Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Expeditious synthesis and preliminary antimicrobial activity of deflazacort and its precursors†
Anna Esposito a,
Eliana De Gregorio
a,
Eliana De Gregorio b,
Maria De Fenza
b,
Maria De Fenza a,
Daniele D'Alonzo
a,
Daniele D'Alonzo a,
Anil Satawanic and
Annalisa Guaragna
a,
Anil Satawanic and
Annalisa Guaragna *a
*a
aDepartment of Chemical Sciences, University of Napoli Federico II, Via Cintia, 80126 Napoli, Italy. E-mail: annalisa.guaragna@unina.it
bDepartment of Molecular Medicine and Medical Biotechnologies, Via S. Pansini, 5, 80131 Napoli, Italy
cSymbiotec Pharmalab Pvt Ltd, Pithampur, Indore, India
First published on 10th July 2019
Abstract
The synthesis of deflazacort (DFZ) and a preliminary evaluation of its microbial activity against the human pathogens Acinetobacter baumannii and Staphylococcus aureus is herein reported. While DFZ is inactive, one of its synthetic precursors showed a strong antibacterial activity against both Gram-negative and -positive bacteria.
Introduction
Mineralocorticoids (MCs) and glucocorticoids (GCs) belong to the group of corticosteroids (hormones with 21 carbon atoms) and are basically classified based on their preferential biological activity, depending on their involvement in the balancing of fluids and electrolytes or in the regulation of carbohydrate metabolism, respectively.1The immunosuppressive and anti-inflammatory properties of GCs, mainly due to inhibition of specific leucocyte functions, are well-established.2 The first efforts in this field led to the synthesis of novel corticosteroids such as prednisolone (1, Fig. 1) and prednisone (2, Fig. 1), which were endowed with a higher anti-inflammatory potency than natural cortisone, along with lower undesired effects. Inspired by these results, a variety of other corticosteroids with different therapeutic applications were synthetized, including fluorinated GCs betamethasone (3) and dexamethasone (4).
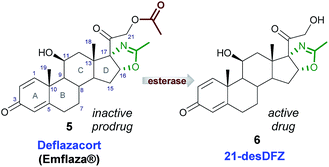
The search of novel anti-inflammatory drugs characterized by high efficacy and good tolerability in each age group, led to identification of deflazacort (5), a heterocyclic corticosteroid, acting as a new anti-inflammatory/immunosuppressive agent. Indeed, 5 is endowed with a lower interference with carbohydrate and phosphocalcium metabolism compared with previous-generation corticosteroids and it is therefore able to produce less serious metabolic events.3,4 DFZ, the oxazoline-derivative prodrug of prednisolone,5 after oral administration is rapidly converted into the active metabolite, 21-desacetyldeflazacort (6, Fig. 2), by cellular esterases.6
To exert its pharmacological effect, DFZ acts as corticosteroid hormone receptor agonist, exploiting the high binding affinity for tissue glucocorticoid receptors.7 Very recently, DFZ was approved8 by the FDA (trade name: Emflaza) for its use in treatment of Duchenne Muscular Dystrophy (DMD), a genetic disorder that affects 1/3600 infants worldwide. However, the precise mechanism by which DFZ exerts its therapeutic effect in patients with DMD is still unknown.
Even though the anti-inflammatory and immunosuppressive activity4 of DFZ is well-known, there are no reports to the best of our knowledge about its direct action on bacterial infections. In this context, our ongoing interest into the synthesis of bioactive compounds9 prompted us to develop a new synthetic protocol for the preparation of DFZ. In addition, the evaluation of the antimicrobial potential of DFZ as well as of its steroidal synthetic precursors against Acinetobacter baumannii and Staphylococcus aureus was assessed by in vitro assays.
Results and discussion
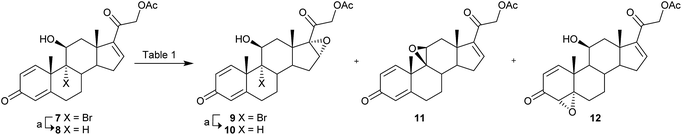
Our synthesis started from 9-bromotriene acetate 7 (Scheme 1), readily obtained from the commercially available tetraene acetate10 (3TR), an industrial key intermediate already used for the synthesis of currently marketed bioactive glucocorticoid drugs.11The use of 7 allowed us to have the proper functionalization at C21 position, as required by the DFZ structure, avoiding the installation of the acetylated 21-hydroxyl function in a late stage of the synthesis, as commonly reported for oxazoline-containing steroids.12
At first, double bond epoxidation at C16–C17 positions of the starting material was studied, to be then further elaborated for conversion into the oxazoline moiety of 5. The epoxidation reaction was attempted on both 7 and its debrominated derivative 8, in turn obtained by treatment of 7 with Bu3SnH and AIBN in refluxing THF (93–98% yield) (Table 1).
