the cell cycle: G0/G1, S and G2/M, in turn. The stages of the cell cycle is divided according to the content of DNA in cells. Cancer is a periodic disease, and disorder in cell cycle regulation can lead to excessive cell proliferation.17 Therefore, the induction of cell cycle arrest can play a key role in anti-tumor activity.To investigate whether or not the anti-proliferative effect of GCP-1 on SGC-7901 cells was triggered by cell cycle arrest, we measured the cell cycle phase ratios by flow cytometry using PI staining. The populations of cells in the G0/G1 phase is 44.2%. Interestingly, with GCP-1 treatments, the populations of cells in the G0/G1 phase were increased by 3.44%, 9.59% and 19.5%, for GCP-1-L, GCP-1-M and GCP-1-H, respectively (Fig. 3A). We inferred that significant numbers of cells could be blocked in the G0/G1 phase. After the GCP-1 treatments, we also analyzed the expression levels of proteins involved in cell cycle regulation. We found that GCP-1 treatment inhibited cyclin D expression and reduced the expression levels of CDK2 and CDK4 in SGC-7901 cells (Fig. 3B). The expression level of Cell Division Cycle 25A (CDC25A), which acts as an upstream regulator of the CDK/cyclin complex, was also inhibited by GCP-1. Thus, we concluded that GCP-1 contributes to cell cycle arrest.
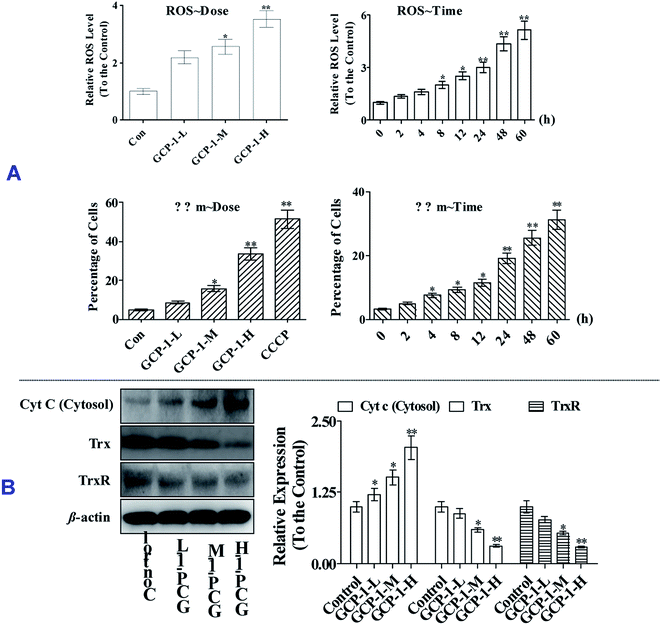
3.5 GCP-1 induces Δψm collapse and suppresses the Trx system
ROS overproduction or antioxidant capacity decline lead to balance disorders and cause ROS accumulation and oxidative stress that can lead to fatal damage to DNA and proteins within the cell, and subsequently induce apoptosis.18To examine the intracellular ROS, we detected the ROS levels in SGC-7901 cells after GCP-1 treatments. As shown in Fig. 4A, GCP-1 caused clear ROS accumulation in a dose and time-dependent manner.
The balance of the mitochondrial membrane potential (Δψm) and mitochondrial integrity are significant for the physiological functions of cells. Mitochondrial membrane permeabilization is a critical event in the process leading to physiological or chemotherapy-induced apoptosis. The mitochondrion-dependent pathway is the most common apoptotic pathway in vertebrate tumor cells.19 To investigate the mechanism underlying GCP-1-induced SGC-7901 cell apoptosis, we measured the loss of ΔΨm using JC-1.
As shown in Fig. 4, GCP-1 treatments increased the population of cells showing a decrease in the Δψm from 4.68% to 8.56%, 15.7% and 33.4%, for GCP-1-L, GCP-1-M and GCP-1-H, respectively. Interestingly, GCP-1 also induced the Δψm collapse in a time-dependent manner. Therefore, we inferred that GCP-1 may induce cell apoptosis by interfering with the function of the mitochondria.
Mitochondrial dysfunction, as indicated by the decline of ΔΨm, could subsequently cause the release of cytochrome c (Cyt c) from the mitochondria into the cytosol. Therefore, we evaluated the cytosolic Cyt c level.20 Western blot analysis showed that treatment with GCP-1 for 24 h resulted in a striking increase in the level of cytosolic Cyt c compared with that in control cells (Fig. 4B). The Trx system plays a pivotal role in regulating the balance of the intracellular redox state. Mechanistic studies have shown that Trx/TrxR is often over-expressed in tumor cells.6 The effect of GCP-1 on Trx and TrxR expression was also examined in SGC-7901 cells. As shown in Fig. 4B, the expression levels of Trx and TrxR were markedly down-regulated by GCP-1 compared to those in the control group.
