Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microporous organic hydroxyl-functionalized polybenzotriazole for encouraging CO2 capture and separation†
Qiang Yina,
Chunlin Lua,
Shuai Zhang a,
Meifang Liu*a,
Kai Dua,
Lin Zhanga and
Guanjun Chang
a,
Meifang Liu*a,
Kai Dua,
Lin Zhanga and
Guanjun Chang *b
*b
aResearch Center of Laser Fusion, China Academy of Engineering Physics, Mianyang, 621900, P. R. China. E-mail: liumeifang@caep.cn
bState Key Laboratory of Environment-friendly Energy Materials, School of Material Science and Engineering, Southwest University of Science and Technology, Mianyang, 621010, P. R. China. E-mail: gjchang@mail.ustc.edu.cn
First published on 22nd July 2019
Abstract
We report a mild, hydroxyl functionalized and thermal stable benzotriazole-based aerogel (HO-PBTA), which is inspired by phenolic resin chemistry. Taking advantage of the synergistic adsorption interactions between hydroxy-benzotriazole and CO2, and the phobic effect between benzotriazole and nitrogen (N2), the CO2 uptake capacity of the HO-PBTA reaches an encouraging level (6.41 mmol g−1 at 1.0 bar and 273 K) with high selectivity (CO2/N2 = 76 at 273 K).
Nowadays, global warming caused by increased concentrations of carbon dioxide (CO2) in the atmosphere is one of the most serious environmental problems.1–3 The development of novel functional materials and new technologies for CO2 capture and storage has gained great attention. Microporous organic polymers (MOPs) with intrinsic properties including large specific surface area, narrow pore size distribution, good chemical stability, and low skeleton density have exhibited potential applications in gas storage and separation.4–7 In addition, microporous porous materials with excellent intrinsic properties also made significant breakthroughs in liquid separation.8,9 The structure and CO2 adsorption of the MOPs have complicated relationships. Therefore, the design of high performance CO2 capture materials often involves sophisticated molecular design and careful adjustments of the ingredient ratio. It has been shown that the incorporation of N-containing groups into the pore wall of MOPs has a profound impact on both CO2 uptake and selectivity by enhancing their physisorption interactions,10–15 however, it still remains a great challenge to make a facile synthesis of functional MOP materials that capture CO2 efficiently and selectively.
In the application of CO2 capture, the nitrogen-containing MOPs act as capable storage media due to physisorption that involves an electron donor–acceptor mechanism between a heteroatom nitrogen and CO2 on the inner surface of the networks.16–18 The first principles study indicated that nitrogen-containing heteroaromatic groups can form strong physical interactions with CO2 via “dispersive π–π stacking” and electrostatic “in-plane” mechanisms.19 This theoretical calculation guides us how to design the functional materials that capture CO2 efficiently and selectively. In previous work, we described a new strategy for CO2 capture based on the synergistic effect of electrostatic in-plane and dispersive π–π stacking interactions of two functional groups with CO2, and the proposed synergistic effect can be considered as a new rationale for the design and fabrication of CO2 capture materials.20 In addition, it has been demonstrated that azo-functionalized MOPs exhibit the N2 phobicity due to the entropic loss of N2 gas molecules upon binding, which endows the networks with the unprecedented CO2 selectivity.21 Inspired by these reported studies, we hypothesized that both the CO2 adsorption capacity and CO2/N2 selectivity can be improved greatly by involving multiple, more than two, functional groups in MOP networks where multiple mechanisms work for CO2 capture and separation. In this work, the synergistic adsorption interactions between the HO-PBTA polymer and CO2 molecules, the N2-phobic effect between the HO-PBTA polymer and N2 have been investigated in details. It is expected that the comprehensive effects of the CO2-philic and N2-phobic behaviors will provide new design principles for the development of next-generation functional porous polymers with high CO2 adsorption capacity and selectivity.