| Entry | Compd | Reacting system | Solvent | Temp [°C] | t [h] | Yield [%] |
|---|---|---|---|---|---|---|
| a Bu3SnH, AIBN, THF, reflux, 30′, 93–98%. | ||||||
| 1 | 7 | MCPBA | DCM | Δ | 16 | 9: 47 |
| 2 | 7 | Oxone/NaHCO3/aq. Na2EDTA | CF3COCH3/ACN | rt | 18 | 11: 51 |
| 3 | 7 | H2O2/NH4HCO3 | ACN/H2O | rt | 16 | 11: 59 |
| 4 | 8 | MCPBA | DCM | Δ | 16 | 10: 40 |
| 5 | 8 | Oxone/NaHCO3/aq. Na2EDTA | CF3COCH3/ACN | rt | 18 | 12: 55 |
| 6 | 8 | H2O2/NH4HCO3 | ACN/H2O | rt | 18 | — |
Nearly neutral or acidic conditions were tested, since alkaline double bond epoxidation was hampered by the presence of the acetyl group at C21 position. The best results were obtained by treatment of 7 or 8 with mCPBA in refluxing DCM (entries 1 and 4, Table 1), obtaining in both cases the desired α-epoxides 9 and 10 in 47% and 40% yield. On the contrary, the use of in situ generated TFDO (Oxone/NaHCO3/aq. Na2EDTA) (entries 2 and 5, Table 1) demonstrated to be unsuited, as it led to the formation of undesired epoxides 11 and 12 (from 7 and 8, respectively). The use of bicarbonate-catalyzed epoxidation with aqueous hydrogen peroxide,13 at near neutral pH, afforded only a minor amount of 9 (13%, from 7), while epoxide 11 was isolated as the main product (59%) (entry 3, Table 1). Under the same conditions, the reaction from olefin 8 led to the recovery of totally unreacted starting material (entry 6, Table 1).
With epoxide 10 in hand, conversion of the latter into cis-17-amino-16-hydroxy derivative 16 was accomplished either by a stepwise route (path a, Scheme 2) or by a one-pot procedure (path b, Scheme 2), exploiting the formation of the corresponding carboethoxy hydrazone 13. Indeed, hydrazone groups-containing oxidosteroids are reported to be able to efficiently drive the regio- and stereoselective ring opening of neighbouring three-membered heterocycles.14 Thus, hydrazone 13 could represent a valuable intermediate to selectively introduce a vicinal amino-alcohol function in C16 and C17 positions with the desired cis-configuration.
As shown in Table 2, different reaction conditions were explored, treating 10 with ethyl carbazate, varying both the reaction solvent and the activating agent. In almost all cases, unsatisfactory results were obtained (entries 1–3), since only limited conversions were achieved, while the recovery of unreacted staring material was mainly found. On the other hand, when the reaction was carried out with Py·HCl in pyridine (entry 4), the protection of both C20 and C3 carbonyl functions occurred, affording hydrazone 14 in 30% yield. The desired C20 monoprotected derivative 13 was instead easily obtained in good yield (59%), using ethyl carbazate and p-toluenesulfonic acid in anhydrous 1,4 dioxane (entry 5).
The epoxide ring opening from hydrazone 13 was then considered. Treatment of 13 with NH3 in dioxane afforded the desired cis-17-amino-16-hydroxy derivative 16 in 97% yield (Scheme 2, path a). Differently from the well-known acid-catalysed epoxide ring opening carried out on similar steroid derivatives, the stereochemical outcome of the reaction under basic conditions can be rationalized assuming that NH3 deprotonates hydrazone 13, enabling epoxide ring opening to give the intermediate 15 (PM3; HyperChem™, rel 8.0.3). The latter then undergoes nucleophilic attack by ammonia from the less hindered side of ring D, leading to amino-alcohol 16. As previously mentioned, C20 protection and epoxide ring opening steps were also performed in a one-pot procedure (Scheme 2, path b) leading to the same results in terms of reaction times and yields.
Oxazoline ring formation from 16 was eventually studied, using the well-known polymer supported triphenylphosphine/iodine/imidazole15 (PS-DPP/I2/ImH) system as the activating agent.16 Treatment of AcOH with 2.0 equivalents of the previously mixed PS-DPP/I2 followed by the addition of 16 and ImH to the reaction mixture provided, already after 2 h in refluxing DCM, the desired 17 in high yield (96%) and purity. The first equivalent of PS-TPP/I2 is needed to activate acetic acid, whereas a second equivalent of the complex promotes the hydroxyl group activation. The addition of ImH to the reaction medium ensures neutral/mild alkaline environment by trapping protons released during the course of the reaction and the amide proton abstraction, thus promoting oxazoline ring closure. It's worth noting that the only reaction by-product, i.e. the resin-bound phosphine oxide, can be easily filtered off and eventually recycled, providing the original phosphine by reduction with trichlorosilane.17
Lastly, removal of C20 hydrazone function (HCl/acetone) on 17 smoothly provided the pure Deflazacort (5) in 72% yield.
DFZ and its synthetic precursors were evaluated in experiments aimed to determine the minimum inhibitory concentration (MIC) against Staphylococcus aureus and Acinetobacter baumannii,18 two bacterial pathogens that have become one of major causes of life-threatening infections. A. baumannii and S. aureus were classified as critical and high priority pathogens, respectively, as they rapidly developed multi-resistance to antibiotics.19 Compounds 5 and 16 were inactive as growth inhibitors even at the high experimental concentrations (1 mg mL−1), whereas compounds 13 and 17 showed only weak antimicrobial activity (Table 3). On the contrary, epoxide 10 showed inhibition at concentrations as low as 16 μg mL−1 for both S. aureus and A. baumannii strains. Interestingly, compounds 10 and 13 also work as bactericidal agents, having minimum bactericidal concentration (MBC) values generally equal or three-fold higher than their MIC values (Table 3).
| Compound | Bacteria | |||
|---|---|---|---|---|
| S. aureus ATCC 29213 | A. baumannii ATCC 17978 | |||
| MIC | MBC | MIC | MBC | |
| a MIC experiments were carried out in triplicates and was determined by broth microdilution assay as previously describes;20 ND: not determined. | ||||
| 10 | 0.016 | 0.016 | 0.016 | 0.016 |
| 13 | 0.375 | 1 | >1 | >1 |
| 16 | >1 | >1 | >1 | >1 |
| 17 | 0.75 | >1 | >1 | >1 |
| DFZ | >1 | >1 | >1 | >1 |
| Vancomycin | 0.001 | ND | ND | ND |
| Methicillin | 0.0025 | ND | ND | ND |
| Gentamicin | 0.0005 | ND | 0.0015 | ND |
| Ampicillin | ND | ND | >0.032 | ND |
| Amikacin | ND | ND | 0.004 | ND |
Cytotoxicity of 10 was assessed in vitro using hemolytic assay measuring its hemolytic activity against horse RBCs at final concentrations ranging from 1 to 512 μg mL−1. The glucocorticoid epoxide 10 showed no hemolytic activity at concentrations of up to 128 μg mL−1, whereas was 62.8% and 78.6% at 256 μg mL−1 and 512 μg mL−1, respectively.21
Conclusions
In summary, we tuned up a novel and efficient synthesis of deflazacort in few steps from 9-bromotriene acetate 7 by regio- and stereoselective functionalization of C16 and C17 positions. In search for antibacterial activity of DFZ and its synthetic precursors, we found that epoxide 10 displayed interesting biological properties. Further studies will be aimed to an in-depth evaluation of the antimicrobial properties of 10, as well as the determination of the mechanism of action.Experimental
Chemical synthesis
1,4-Pregnadiene-9-bromo-11-hydroxy-16α,17α-epoxy-3,20-dione (9). To a stirred solution of 9-bromotriene acetate 7 (1.0 g, 2.16 mmol) in anhydrous CH2Cl2 (30 mL), m-CPBA (0.75 g, 4.32 mmol) was added at room temperature. The mixture was warmed to reflux and stirred for 16 h. Then, aq. NaHCO3 was added and the mixture extracted with CH2Cl2; the organic layer was dried (Na2SO4) and the solvent evaporated under reduced pressure. The solid residue was recrystallized from AcOEt to give the final compound 9 (0.49 g, 47% yield) as a white solid. 1H NMR (400 MHz): δ 1.41 (s, 3H), 1.48–1.55 (m, 1H), 1.70 (s, 3H), 1.71–1.82 (m, 2H), 1.94–2.05 (m, 3H), 2.15 (s, 3H), 2.16–2.25 (m, 1H), 2.36–2.49 (m, 2H), 2.55–2.68 (m, 1H), 3.85 (s, 1H), 4.59 (d, J = 13.4, 1H), 4.67 (d, J = 13.4, 1H), 4.76 (bs, 1H), 6.07 (bs, 1H), 6.32 (d, J = 10.1, 1H), 7.21 (d, J = 10.1, 1H). 13C NMR (100 MHz): 18.3, 20.4, 24.9, 27.0, 28.3, 30.4, 33.5, 37.3, 39.3, 42.1, 50.2, 61.1, 65.7, 70.5, 75.9, 85.3, 125.1, 129.3, 152.3, 165.5, 170.4, 186.2, 198.9. Anal. calcd for C23H27BrO6: C, 57.63; H, 5.68; Br, 16.67. Found: C, 57.75; H, 5.66; Br, 16.62.
1,4-Pregnadiene-11-hydroxy-16α,17α-epoxy-3,20-dione (10). To a boiling and stirring suspension of epoxide 9 (0.50 g, 1.04 mmol) in anhydrous THF (13.0 mL), a solution of Bu3SnH (0.36 mL, 1.25 mmol) and AIBN (catalytic amount, 33.3 mg, 0.21 mmol) in THF (10 mL) was added dropwise under nitrogen atmosphere. The reaction mixture was stirred for 30 min at reflux temperature. The crude residue was then concentrated under reduced pressure and recrystallized from AcOEt to give 10 (0.41 g, 98% yield). Epoxide 10 (40% yield) was also obtained from compound 8 by epoxidation reaction using the same procedure reported for 9. Data for 10: white solid, 1H NMR (400 MHz): δ 1.02–1.14 (m, 2H), 1.39 (s, 3H), 1.46 (s, 3H), 1.46–1.51 (m, 2H), 1.62–1.70 (m, 1H), 1.97–2.02 (m, 3H), 2.15 (s, 3H), 2.15–2.19 (m, 1H), 2.29–2.38 (m, 1H), 2.51–2.62 (m, 1H), 3.82 (s, 1H), 4.42 (bs, 1H), 4.57 (d, J = 13.4, 1H), 4.66 (d, J = 13.4, 1H), 6.00 (bs, 1H), 6.26 (dd, J = 1.8, 10.1, 1H), 7.21 (d, J = 10.1, 1H). 13C NMR (100 MHz): 17.4, 20.5, 21.3, 27.8, 28.3, 29.5, 31.9, 33.4, 41.0, 42.0, 44.2, 45.1, 56.2, 61.4, 65.8, 69.9, 122.6, 128.0, 156.1, 169.6, 170.4, 186.6, 190.0. Anal. calcd for C23H28O6: C, 68.98; H, 7.05. Found: C, 69.05; H, 7.03.
1,4,17-Pregnatriene-11-hydroxy-3,20-dione (8). Compound 8 was obtained in 93% yield from 7 by using the same conditions reported above for preparation of 10. Data for 8: white solid, 1H NMR (500 MHz): δ 1.06–1.21 (m, 1H), 1.26 (s, 3H), 1.32–1.38 (m, 1H), 1.49 (s, 3H), 1.59–1.63 (m, 2H), 2.06–2.12 (m, 1H), 2.17 (s, 3H), 2.18–2.30 (m, 2H), 2.33–2.43 (m, 2H), 2.45–2.51 (m, 1H), 2.55–2.67 (m, 1H), 4.40 (bs, 1H), 4.85 (d, J = 16.1, 1H), 5.01 (d, J = 16.1, 1H), 6.01 (bs, 1H), 6.27 (d, J = 10.1, 1H), 6.73 (bs, 1H), 7.31 (d, J = 10.1, 1H). 13C NMR (100 MHz): 18.4, 20.5, 21.2, 30.1, 31.8, 32.8, 33.6, 44.2, 44.6, 46.1, 56.1, 56.4, 65.5, 70.2, 122.5, 128.0, 143.5, 152.2, 156.1, 169.4, 170.2, 186.6, 190.5. Anal. calcd for C23H28O5: C, 71.85; H, 7.34. Found: C, 71.80; H, 7.35.
20-Carboethoxyhydrazone of 1,4-pregnadiene-11-hydroxy-16α,17α-epoxy-3-one (13). To a stirring suspension of 10 (0.50 g, 1.25 mmol) in anhydrous 1,4-dioxane (24 mL), ethyl carbazate (0.26 g, 2.5 mmol) and p-toluenesulfonic acid (0.24 g, 1.25 mmol) were sequentially added at room temperature under argon atmosphere. The resulting mixture was stirred at the same temperature for 8 h. Then aq. NaHCO3 was added and the mixture extracted with EtOAc; the organic layer was dried (Na2SO4) and the solvent evaporated under reduced pressure. The solid residue was recrystallized from Et2O to give the final compound 13 (0.36 g, 59% yield) as a white solid. 1H NMR (500 MHz): δ 1.01–1.12 (m, 2H), 1.15–1.27 (m, 2H), 1.31 (s, 3H), 1.33 (t, J = 7.1, 3H), 1.40–1.44 (m, 1H), 1.47 (s, 3H), 1.68 (dd, J = 3.1, 14.2, 1H), 1.96–2.04 (m, 2H), 2.13 (s, 3H), 2.15–2.18 (m, 1H), 2.32 (dd, J = 3.0, 12.7, 1H), 2.50–2.61 (m, 2H), 3.67 (s, 1H), 4.22–4.29 (m, 2H), 4.31 (d, J = 13.1, 1H), 4.40 (bs, 1H), 4.56 (d, J = 13.1, 1H), 6.00 (bs, 1H), 6.25 (d, J = 10.1, 1H), 7.29 (d, J = 10.1, 1H). 13C NMR (125 MHz): 14.6, 18.2, 20.6, 21.2, 27.2, 29.9, 31.9, 33.5, 41.4, 41.7, 44.2, 45.9, 55.0, 56.2, 59.6, 62.1, 69.8, 70.2, 122.5, 127.8, 142.5, 153.6, 156.2, 169.7, 171.2, 186.6. Anal. calcd for C26H34N2O7: C, 64.18; H, 7.04; N 5.76. Found: C, 64.29; H, 7.02; N 5.75.
20-Carboethoxyhydrazone of 1,4-pregnadiene-16α-amino-11,17α-diol-3-one (16). Method A. Epoxide 13 (0.30 g, 0.62 mmol) was dissolved in anhydrous 1,4-dioxane (15 mL) under nitrogen atmosphere at rt. Anhydrous ammonia was then gently bubbled into the solution for 3 minutes and the reaction was stirred at the same temperature for 18 h. Nitrogen was bubbled in the reaction mixture until the ammonia was eliminated from the solution (pH = 7). The mixture was diluted with CH2Cl2 and the organic layers were washed with brine, dried (Na2SO4) and concentrated under reduced pressure. The solid residue was recrystallized from Et2O to give the final compound 16 (0.30 g, 97% yield) as a white solid. Method B (one-pot procedure). To a stirred suspension of 10 (0.50 g, 1.25 mmol) in anhydrous 1,4-dioxane (24 mL), ethyl carbazate (0.26 g, 2.5 mmol) and p-toluenesulfonic acid (0.24 g, 1.25 mmol) were sequentially added at room temperature under argon atmosphere. The resulting mixture was stirred at the same temperature for 8 h and then anhydrous ammonia was gently bubbled into the solution for 3 minutes. The reaction mixture was stirred at room temperature for 18 h. The mixture was diluted with CH2Cl2 and the organic layers washed with brine, dried (Na2SO4) and concentrated under reduced pressure. The solid residue was recrystallized from Et2O to give the final compound 16 (0.43 g, 69% overall yield). 1H NMR (400 MHz, acetone-d6): δ 1.04–1.16 (m, 5H), 1.21–1.41 (m, 4H), 1.51 (s, 3H), 1.52–1.57 (m, 1H), 1.78–1.99 (m, 4H), 2.03–2.17 (m, 7H), 2.35 (dd, J = 3.4, 13.4, 1H), 2.62 (td, J = 4.9, 13.4, 1H), 3.81 (bs, 1H), 4.17 (q, J = 7.1, 2H), 4.47 (bs, 1H), 4.76 (d, J = 13.0, 1H), 4.90 (d, J = 13.0, 1H), 5.16 (dd, J = 3.0, 9.2, 1H), 5.92 (bs, 1H), 6.14 (dd, J = 1.6, 10.1, 1H), 7.33 (d, J = 10.1, 1H). 13C NMR (100 MHz, acetone-d6): 14.0, 18.2, 19.9, 20.8, 29.7, 30.9, 31.6, 33.9, 40.9, 44.0, 46.4, 48.9, 55.6, 58.2, 60.8, 69.4, 81.2, 83.7, 121.8, 127.3, 149.4, 153.6, 155.9, 170.1, 170.3, 185.1. Anal. calcd for C26H37N3O7: C, 62.01; H, 7.41; N 8.34. Found: C, 62.08; H, 7.38; N 8.36.
20-Carboethoxyhydrazone of 1,4-pregnadiene-11-hydroxy- 16α,17α-oxazole-3-one (17). I2 (0.30 g, 1.19 mmol) was added to a stirred solution of polymer supported triphenylphosphine (PS-TPP; 100–200 mesh, extent of labeling: ∼3 mmol g−1 triphenylphosphine loading) (0.40 g, 1.19 mmol) in anhydrous DCM (20 mL) at rt. Then glacial acetic acid (35 μL, 0.59 mmol) was added and the solution was stirred at room temperature for 20′. Afterwards, 16 (0.30 g, 0.59 mmol) and imidazole (0.16 g, 2.38 mmol) were sequentially added and the solution was warmed to 40 °C and stirred for 2 h. The mixture was then filtered and the solvent removed under reduced pressure. The solid residue was recrystallized from Et2O to give the final compound 17 (0.29 g, 96% yield) as a white solid. 1H NMR (500 MHz, acetone-d6): δ 1.04 (dd, J = 3.7, 11.4, 1H), 1.07 (s, 1H), 1.09–1.18 (m, 1H), 1.27 (t, J = 7.1, 1H), 1.29–1.36 (m, 3H), 1.51 (s, 3H), 1.74 (dd, J = 5.7, 13.6, 1H), 1.81 (dd, J = 7.6, 13.6, 1H), 1.85–1.90 (m, 4H), 2.12 (s, 3H), 2.18–2.25 (m, 1H), 2.35 (dd, J = 3.4, 13.5, 1H), 2.65 (td, J = 5.7, 13.5, 1H), 3.83 (bs, 1H), 4.17 (q, J = 7.1, 2H), 4.47 (bs, 1H), 4.73 (d, J = 13.0, 1H), 4.92 (d, J = 13.0, 1H), 5.66 (d, J = 5.7, 1H), 5.92 (bs, 1H), 6.14 (dd, J = 1.8, 10.1, 1H), 7.31 (d, J = 10.1, 1H), 9.37 (bs, 1H). 13C NMR (125 MHz, acetone-d6): 13.2, 14.0, 19.1, 19.9, 20.8, 29.7, 30.6, 31.5, 33.9, 41.7, 43.9, 47.0, 50.4, 55.5, 57.5, 60.9, 69.1, 83.7, 91.6, 121.9, 127.4, 145.9, 153.4, 155.7, 165.1, 169.5, 170.2, 185.0. Anal. calcd for C28H37N3O7: C, 63.74; H, 7.07; N 7.96. Found: C, 63.81; H, 7.02; N 7.99.
Deflazacort (5). To a stirred solution of 17 (0.30 g, 0.57 mmol) in acetone (8 mL), HCl 37% solution (0.09 mL, 1.14 mmol) was added and stirring at room temperature for 24 h. Then aq. NaHCO3 was added and the mixture extracted with CH2Cl2; the combined organic layers were washed with brine, dried (Na2SO4) and the solvent evaporated under reduced pressure. Chromatography of the crude residue over silica gel (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone = 7
acetone = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gave the pure DFZ (5) (0.18 g, 72% yield) as a white solid. 1H NMR (400 MHz, CD3OD): δ 1.01 (dd, J = 3.6, 11.3, 1H), 1.02 (s, 3H), 1.06–1.25 (m, 2H), 1.49 (s, 3H), 1.75 (dd, J = 5.9, 13.8, 1H), 1.79–1.85 (m, 1H), 1.89 (dd, J = 3.9, 14.2, 1H), 1.97 (s, 3H), 2.01 (dd, J = 2.7, 13.8, 1H), 2.06–2.12 (m, 1H), 2.13 (s, 3H), 2.20 (dd, J = 4.1, 11.6, 1H), 2.37 (ddd, J = 1.8, 4.6, 13.4, 1H), 2.65 (td, J = 5.7, 13.4, 1H), 4.41 (dd, J = 3.2, 6.3, 1H), 4.93 (s, 2H), 5.30 (d, J = 5.5, 1H), 6.00 (bs, 1H), 6.25 (dd, J = 1.9, 10.1, 1H), 7.45 (d, J = 10.1, 1H). 13C NMR (125 MHz, CD3OD): 12.5, 17.2, 18.9, 20.1, 30.4, 31.6, 33.9, 34.0, 40.9, 44.5, 50.4, 55.5, 67.0, 69.0, 84.8, 94.2, 121.2, 126.5, 158.3, 168.0, 170.6, 172.8, 187.5, 200.9. Anal. calcd for C25H31NO6: C, 68.01; H, 7.08; N 3.17. Found: C, 68.09; H, 7.06; N 3.15.
3) gave the pure DFZ (5) (0.18 g, 72% yield) as a white solid. 1H NMR (400 MHz, CD3OD): δ 1.01 (dd, J = 3.6, 11.3, 1H), 1.02 (s, 3H), 1.06–1.25 (m, 2H), 1.49 (s, 3H), 1.75 (dd, J = 5.9, 13.8, 1H), 1.79–1.85 (m, 1H), 1.89 (dd, J = 3.9, 14.2, 1H), 1.97 (s, 3H), 2.01 (dd, J = 2.7, 13.8, 1H), 2.06–2.12 (m, 1H), 2.13 (s, 3H), 2.20 (dd, J = 4.1, 11.6, 1H), 2.37 (ddd, J = 1.8, 4.6, 13.4, 1H), 2.65 (td, J = 5.7, 13.4, 1H), 4.41 (dd, J = 3.2, 6.3, 1H), 4.93 (s, 2H), 5.30 (d, J = 5.5, 1H), 6.00 (bs, 1H), 6.25 (dd, J = 1.9, 10.1, 1H), 7.45 (d, J = 10.1, 1H). 13C NMR (125 MHz, CD3OD): 12.5, 17.2, 18.9, 20.1, 30.4, 31.6, 33.9, 34.0, 40.9, 44.5, 50.4, 55.5, 67.0, 69.0, 84.8, 94.2, 121.2, 126.5, 158.3, 168.0, 170.6, 172.8, 187.5, 200.9. Anal. calcd for C25H31NO6: C, 68.01; H, 7.08; N 3.17. Found: C, 68.09; H, 7.06; N 3.15.
Biology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) after 24 h of incubation as compared to the colony count of the starting inoculum. All tests were performed in triplicate and repeated three times.
10) after 24 h of incubation as compared to the colony count of the starting inoculum. All tests were performed in triplicate and repeated three times.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AG and AE wish to thank Dr Mario Micciarelli and Prof. Giovanni Palumbo for valuable suggestions and helpful discussions regarding various aspects of this project.Notes and references
- S. Samuel, T. Nguyen and H. A. Choi, J. Neurocrit. Care, 2017, 10, 53 CrossRef.
- S. Gessi, S. Merighi and P. A. Borea, Curr. Pharm. Des., 2010, 16, 3540 CrossRef CAS.
- G. Nathansohn, G. Winters and E. Testa, J. Med. Chem., 1967, 10, 799 CrossRef CAS.
- A. Markham and H. M. Bryson, Drugs, 1995, 50, 317 CrossRef CAS.
- L. Parente, BMC Pharmacol. Toxicol., 2017, 18, 1 CrossRef.
- A. Assandri, G. Buniva, E. Martinelli, A. Perazzi and L. Zerilli, Adv. Exp. Med. Biol., 1984, 171, 9 CAS.
- H. Coirini, D. Flores, M. C. Vega, M. C. Gonzalez Deniselle and A. F. De Nicola, J. Steroid Biochem. Mol. Biol., 1994, 49, 43 CrossRef CAS.
- (a) K. Traynor, Am. J. Health Syst. Pharm., 2017, 74, 368 CrossRef; (b) W. D. Biggar, L. Politano, V. A. Harris, L. Passamano, J. Vajsar, B. Alman, A. Palladino, L. I. Comi and G. Nigro, Neuromuscul. Disord., 2004, 14, 476 CrossRef CAS.
- (a) D. D'Alonzo, M. De Fenza, C. Porto, R. Iacono, M. Huebecker, B. Cobucci-Ponzano, D. A. Priestman, F. Platt, G. Parenti, M. Moracci, G. Palumbo and A. Guaragna, J. Med. Chem., 2017, 60, 946 Search PubMed; (b) R. Caputo, U. Ciriello, P. Festa, A. Guaragna, G. Palumbo and S. Pedatella, Eur. J. Org. Chem., 2003, 14, 2617 CrossRef; (c) M. F. Caso, D. D'Alonzo, S. D'Errico, G. Palumbo and A. Guaragna, Org. Lett., 2015, 17, 2626 CrossRef CAS; (d) R. Caputo, A. Guaragna and G. Palumbo, Eur. J. Org. Chem., 1999, 6, 1455 CrossRef.
- See for example: P. Jia, X. Wu, Y. Yu and G. Zhang, Steroids, 2009, 74, 229 CrossRef CAS.
- R. Jouve, V. Thery, S. Ducki, J. Helfenbein, J.-C. Thiery, A. Job, El. Picard, C. Mallet, I. Ripoche and K. Bennis, Steroids, 2018, 137, 14 CrossRef CAS.
- See for example: X. Runxing, CN Pat., 108484714A, 2018; D. Lianqiang, and L. Mingqing, CN Pat., 106397532A, 2017.
- H. Yao and D. E. Richardson, J. Am. Chem. Soc., 2000, 122, 3220 CrossRef CAS.
- A. V. Kamernitskii and A. M. Turuta, Pharm. Chem. J., 1992, 26, 526 CrossRef.
- D. H. Drewry, D. M. Coe and S. Poon, Med. Res. Rev., 1999, 19, 97 CrossRef CAS.
- (a) M. De Fenza, D. D'Alonzo, A. Esposito, S. Munari, N. Loberto, A. Santangelo, I. Lampronti, A. Tamanini, A. Rossi, S. Ranucci, I. De Fino, A. Bragonzi, M. Aureli, M. Tironi, G. Lippi, R. Gambari, G. Cabrini, G. Palumbo, M. C. Dechecchi and A. Guaragna, Eur. J. Med. Chem., 2019, 175, 63 CrossRef CAS; (b) R. Caputo, G. Cecere, A. Guaragna, G. Palumbo and S. Pedatella, Eur. J. Org. Chem., 2002, 17, 3050 CrossRef; (c) S. Pedatella, A. Guaragna, D. D'Alonzo, M. De Nisco and G. Palumbo, Synthesis, 2006, 2, 305 Search PubMed.
- S. L. Regen and D. P. Lee, J. Org. Chem., 1975, 40, 1669 CrossRef CAS.
- E. De Gregorio, E. Roscetto, V. D. Iula, M. Martinucci, R. Zarrilli, P. P. Di Nocera and M. R. Catania, New Microbiol., 2015, 38, 251 CAS.
- E. Tacconelli, E. Carrara, A. Savoldi, S. Harbarth, M. Mendelson, D. L. Monnet, C. Pulcini, G. Kahlmeter, J. Kluytmans, Y. Carmeli, M. Ouellette, K. Outterson, J. Patel, M. Cavaleri, E. M. Cox, C. R. Houchens, M. L. Grayson, P. Hansen, N. Singh, U. Theuretzbacher and N. Magrini, Lancet Infect. Dis., 2018, 18, 318 CrossRef.
- K. Pane, V. Cafaro, A. Avitabile, M. T. Torres, A. Vollaro, E. De Gregorio, M. R. Catania, A. Di Maro, A. Bosso, G. Gallo, A. Zanfardino, M. Varcamonti, E. Pizzo, A. Di Donato, T. K. Lu, C. de la Fuente-Nunez and E. Notomista, ACS Synth. Biol., 2018, 7, 2105 CrossRef CAS.
- A. Ebbensgaard, H. Mordhorst, M. T. Overgaard, F. M. Aarestrup and E. B. Hansen, PLoS One, 2018, 13(5), e0197742 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of 1H, 13C and 1H–1H–COSY NMR and LC-MS spectra of all compounds and biological details. See DOI: 10.1039/c9ra03673c |
| This journal is © The Royal Society of Chemistry 2019 |