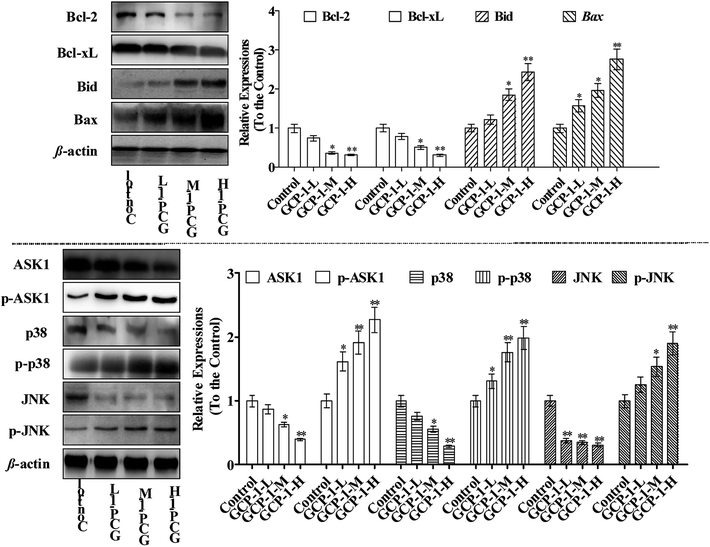
3.6 GCP-1 regulates expression levels of Bcl-2 family members and MAPKs
Bcl-2 family members can be functionally divided into pro-apoptotic factors (e.g. Bax, Bid) and anti-apoptotic factors (e.g. Bcl-2, Bcl-xL), and extensive research has been carried out on the molecular mechanisms of the apoptotic stimulus of the Bcl-2 family. Bax is generally sequestered in the cytosol and translocated into the mitochondria in large aggregates during apoptosis. Bax translocates into the mitochondria targets the mitochondrial intermembrane contact sites and releases Cyt c. The ratio of anti-apoptotic Bcl-2 and Bcl-xL to pro-apoptotic Bax and Bid determines the point at which apoptosis is triggered.21 Moreover, studies have shown that many chemotherapeutics trigger mitochondria-mediated apoptosis in tumor cells through down-regulation of Bcl-2 and Bcl-xL and up-regulation of Bax and Bid.22,23In the present study, the expression levels of Bcl-2 and Bcl-xL were down-regulated and, in contrast, the Bax and Bid expressions were increased which could be a sign of the induction of apoptosis. Studies have reported that ASK1 and MAPK are involved in many cellular and immune responses, such as cell cycle regulation and apoptosis.24 ASK1 is activated by a variety of stress factors, such as the accumulation of ROS. The reduced form of Trx is a significant inhibitor of ASK1. ASK1 is activated by phosphorylation and, upon Trx disassociation, it may cause the downstream activation of the JNK and p38 MAPK pathways.25 When Trx is oxidized, it dissociates from ASK1, which is then activated by the autophosphorylation of the kinase domain.26 It is interesting that ASK1 expression decreases in a dose-dependent manner following treatment with GCP-1 (Fig. 5). Therefore, we used western blotting to investigate how GCP-1 affects the phosphorylation status of the MAPK proteins (p38, JNK) in SGC-7901 cells. As shown in Fig. 5, p38 and JNK were down-regulated in SGC-7901 cells in a dose-dependent manner following the GCP-1 treatments, while the phosphorylation of p38 and JNK were up-regulated. These results indicate that the inhibition of the Trx system and activation of MAPK might be involved in GCP-1-mediated apoptosis.
4 Conclusion
The anti-tumor potency of natural peptides offers great promise in the development of chemotherapeutic drugs. Therefore, we focused predominantly on the anti-proliferative activities of the ginseng peptides, rather than the ginseng saponin. In the present study, GCP-1 significantly induced apoptosis in SGC-7901 cells. The cells were blocked in the G0/G1 phase, which indicated that the anti-proliferative effect of GCP-1 is associated with apoptosis and cell cycle arrest. GCP-1 also increased the phosphorylation of ASK1, which further phosphorylated the downstream MAPKs JNK and p38, suggesting that GCP-1 may activate the ASK1-JNK/p38 signaling axis to exert its pro-apoptotic effects. Notably, GCP-1 inhibited the Trx system and subsequently activated a series of Trx-dependent pathways, including the ASK1, MAPK-p38 and JNK pathways, which may lead to GCP-1-mediated apoptosis. However, further investigation is required to ascertain whether or not GCP-1 exhibits similar effects on other cellular systems.Conflicts of interest
The authors certify that there is no conflict of interest with any individual/organization for the present work.Acknowledgements
We thank Professor Chen for technical assistance as well as critical editing of the manuscript.References
- K. Renner, et al., Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment, Front. Immunol., 2017, 8(6), 248–259 Search PubMed.
- E. P. Weledji, Centralization of Liver Cancer Surgery and Impact on Multidisciplinary Teams Working on Stage IV Colorectal Cancer, Oncol. Rev., 2017, 11(2), 28–39 CrossRef PubMed.
- R. S. Narayan, et al., The allosteric AKT inhibitor MK2206 shows a synergistic interaction with chemotherapy and radiotherapy in glioblastoma spheroid cultures, BMC Cancer, 2017, 17(1), 204–215 CrossRef PubMed.