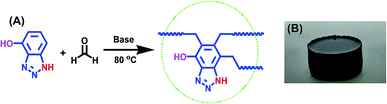
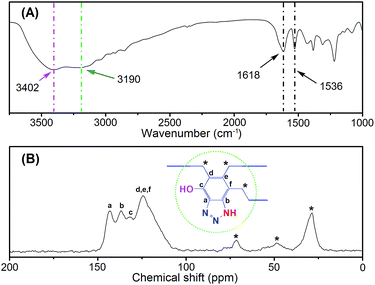
To achieve this objective, a benzotriazole-based microporous aerogel (HO-PBTA), with azo, hydroxyl and imino groups in the polymer chains, was fabricated via sol–gel technology involving phenolic resin-inspired chemistry22–24 and followed by CO2 supercritical drying (Fig. 1A). The material preparation and characterization are detailed in the ESI.† The as-prepared HO-PBTA is a dark gray, porous ultralight material, as shown in Fig. 1B. HO-PBTA aerogel was characterized by Fourier transform infrared and 13C CP/MAS NMR, and the results were in good agreement with the proposed structures (Fig. 2). The FTIR spectrum of the HO-PBTA is shown in Fig. 2a, in which the absorption peaks at about 3190 cm−1 and 3402 cm−1 correspond to the structure of NH and the OH groups. The peak at 1618 cm−1 is attributed to the vibration of the aromatic ring skeleton. And the absorption at 1536 cm−1 corresponds to the structure of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the network. As shown in Fig. 2B, the broad peaks at 150–110 ppm are ascribed to the benzotriazole group carbons, and the peaks at 75–25 ppm corresponds to methylene carbons.
N in the network. As shown in Fig. 2B, the broad peaks at 150–110 ppm are ascribed to the benzotriazole group carbons, and the peaks at 75–25 ppm corresponds to methylene carbons.
 | ||
| Fig. 2 Chemical structure characterization of the HO-PBTA polymer material. (A) FTIR spectrum, recorded as KBr pellet; (B) 13C CP/MAS NMR spectrum. | ||
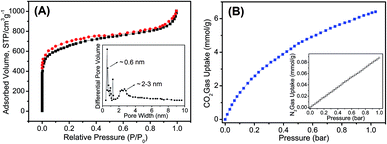
The porosity of the HO-PBTA aerogel was quantified by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and sorption analysis. A SEM image shows that the HO-PBTA aerogel consists of aggregated particles with submicrometer sizes (Fig. 3A). The TEM image (Fig. 3B) reveals the micropore structure, which is an essential requirement for CO2 capture. The porosity of aerogel was further quantified by sorption analysis using nitrogen (N2) as the sorbate molecule, and the HO-PBTA aerogel is microporous and exhibits a combination of type I and II N2 sorption isotherms according to the IUPAC classification (Fig. 4A).25 The increase in the nitrogen sorption at a high relative pressure above 0.9 may arise in part from interparticulate porosity associated with the meso- and macrostructures of the samples and interparticular void.26 According to ref. 27, the specific surface areas calculated in the relative pressure (P/P0) range from 0.01 to 0.1 shows that the Brunauer–Emmett–Teller (BET) specific surface area of HO-PBTA is up to 2160 m2 g−1 (Fig. S3†). The pore size distribution (PSD) of the network calculated from the adsorption branch of the isotherms with the nonlocal density functional theory (NLDFT) approach indicates that HO-PBTA aerogel exhibits a dominant pore diameter centered at about 0.60 nm (inset in Fig. 4A). Thermal property of HO-PBTA aerogel was evaluated via thermogravimetric analysis (TGA) in nitrogen and air conditions, and the typical TGA curves are shown in Fig. S4.† HO-PBTA exhibits great thermal stability with high decomposition temperature (Td, 5% = ∼500 °C) at N2 atmosphere. The TGA curve indicated that the HO-PBTA aerogel still exhibited good thermal stability at air condition.
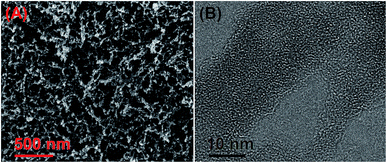 | ||
| Fig. 3 Microstructural characterization of HO-PBTA aerogel. (A) Scanning electron microscopy (SEM) image of HO-PBTA aerogel; (B) transmission electron microscopy (TEM) image of HO-PBTA aerogel. | ||
The CO2 adsorption capacity and selectivity (CO2/N2) in HO-PBTA aerogel were evaluated by adsorption isotherm measurements. As shown in Fig. 4B, the CO2 capture exhibits an increase with the increasing of the pressure. The CO2 adsorption capacity of the HO-PBTA aerogel is as high as 6.41 mmol g−1 at 1.0 bar and 273 K, while the adsorption capacity of N2 is only 0.09 mmol g−1 at the same conditions (inset in Fig. 4B). The CO2 adsorption capacity of the HO-PBTA aerogel is up to 2.3 mmol g−1 at 1 bar and 323 K, and 0.02 mmol g−1 for N2 at the same conditions (Fig. S5†). Equilibrium CO2 adsorption capacity is found to decrease with an increase in temperature due to the exothermic nature of the adsorption process, as expected for physical adsorbents. We found that the CO2 adsorption capacity of the HO-PBTA aerogel is higher than most of the CO2 capture MOP materials, and close to some of the MOF materials (Table S1†).28–30 This high affinity is a consequence of the favourable interactions of the polarizable CO2 molecules through multiple adsorption interactions with the framework, and also the inherent microporosity of HO-PBTA aerogel. Additionally, HO-PBTA aerogel also has great CO2 adsorption capacities and selectivities at 298 and 323 K (Fig. S5†), and these values are still comparable to the high surface area MOP networks.31–33
For the further CO2 adsorption, CO2 was also adsorbed preferentially over N2 at high temperatures. The selectivities were calculated using the Ideal Adsorbed Solution Theory (IAST) for CO2/N2 mixture in the ratio of 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85. At 1 bar, the IAST CO2/N2 selectivities of the HO-PBTA were 76 at 273 K, 97 at 298 K and become 110 at 323 K, which close to the highest one reported to date under the same conditions. Additionally, the HO-PBTA aerogel have the high selectivity of CO2/N2 at high temperature than other porous materials (Table S2†). It is worth noting that the CO2/N2 selectivities increased with the temperature increasing, which are essential requirements for high temperature post-combustion CO2 adsorption. N2 uptake drops ∼70% at a temperature increase from 273 to 298 K, compared with a ∼60% drop for CO2. This phenomenon is in line with the conventional CO2 affinity and the concept of N2 phobic in nitrogen-rich porous polymers where nitrogen-rich groups (–N
0.85. At 1 bar, the IAST CO2/N2 selectivities of the HO-PBTA were 76 at 273 K, 97 at 298 K and become 110 at 323 K, which close to the highest one reported to date under the same conditions. Additionally, the HO-PBTA aerogel have the high selectivity of CO2/N2 at high temperature than other porous materials (Table S2†). It is worth noting that the CO2/N2 selectivities increased with the temperature increasing, which are essential requirements for high temperature post-combustion CO2 adsorption. N2 uptake drops ∼70% at a temperature increase from 273 to 298 K, compared with a ∼60% drop for CO2. This phenomenon is in line with the conventional CO2 affinity and the concept of N2 phobic in nitrogen-rich porous polymers where nitrogen-rich groups (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) will reject N2 gas selectively.21 To further akin to capture from post-combustion gas streams, CO2 adsorption capacity of HO-PBTA aerogel at higher temperature have been characterized (Fig. S6†). The HO-PBTA shown the good CO2 adsorption capacity of 1.8 mmol g−1 at 333 K and 1.5 mmol g−1 343 K. The N2 uptakes of HO-PBTA aerogel under the same conditions were 0.016 mmol g−1 and 0.015 mmol g−1, resulting in selectivity of 112 and 101, respectively.
N–) will reject N2 gas selectively.21 To further akin to capture from post-combustion gas streams, CO2 adsorption capacity of HO-PBTA aerogel at higher temperature have been characterized (Fig. S6†). The HO-PBTA shown the good CO2 adsorption capacity of 1.8 mmol g−1 at 333 K and 1.5 mmol g−1 343 K. The N2 uptakes of HO-PBTA aerogel under the same conditions were 0.016 mmol g−1 and 0.015 mmol g−1, resulting in selectivity of 112 and 101, respectively.
The isosteric heat of adsorption (Qst) for HO-PBTA aerogel was calculated using the virial equations.34 As shown in Fig. S7,† HO-PBTA has a Qst value of 33.9 kJ mol−1, and this value can be considered as the optimum for gas adsorption and separation because of a balance between the reversibility and selectivity. The Qst values is highest among reported values for organic porous polymers and comparable to some MOFs compounds.21,35,36 The impressive Qst of 33.9 kJ mol−1 further indicates the strong interaction of HO-PBTA polymers with CO2 guest molecules. It should be noted that the higher and more optimized Qst value of the HO-PBTA aerogel can be ascribed to the synergistic effect of azo (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), hydroxyl (–OH) and imino (–NH) units arising from different interaction mechanisms.
N–), hydroxyl (–OH) and imino (–NH) units arising from different interaction mechanisms.
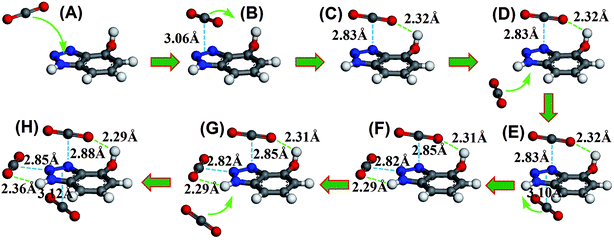
To illustrate the synergistic adsorption mechanism, we used density functional theory (DFT)19,20,37–39 to investigate the interaction of HO-PBTA with CO2 and to track the CO2 capture process. The calculation is detailed in the ESI. Fig. 5 shows a series of snapshots for CO2 capture by 4-hydroxybenzotriazole, as the model compound, where benzotriazole and hydroxyl work synergistically to adsorb multiple CO2 molecules. The minimum energy structure of the CO2–azo complex is obtained when CO2 lies on the azo at a bond distance of 3.06 Å to form the π–π stacking conformation (Fig. 5B). The electrostatic “in-plane” equilibrium conformation of CO2–HO-PBTA involves two sites: one is the electron deficient central carbon atom of CO2 to the lone pair of electrons on a nitrogen atom of azo group via dipole–quadrupole interaction; the other is lone pairs of oxygen on CO2 to a hydrogen atom on the imino group (or a hydrogen atom on the hydroxyl) via hydrogen bonding (Fig. 5C and F). Either dipole–quadrupole interaction or hydrogen bonding cannot stabilize the CO2–HO-PBTA complex because of their low binding energies.19 However, previous works and our calculation indicated that simultaneous formation of dipole–quadrupole interaction and hydrogen bonding at both sites cause a much more stable in-plane conformation of CO2–HO-PBTA complex (Fig. S8†).19,20 However, the capture of flowing CO2 by electrostatic “in-plane” interactions is difficult due to a small binding area by only two atomistic sites. On the other hand, CO2 can be rapidly adsorbed on the azo group because of its relatively large binding area (Fig. 5A). To our knowledge, the desorption occurs frequently driven by thermal fluctuation. Once CO2 desorption, the starting speed should be much slower than the bulk speed, resulting in a high probability to be captured by an adjacent hydroxyl and imino groups. The in-plane conformation of CO2–HO-PBTA complex is, therefore, formed much easily and efficiently with help of the functional azo while retaining the high selectivity of CO2 over other gas molecules (Fig. 5H). Final coordinates of DFT geometry optimization was shown in Table S3.†
Conclusions
In summary, we designed measurements to show that hydroxyl functionalized benzotriazole-based microporous aerogel (HO-PBTA) can efficiently capture CO2 with encouraging adsorption capacity and selectivity, which are essential requirements for high temperature CO2 adsorption. The key innovation of this work includes three aspects: first, taking advantage of the synergistic adsorption interactions between benzotriazole and CO2, and the phobic effect between benzotriazole and nitrogen (N2), the CO2 uptake capacity of the HO-PBTA reaches an encouraging level (6.41 mmol g−1) at 1.0 bar and 273 K, with a high selectivity CO2/N2 = 76 at 273 K and 110 at high temperature 323 K. Second, in comparison to the reported porous organic polymers, the facile synthetic strategy exhibits cost-effective advantages, making the HO-PBTA network the one of most promising microporous materials for application in CO2 capture and separation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21504073, 21202134, 11447215), the Scientific Research Fund of Sichuan Provincial Education Department (16ZA0136, 18ZA0495). We thank the Southwest Computing Center of the China Academy of Physics Engineering for their support of computer simulation.Notes and references
- K. B. Karnauskas, J. K. Lundquist and L. Zhang, Nat. Geosci., 2018, 11, 38 CrossRef CAS.
- A. Ballantyne, W. Smith, W. Anderegg, P. Kauppi, J. Sarmiento, P. Tans, E. Shevliakova, Y. Pan, B. Poulter, A. Anav, P. Friedlingstein, R. Houghton and S. Running, Nat. Clim. Change, 2017, 7, 148 CrossRef CAS.
- A. Hassanpouryouzband, J. Yang, B. Tohidi, E. Chuvilin, V. Istomin, B. Bukhanov and A. Cheremisin, Environ. Sci. Technol., 2018, 52, 4324 CrossRef CAS PubMed.
- L. Yang, C. Wang, G. Chang and X. Ren, Sens. Actuators, B, 2017, 240, 212 CrossRef CAS.
- Q. He, Y. Xu and X. Yang, RSC Adv., 2019, 9, 11851 RSC.
- J.-S. M. Lee, M. E. Briggs, T. Hasell and A. I. Cooper, Adv. Mater., 2016, 28, 9804 CrossRef CAS PubMed.
- S. Hou and B. Tan, Macromolecules, 2018, 51, 2923 CrossRef CAS.
- G. Ignacz, F. Fei and G. Szekely, ACS Appl. Nano Mater., 2018, 1, 6349–6356 CrossRef CAS.
- J. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 6349–6356 Search PubMed.
- K. Yuan, C. Liu, L. Zong, G. Yu, S. Cheng, J. Wang, Z. Weng and X. Jian, ACS Appl. Mater. Interfaces, 2017, 9, 13201 CrossRef CAS PubMed.
- H. Wang, Z. Cheng, Y. Liao, J. Li, J. Weber, A. Thomas and C. F. J. Faul, Chem. Mater., 2017, 29, 4885 CrossRef CAS.
- G. Chang, Z. Shang, T. Yu and L. Yang, J. Mater. Chem. A, 2016, 4, 2517 RSC.
- M. Oschatz and M. Antonietti, Energy Environ. Sci., 2018, 11, 57 RSC.
- K. Wang, L. Yang, W. Wei, L. Zhang and G. Chang, J. Membr. Sci., 2018, 549, 23 CrossRef CAS.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840 CrossRef PubMed.
- J. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schröder, J. Am. Chem. Soc., 2014, 136, 12828 CrossRef PubMed.
- L. Zou, Y. Sun, S. Che, X. Yang, X. Wang, M. Bosch, Q. Wang, H. Li, M. Smith, S. Yuan, Z. Perry and H.-C. Zhou, Adv. Mater., 2017, 29, 1700229 CrossRef PubMed.
- F.-F. Chen, K. Huang, Y. Zhou, Z.-Q. Tian, X. Zhu, D. Tao, D. Jiang and S. Dai, Angew. Chem., Int. Ed., 2016, 55, 7166 CrossRef CAS PubMed.
- H. M. Lee, I. S. Youn, M. Saleh, J. W. Lee and K. S. Kim, Phys. Chem. Chem. Phys., 2015, 17, 10925 RSC.
- L. Yang, G. Chang and D. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15213 CrossRef CAS PubMed.
- H. A. Patel, S. H. Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun., 2013, 4, 1357 CrossRef PubMed.
- G. Hasegawa, T. Shimizu, K. Kanamori, A. Maeno, H. Kaji and K. Nakanishi, Chem. Mater., 2017, 29, 2122 CrossRef CAS.
- F. Liu, K. Huang, Q. Wu and S. Dai, Adv. Mater., 2017, 29, 1700445 CrossRef PubMed.
- W. Wei, G. Chang, Y. Xu and L. Yang, J. Mater. Chem. A, 2018, 6, 18794 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CAS.
- J. Weber, J. Schmidt, A. Thomas and W. Böhlmann, Langmuir, 2010, 26, 15650 CrossRef CAS PubMed.
- K. S. Walton and R. Q. Snurr, J. Am. Chem. Soc., 2007, 129, 8552–8556 CrossRef CAS PubMed.
- Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630 CrossRef CAS.
- G. Feng, Y. Peng, W. Liu, F. Chang, Y. Dai and W. Huang, Inorg. Chem., 2017, 56, 2363 CrossRef CAS PubMed.
- J. W. F. To, J. He, J. Mei, R. Haghpanah, Z. Chen, T. Kurosawa, S. Chen, W.-G. Bae, L. Pan, J. B.-H. Tok, J. Wilcox and Z. Bao, J. Am. Chem. Soc., 2016, 138, 1001 CrossRef CAS PubMed.
- R. Bera, M. Ansari, S. Mondal and N. Das, Eur. Polym. J., 2018, 99, 259 CrossRef CAS.
- N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372 CrossRef CAS PubMed.
- X. Su, L. Bromberg, V. Martis, F. Simeon, A. Huq and T. A. Hatton, ACS Appl. Mater. Interfaces, 2017, 9, 11299 CrossRef CAS PubMed.
- J. A. Dunne, R. Mariwala, M. Rao, S. Sircar, R. J. Gorte and A. L. Myers, Langmuir, 1996, 12, 5888 CrossRef CAS.
- R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2001, 2, 1173 RSC.
- G. Chang, L. Yang, J. Yang, Y. Huang, K. Cao, J. Ma and D. Wang, Polym. Chem., 2016, 7, 5768 RSC.
- G. Chang, L. Yang, J. Yang, M. P. Stoykovich, X. Deng, J. Cui and D. Wang, Adv. Mater., 2018, 30, 1704234 CrossRef PubMed.
- L. Mino, G. Spoto and A. M. Ferrari, J. Phys. Chem. C, 2014, 118, 25016 CrossRef CAS.
- W. Wang, L. Fan, G. Wang and Y. Li, Appl. Surf. Sci., 2017, 425, 972 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis and characterizations of 4-OH-BTA and the HO-PBTA polymer; main materials and measurements; BET; thermal stability; isosteric heat, simulation method. See DOI: 10.1039/c9ra03741a |
| This journal is © The Royal Society of Chemistry 2019 |