- X. R. Wang, et al., The functions of caspase in whitefly Bemisia tabaci apoptosis in response to UV irradiation, Insect Mol. Biol., 2018, 27(6), 2223–2235 CrossRef PubMed.
- L. Zhang, et al., Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells, J. Cell. Biochem., 2010, 106(4), 553–562 CrossRef PubMed.
- W. Li, et al., Two thioredoxin reductases (TrxRs), trxr-1 and trxr-2, have differential physiological roles in Caenorhabditis elegans, Mol. Cells, 2012, 34(2), 209–218 CrossRef CAS PubMed.
- E. A. Liedhegner, K. M. Steller and J. J. Mieyal, Levodopa activates apoptosis signaling kinase 1 (ASK1) and promotes apoptosis in a neuronal model: implications for the treatment of Parkinson's disease, Chem. Res. Toxicol., 2011, 24(10), 1644–1652 Search PubMed.
- S. E. Kim, Y. H. Lee, J. H. Park and S. K. Lee, Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepatoma SK-HEP-1 cells, Eur. J. Cancer, 1999, 35(3), 507–511 CrossRef CAS PubMed.
- B. Li, et al., Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53, Cancer Lett., 2011, 301(2), 185–192 CrossRef CAS PubMed.
- P. Wang, et al., Effect of ginsenoside Rg1 on proliferation and differentiation of human dental pulp cells in vitro, Aust. Dent. J., 2012, 57(2), 157–165 CrossRef CAS PubMed.
- X. Zhang, et al., Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways, Front. Pharmacol., 2013, 4, 152–166 Search PubMed.
- B. L. Ye, et al., Chitosan-coated doxorubicin nano-particles drug delivery system inhibits cell growth of liver cancer via p53/PRC1 pathway, Biochem. Biophys. Res. Commun., 2017, 495(1), 1248–1255 Search PubMed.
- D. Li and P. Zhang, Protective effect and molecular mechanism of liquiritin on oxybuprocaine-induced apoptosis of human corneal endothelial cells, Exp. Ther. Med., 2018, 15(4), 3432–3438 CAS.
- F. R. Petry, et al., A Simple Method to Avoid Nonspecific Signal When Using Monoclonal Anti-Tau Antibodies in Western Blotting of Mouse Brain Proteins, Methods Mol. Biol., 2017, 263–272 CrossRef CAS PubMed.
- Z. Yang and D. J. Klionsky, Mammalian autophagy: core molecular machinery and signaling regulation, Curr. Opin. Cell Biol., 2010, 22(2), 124–131 CrossRef CAS PubMed.
- N. A. Thornberry, The caspase family of cysteine proteases, Br. Med. Bull., 1997, 53(3), 478–489 CrossRef CAS PubMed.
- N. Solvason, et al., Induction of cell cycle regulatory proteins in anti-immunoglobulin-stimulated mature B lymphocytes, J. Exp. Med., 1996, 184(2), 407–417 CrossRef CAS PubMed.
- E. Soleimani, R. H. Moghadam and A. Ranjbar, Occupational exposure to chemicals and oxidative toxic stress, J. Toxicol. Environ. Health Sci., 2015, 7(1), 1–24 CrossRef.
- D. C. Joshi and J. C. Bakowska, Determination of Mitochondrial Membrane Potential and Reactive Oxygen Species in Live Rat Cortical Neurons, J. Visualized Exp., 2011, 51(51), 2704 Search PubMed.
- J. A. Heibein, et al., Granzyme B-Induced Loss of Mitochondrial Inner Membrane Potential (ΔΨm) and Cytochrome c Release Are Caspase Independent, J. Immunol., 1999, 163(9), 4683–4693 CAS.
- Q. Kang, et al., Characterization and prognostic significance of mortalin, Bcl-2 and Bax in intrahepatic cholangiocarcinoma, Oncol. Lett., 2018, 15(2), 2161–2168 Search PubMed.
- Y. Liu, et al., Ethanol promotes apoptosis in rat ovarian granulosa cells via the Bcl-2 family dependent intrinsic apoptotic pathway, Cell. Mol. Biol., 2018, 118–125 CrossRef PubMed.
- A. Inoueyamauchi, et al., Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy, Nat. Commun., 2017, 8, 16078–16088 CrossRef CAS PubMed.
- K. P. Sarker, et al., Ebselen inhibits NO-induced apoptosis of differentiated PC12 cells via inhibition of ASK1-p38 MAPK-p53 and JNK signaling and activation of p44/42 MAPK and Bcl-2, J. Neurochem., 2010, 87(6), 1345–1353 CrossRef PubMed.
- W. Yan, et al., ASK1 is activated by arsenic trioxide in leukemic cells through accumulation of reactive oxygen species and may play a negative role in induction of apoptosis, Biochem. Biophys. Res. Commun., 2007, 355(4), 1038–1044 CrossRef CAS PubMed.
- A. Shiyong Wu and R. J. Kaufman, trans-Autophosphorylation by the Isolated Kinase Domain Is Not Sufficient for Dimerization or Activation of the dsRNA-Activated Protein Kinase PKR, Biochemistry, 2004, 43(34), 11027–11034 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |