 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protective effect of ginger (Zingiber officinale) against PCB-induced acute hepatotoxicity in male rats
Khedher Ahda,
Sabah Dhibi *ab,
Sarra Akermiab,
Hafsia Bouzennaab,
Noura Samoutab,
Abdelfattah Elfekib and
Najla Hfaiedhab
*ab,
Sarra Akermiab,
Hafsia Bouzennaab,
Noura Samoutab,
Abdelfattah Elfekib and
Najla Hfaiedhab
aUnity of Macromolecular Biochemistry and Genetics Faculty of Sciences, Sidi Ahmed Zarrouk, 2112, Gafsa, Tunisia. E-mail: sabahdhibi7@gmail.com
bLaboratory of Environmental Physiopathology, Valorization of Bioactive Molecules and Mathematical Modeling, Faculty of Sciences of Sfax, Road Soukra km 3.5, PB no 1171-3000, Sfax, Tunisia
First published on 17th September 2019
Abstract
After absorption by the organism, polychlorinated biphenyls (PCBs) cross cellular membranes and pass into blood vessels and the lymphatic system. It is generally in the liver, adipose tissues, brain and skin that we find the strongest concentrations of PCBs. Herbal medicine remains as a discipline intended to treat and to prevent certain functional disorders and/or pathologies caused by oxidative stress, which can be induced by pesticides, medicines or pollutants. The objective of this study is to verify the toxic and oxidative effects of PCBs and to investigate the protective effect of ginger (Zingiber officinale) in the liver of male rats of the “Wistar” strain. These rats are divided into 6 groups: a control group (T), two groups treated with PCB at two different concentrations (P1 and P2), a group treated with ginger extract (G), a group pretreated with ginger extract and then injected with the first concentration of PCBs (P1G), and a group pretreated with ginger and then injected with the second concentration of PCBs (P2G). The results showed that the administration of PCBs led to an increase in the relative weight of the liver, and a significant increase in all of the hepatic biomarker levels (glucose, cholesterol, triglycerides, AST, ALT, and LDH) in the serum. Furthermore, an increase in the rate of lipid peroxidation and a decrease in the antioxidant enzyme activities (catalase, superoxide dismutase and glutathione peroxidase) were observed under the influence of PCBs in the liver. The histological test showed that the PCBs induced hepatocyte vacuolization, prominent and peripheralized nuclei, hepatocellular hypertrophy and turgor of the vein in the centriacinar regions. Pretreatment with ginger extract restored all of the biochemical and oxidative parameters to the normal values and reduced the injuries caused by the PCBs. In conclusion, in our experimental conditions, ginger effectively protects the liver against the hepatotoxic effects induced by PCBs.
1. Introduction
Polychlorinated biphenyls (PCBs) are a group of POPs that include chemicals such as aldrin, heptachlor, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans, as defined by the Stockholm Convention.1PCBs are a class of synthetic organic chemicals containing 209 congeners with one to ten chlorine atoms attached to the biphenyl ring, and were previously used in a variety of commercial applications.2 They are ubiquitous pollutants chemically characterized by their lipophilicity, high persistence, chemical stability and low biodegradability.3 They have a renowned toxicity; they are bioaccumulative contaminants and are found in certain fatty tissues in humans, including human milk.4–7
In the body, polychlorinated biphenyls cross cellular membranes and pass into the blood vessels and lymphatic system; then, they are stored in the liver, adipose tissues, brain and skin.8,9 PCBs can undergo anaerobic reductive dechlorination and lead to the formation of less chlorinated congeners.10 The highly chlorinated PCBs accumulate more in the body than the lower chlorinated ones, but they are considered less toxic.11,12 The biotransformation of PCBs could also occur via enzyme-mediated oxidation, which leads to the formation of hydroxylated polychlorinated biphenyls (OH-PCBs).11–13 OH-PCBs are considered to be more toxic than their parent PCBs and could lead to the disruption of thyroid hormone metabolism.14
Some research work has shown that PCBs as endocrine disruptors and enzyme inducers can perturb metabolism.15 Exposure to PCBs has harmful effects on the nervous system,16 reproduction because of the hormonal disruption, alteration in thyroid function, and the development of the immune system, and PCBs also have carcinogenic effects.17,18 Most of the past studies also reported that PCBs induce oxidative stress by the high generation of free radicals such as O2˙− and H2O2.19 These ROS are thought to contribute to lipid peroxidation, DNA damage and protein degradation.20 Indeed, in physiological conditions, there is a perfect balance between the production of reactive oxygen species (ROS) and antioxidant defense systems. Thus, oxidative stress will be defined when there is a serious imbalance between pro-oxidants and antioxidants in favor of the latter.21
The liver, being the primary site for xenobiotic detoxification, is the principal target organ for toxic effects induced by environmental pollutants, including PCBs.22 It has been shown that polychlorinated biphenyls disrupt the function of the liver. Commercial mixtures of PCBs and their congeners have been discovered to be hepatically carcinogenic by their induction of mono-oxygenase cytochrome P450-dependent pathways in the liver.23 This is due to the activity of tumor initiation of the lower chlorinated congeners and tumor promoting activity of highly chlorinated PCB congeners.24 Furthermore, it was found that PCB3 is responsible for the induction of gene mutation, which is a typical characteristic of tumor initiation, in the liver and lungs of rats by increasing ROS levels.25
For several years, the wealth of medicinal plants was the remedy and solution to health problems and increasing attention has been paid to the protective effects of these natural antioxidants on drug-induced toxicities.
Ginger, Zingiber officinale, has been used for 6000 years, and it is the panacea of Asian medicine. It has been used to treat transport and pregnancy nausea, has been used as an antioxidant, and has antimicrobial and antifungal properties, in addition to its culinary uses.26,27 Several studies have shown that gingerol, the active ingredient of ginger, has anti-inflammatory and analgesic activities.28 Besides, “in vitro” it has been shown that Zingiber officinale has an antioxidant action and can protect against free radicals in animals,29 hence its anticancer activity.30 In addition, it was shown that ginger acts on the liver to reduce cholesterol biosynthesis, stimulate its conversion into bile acids and increase its fecal excretion.31 Therefore, we assume that ginger can prevent against the hepatotoxic effects of PCBs.
In this study, we investigated whether pretreatment with an aqueous extract of ginger for 6 weeks could prevent PCB-induced hepatotoxicity in male Wistar rats. The serum levels of glucose, cholesterol, triglycerides, lactate dehydrogenase (LDH), aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and activities of antioxidant enzymes (catalase, SOD, GPx, TBARS) were measured in the liver.
2. Materials and methods
2.1. Preparation of the aqueous extract
Commercialized powder of Zingiber officinale (ZOE) was purchased from a local market, homogenized in boiling distilled water and soaked for about 24 h. Then the mixture was filtered, and the filtrates were stored in a refrigerator for subsequent analysis.2.2. PCB sample
In this study, we used Aroclor 1260 provided from an electricity company's dead stock.2.3. Phytochemical studies of Zingiber officinale extract
2.4. In vitro antioxidant activity of ZOE
| ARA% = 1 − [(Asample − Acontrol)/ADPPH] × 100 |
2.5. Acute toxicity test
The ZOE was investigated in toxicity studies. A total of 30 rats were divided randomly into 5 groups supplemented orally with gradually increasing concentrations (100 mg to 1000 mg per rat). The animals were directly observed for toxic symptoms, after the first 4 h of dosing. After 24 h, the surviving animals were maintained under daily observation for two weeks.2.6. Animal treatments
Three-month-old Wistar male rats, about 111 g in body weight, fed on 15% protein food (SNA, Sfax, Tunisia), were kept in a breeding farm, at 22 °C, with a stable hygrometry, under a constant photoperiod.These rats were divided into 6 batches each containing 6 rats:
• Group T, which served as the control group.
• Group G, which was treated by drinking 200 mg per kg b.w. of the aqueous ginger extract37 throughout the duration of the treatment.
• Two groups P1 and P2, which were treated with PCBs at two different concentrations (P1 = 470 mg per kg of b.w. and P2 = 980 mg per kg of b.w.) by using intra-gastric intubation,38 for 7 and 5 days, respectively.
• Two groups P1G and P2G, which were pretreated with the aqueous extract of ginger in drinking water for 6 weeks and then administered the PCBs at concentrations P1 and P2 for 7 and 5 days, respectively.
The animals were weighed daily and after 49 days of treatment they were sacrificed by cervical disruption. The liver was quickly removed and weighed, a portion was stored at −80 °C until analysis and a portion was fixed in formalin immediately for histopathological examination. Blood was centrifuged and serum aliquots were stored at −80 °C.
2.7. Preparation of the liver extracts
About 1 g of liver was cut into small pieces and homogenized in 2 ml of ice-cold Tris buffer (TBS, pH 7,4) using a crusher (homogenizing Ultra-Turax), and then centrifuged at 9000 rpm for 15 min at 4 °C. The supernatants (S1) were collected and stored at −80 °C until use.2.8. Biochemical assays
The absorbance was measured at 530 nm. The amount of TBARS was calculated using an extinction coefficient of 156 mM−1 cm−1 and expressed in nmol mg−1 of protein.
2.9. Histopathological examination
Formalin-fixed livers were processed routinely, embedded in paraffin, sectioned at 3–4 μm and stained with hematoxylin and eosin (H&E). An expert in histopathological evaluation44 examined the slides.2.10. Statistical analysis
Two independent experiments were performed. Data were expressed as means ± standard deviation (SD). Statistical significance was assessed using Student's t-test. p ≤ 0.05 was considered significant.3. Results
3.1. Acute toxicity test
The tested animals were administered with different doses of ginger extract (100–1000 mg kg−1). The data of the acute toxicity test showed no toxicity or lethality observed up to 1000 mg kg−1. In this study, a dose of 200 mg kg−1 BW was chosen to investigate the antioxidant and hepatoprotective activities of the aqueous extract of Z. officinale in experimental animals.3.2. Phytochemical studies of ZOE
The phytochemical studies of ZOE revealed a significant amount of polyphenols (179.5 ± 2.47 mg GAE per g DW ZOE) and to a lesser degree flavonoids and condensed tannins (59.1 ± 0.7 mg RE per g DW ZOE and 28.38 ± 5.66 mg CE per g DW ZOE, respectively) (Table 1).| Total phenolicsa (mg GAE per g DW) | Total flavonoidsb (mg RE per g DW) | Total condensed tanninsc (mg CE per g DW) | |
|---|---|---|---|
| a Total phenolic content as the gallic acid equivalent.b Total flavonoid content as the rutin equivalent.c Condensed tannin as the catechin equivalent. Values are expressed as mean ± standard deviation (n = 3). | |||
| Zingiber officinale | 179.5 ± 2.47 | 59.1 ± 0.7 | 28.38 ± 5.66 |
3.3. In vitro antioxidant capacity
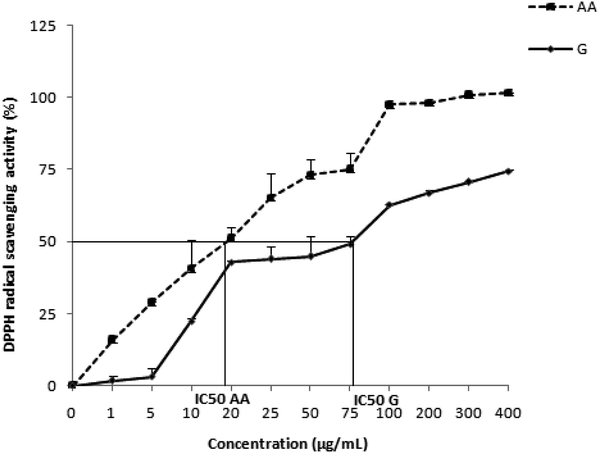 | ||
| Fig. 1 The antiradical activity of Zingiber officinale against the radical DPPH. Values are represented as mean ± standard deviation (n = 3). | ||
| DPPH (IC50, μg mL−1) | FRAP (EC50, mg mL−1) | |
|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 3). | ||
| Zingiber officinale | 79.70 ± 0.27 | 19.4 ± 1.23 |
| Ascorbic acid | 19.41 ± 2.71 | 0.47 ± 0.002 |
3.4. Liver weight
After sacrifice of the rats, the livers were weighed and the relative weight was estimated. The results showed an important increase in liver relative weight in all PCB-treated groups: P1, P2, P1G and P2G (+77%, 68%, 87% and 54%, respectively). There was no significant improvement noticed even with ginger pretreatment (Fig. 3).3.5. Serum markers of cell damage
Lactate dehydrogenase (LDH), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are released into the blood when certain organs or tissues, particularly the liver and heart, are injured. As shown in Fig. 4, PCB treatment induced a significant increase in the serum levels of LDH, AST and ALT (+33%, 42% and 64%, respectively) in (P1) and (P2) rats, as compared to controls (T). These effects were significantly decreased in the PCB-treated rats drinking ginger extract (P1G and P2G groups) (−26%, 20% and 19%, respectively) compared to the PCB-treated groups (Fig. 4).The serum levels of glucose (+24%), triglycerides (+180%) and total cholesterol (+98%) were also found to be significantly increased in (P1) and (P2) rats, as compared to controls. However, these levels were significantly decreased in the rats pretreated with ginger extract (P1G and P2G) (Table 3).
| Controls (T) | G | P1 | P2 | P1G | P2G | |
|---|---|---|---|---|---|---|
| a Student test: **(p ≤ 0.01) indicates significant differences between (P1 and P2) and control rats (T). ++(p ≤ 0.01) indicates significant difference between (P1G and P2G) and (P1 and P2) rats. Values correspond to the mean of 6 measurements ± SD. | ||||||
| Glucose (mmol L−1) | 4.8 ± 0.98 | 5.36 ± 0.86 | 6.84 ± 0.46** | 5.04 ± 0.05 | 5.58 ± 0.73+ | 5.1 ± 0.14 |
| +42.5% | +5% | −18% | ||||
| Triglycerides (mmol L−1) | 0.65 ± 0.12 | 0.48 ± 0.16 | 2.18 ± 0.2** | 1.69 ± 0.44** | 1.07 ± 0.73++ | 1.11 ± 0.12++ |
| +200% | +160% | −51% | −34% | |||
| Total cholesterol (mmol L−1) | 1.56 ± 0.14 | 1.82 ± 0.08 | 3.04 ± 0.53** | 3.14 ± 0.24** | 2.19 ± 0.73++ | 1.88 ± 0.11++ |
| +95% | +101% | −28% | −40% | |||
3.6. Estimation of lipid peroxidation levels (TBARS) in liver extract
In this study, we showed that the administration of PCBs (at concentrations P1 and P2) induces an important increase in the TBARS rates (+117% and 140%, respectively) in the liver as compared to control rats (T). However, in rats pretreated with singer extract (P1G and P2G), the rates of TBARS decreased and the results are comparable with those obtained for the control rats (T) (Fig. 5).3.7. Changes of antioxidant enzyme activities in liver extracts
PCB treatment induced a significant increase (+142%) in SOD activity in the liver of (P1) and (P2) rats and about a −53% decrease in CAT and GPx activities (Fig. 5). However, the ginger extract pretreatment (P1G and P2G groups) showed important changes in the activities of these enzymes (−45% decrease in SOD activity, +88.5% and 63.5% increase, respectively, in CAT and GPx activities).3.8. Liver histopathological changes
Histological examination showed that the PCBs (for both concentrations of 470 mg kg−1 and 940 mg kg−1) lead to an increase in the size of the hepatic lobules due to hepatocyte hypertrophy characterized by large areas of cytoplasmic pallor (hydropic degeneration), mild vacuolation of hepatocytes (arrows, c and d, Fig. 6), prominent and peripheralized nuclei, and hypertrophy and turgor in the central vein (CL, c and d, Fig. 6). These changes were moderately improved in ginger-pretreated rats (e and f). In the control (a) and ginger groups (b), there was no evidence of hepatic abnormality; we observed mild cytoplasmic clearing without vacuolization and with centrally located nuclei consistent with glycogen.4. Discussion
In this study, we intended to determine the toxic effect of polychlorinated biphenyls (PCBs) on hepatic function, and to verify the protective effects of ginger extract against the toxicity induced by PCBs.In the animal study, we found that the oral administration of PCBs to male rats from the “Wistar” strain induced liver hypertrophy. In accordance with our study, Lai et al.45 explained this liver hypertrophy by the accumulation of lipids due to disruption of hepatic lipid intake and metabolism.
The PCB treatment induced a highly significant increase in the serum levels of glucose, cholesterol and triglycerides, which is associated with an increase in the hepatic biomarkers AST, ALT and LDH. This could be explained by the strong accumulation of PCBs in the liver and the severe alteration of hepatocytes. Indeed, the transaminases (AST and ALT), which are involved in protein renewal and the synthesis of new peptides, strongly affect the metabolism due to their inhibition by PCBs. Consistent with our results, Pereira & Rao46 found that the administration of PCBs (Clophen A60) at 2.8 mg per kg of b.w. per day significantly increased the level of glucose, LDH, cholesterol and triglycerides. In addition, our results are harmonized with other studies reporting negative effects of PCBs in the liver, such as that by Wang et al.47 AST, being a primarily mitochondrial enzyme, allows us to deduce that, at the cellular level, there was an increase in respiratory burst and mitochondrial involvement in the hepatocytes of rats treated with PCB. Pereira & Rao46 explained the increase of hepatic biomarkers with the increase of lipid peroxidation that could affect mitochondrial function and the leakage of mitochondrial enzymes due to the injury of the mitochondrial membranes.
After 49 days of treatment, we observed in rats pretreated with ginger aqueous extract (P1G and P2G) that the toxicity of PCB was greatly reduced. The aqueous extract of ginger decreased significantly the AST, ALT, cholesterol, triglyceride, glucose and LDH levels. Several studies clearly reinforce our results, showing the hepatoprotective effect of ginger against liver toxicity induced by ethanol, carbon tetrachloride, bromobenzene and acetaminophen, accompanied by a significant decrease of AST and ALT.48–51 It is the same for the hypocholesterolemic effect of ginger, which is probably due to the inhibition of cellular synthesis of cholesterol. The possible mechanism of the plant to reduce serum triglycerides is due to the increase in the expression and activity of the lipoprotein lipase enzyme in the vessels. This enzyme increases the breakdown of triglycerides in the blood vessels and reduces the blood levels of triglycerides.52 Ginger also inhibits hepatic fatty acid and triglyceride synthesis by lowering key enzyme activity.53 In the research of Heeba & Abd-Elghany,54 it was also shown that ginger stimulates the conversion of cholesterol into bile acids, and increasing the excretion of cholesterol and phospholipids in the stools after taking ginger can also be considered as a potential mechanism for the effects of ginger in reducing serum cholesterol levels.55,56 In the study of Gao et al.,57 it was shown that ginger improves insulin sensitivity in the body, which may explain the mechanism of the decrease in blood glucose levels in rats pretreated with ginger.
Our experimental study showed that the induction of oxidative stress by PCBs has been demonstrated by the highly significant increase of TBARS in liver tissues. We found that the levels of lipid peroxidation are responsible for the formation of lipid hydroperoxides in membranes, leading to lesions in the membrane structure and inactivation of enzyme membrane binding.58 Research by Twaroski et al.59 showed that the toxic manifestations induced by PCBs may be associated with the high production of ROS and the initiation and self-propagating reaction of lipid peroxidation. The oxygen radicals react with poly-unsaturated fatty acid residues in the phospholipids resulting in the production of excessive amounts of products, which can damage proteins and DNA. In fact, PCBs may interact with hydrogen peroxide to form hydroxyl radicals, which are the most active form of ROS in biological systems.
It seems quite clear that the presence of ginger reduced the TBARS in pretreated rats (P1G and P2G). This urged us to deduce that ginger plays a protective role against oxidative stress induced by PCBs. The research of Rajkumar & Rao60 showed an important inhibition of lipid peroxidation by dehydro-zingerone, which is a synthetic analogue of zingerone. It is important to note that dietary ginger concomitant to 1% w/w during the administration of malathion (20 ppm) for 4 weeks significantly attenuated lipid peroxidation in the liver.61
The high lipid peroxidation may also be due to decreased activities of catalase and superoxide dismutase, which are scavenger enzymes of free radicals. In our study, we found that PCBs increased the activity of superoxide dismutase and decreased the catalase and glutathione peroxidase activities. Several studies have associated changes in the activity of superoxide dismutase with the reduced synthesis, high degradation or inactivation of this enzyme.16,20,62 Other studies have investigated the increase in superoxide dismutase activity and they explained these results by the increase of the concentration of superoxide anions (O2˙−), which causes an increase in the concentration of H2O2 due to the inhibition of catalase and glutathione peroxidase, as they are the responsible enzymes for scavenging H2O2. In the work of Venkataraman et al.,16 the decrease in glutathione peroxidase activity in rats treated with PCBs is correlated to the reduction of the substrate, meaning reduced glutathione (GSH) level and high peroxide level. In other research studies, the decrease observed in our results is evaluated by decreased synthesis and/or inactivation of the enzyme.20
However, the administration of ginger as a pretreatment (200 mg per kg of b.w.) for 6 weeks restored the activities of antioxidant enzymes. This could be explained by the presence of several antioxidant compounds in ginger, such as gingerols, shogaol derivatives, ketones, phenolics, flavonoids and volatile oils.58 [6]-Gingerol as the major constituent of ginger has been shown to exert an inhibitory effect on xanthine oxidase, the enzyme responsible for the generation of reactive oxygen species.63 This antioxidant activity of ginger extract is explained by the results found in our phytochemical study, which revealed significant levels of polyphenols and a lesser amount of flavonoids and tannins, which is correlated with the findings of Gabr et al.64 These antioxidant substances are well known to protect the body against free radicals. The antioxidant activity of Z. officinale aqueous extract was evaluated in vitro using the DPPH and FRAP tests. Our results showed important reducing properties due to the presence of compounds that reduce the ferricyanide complex of Fe3+ to the ferrous (Fe2+) form by donating a proton. In addition, the ginger aqueous extract exhibits a significant scavenging activity based on the reduction of the stable and free radical DPPH (purple color) thanks to the hydrogen atoms existing on the antioxidant contents of the ZOE. Our observations are consistent with those demonstrated by Gabr et al.64 These antioxidant potentials of the ginger extract might be due to its richness in polyphenols and flavonoids. These bioactive compounds are known by their redox properties and might play an important role in chelating transition metals and scavenging free radicals.65 The studies of Shanmugam et al.58 showed that the activities of antioxidant enzymes SOD and GPx were improved in the liver tissue of animals pretreated with an ethanolic extract of ginger at a concentration of 100 mg per kg b.w. compared to animals exposed to bromobenzene. The same research team reported that the extract of ginger decreases the activity of cytochrome P450 by reducing the metabolism of bromobenzene into reactive metabolites.
Histological examination showed that the PCBs (at both concentrations of 470 mg kg−1 and 940 mg kg−1) induced vacuolization of hepatocytes, prominent and peripheralized nuclei and hypertrophy and turgor of the central vein. The results of this study are consistent with the works of Lai et al.45 and Wang et al.,47 which showed severe changes in hepatocytes with an increase in the size of the lobules and capsular irregularity. However, the aqueous extract of ginger slightly attenuated these cytological manifestations. This has been demonstrated in the studies of Heeba & Abd-Elghany,54 showing that the administration of ginger plays an important role in reducing liver damage and preserves the integrity of the hepatocyte membranes.
In conclusion, it seems that ginger is able to protect the liver against oxidative stress and biochemical manifestations induced by polychlorinated biphenyls. The high protection capacity of ginger could be due to its content of antioxidant phytochemicals, which neutralize the high production of free radicals generated by the PCBs.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This research was funded by the Tunisian Ministry of Higher Education and Scientific Research through the unit of Macromolecular Biochemistry and Genetics, Faculty of Sciences of Gafsa and Laboratory of Environmental Pathophysiology, Development of Bioactive Molecules and Mathematical Modeling, Faculty of Science of Sfax, with collaboration with the Laboratory of Biochemistry at the Regional Hospital of Gafsa, Tunisia.References
- United Nations Industrial Development Organization, What Are Persistent Organic Pollutants (POPs)?, 2015 Search PubMed.
- J. Warner, J. R. Osuch, W. Karmaus, J. R. Landgraf, B. Taffe, M. O'Keefe, D. Mikucki and P. Haan, Common classification schemes for PCB congeners and the gene expression of CYP17, CYP19, ESR1 and ESR2, Sci. Total Environ., 2012, 414, 81–89 CrossRef CAS PubMed.
- X. B. Zheng, X. J. Luo, Y. H. Zeng, J. P. Wu and B. X. Mai, Chiral polychlorinated biphenyls (PCBs) in bioaccumulation, maternal transfer, and embryo development of chicken, Environ. Sci. Technol., 2015, 49, 785–791 CrossRef CAS PubMed.
- J. L. Cai, L. L. Liu, Y. Hu, X. M. Jiang, H. L. Qiu, A. G. Sha, C. G. Wang, Z. H. Zuo and J. Z. Ren, Polychlorinated biphenyls impair endometrial receptivity in vitro via regulating mir-30d expression and epithelial mesenchymal transition, Toxicology, 2016, 365, 25–34 CrossRef CAS PubMed.
- R. E. Ellsworth, K. A. Mamula, N. S. Costantino, B. Deyarmin, P. J. Kostyniak, L. H. Chi, A. S. El-Sharaky, A. A. Newairy, M. A. Kamel and S. M. Eweda, Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats, Food Chem. Toxicol., 2009, 47, 1584–1590 CrossRef PubMed.
- O. Huetos, M. Bartolome, N. Aragones, M. Cervantes-Amat, M. Esteban, M. Ruiz-Moraga and H. A. Jeng, Exposure to endocrine disrupting chemicals and male reproductive health, Front. Public Health, 2014, 2, 55 Search PubMed.
- B. A. Cohn, M. B. Terry, M. Plumb and P. M. Cirillo, Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50, Breast Cancer Res. Treat., 2012, 136, 267–275 CrossRef CAS PubMed.
- J. Kumar, L. Lind, S. Salihovic, B. Van Bavel, E. Ingelsson and P. M. Lind, Persistent organic pollutants and liver dysfunction biomarkers in a population based human sample of men and women, Environ. Res., 2014, 134, 251–256 CrossRef CAS PubMed.
- J. Kumar, L. Lind, S. Salihovic, B. Van Bavel, K. N. Ekdahl, B. Nilsson, L. Lind and E. Ingelsson, Influence of persistent organic pollutants on the complement system in a population-based human sample, Environ. Int., 2014, 71, 94–100 CrossRef CAS PubMed.
- L. Passatore, S. Rossetti, A. A. Juwarkar and A. Massacci, Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): state of knowledge and research perspectives, J. Hazard. Mater., 2014, 278, 189–202 CrossRef CAS PubMed.
- X. N. Wu, M. Duffel and H. J. Lehmler, Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective, Chem. Res. Toxicol., 2013, 26(l1), 1642–1651 Search PubMed.
- G. S. Zhai, H. J. Lehmler and J. L. Schnoor, Inhibition of cytochromes P450 and the hydroxylation of 4-monochlorobiphenyl in whole poplar, Environ. Sci. Technol., 2013, 47, 6829–6835 CrossRef CAS PubMed.
- J. Rezek, T. Macek, M. Mackova, J. Triska and K. Ruzikova, Hydroxy-PCBs, methoxy-PCBs and hydroxy-methoxy-PCBs: metabolites of polychlorinated biphenyls formed in vitro by tobacco cells, Environ. Sci. Technol., 2008, 42, 5746–5751 CrossRef CAS PubMed.
- I. A. T. M. Meerts, Y. Assink, P. H. Cenijn, J. H. J. Van den Berg, B. M. Weijers, A. Bergman, J. H. Koeman and A. Brouwer, Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat, Toxicol. Sci., 2002, 68, 361–371 CrossRef CAS PubMed.
- H. A. Jeng, Exposure to endocrine disrupting chemicals and male reproductive health, Front. Pub. Health., 2014, 2, 55 Search PubMed.
- P. Venkataraman, R. Muthuvel, G. Krishnamoorthy, A. Arunkumar, M. Sridhar, N. Srinivasan, K. Balasubramanian, M. M. Aruldhas and J. Arunakaran, PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid, NeuroToxicology, 2007, 28(3), 490–498 CrossRef CAS PubMed.
- W. J. Xie, A. P. Chen, J. Y. Li, Q. Liu, H. J. Yang, T. Wu and Z. H. Lu, Topsoil dichlorodiphenyltrichloroethane and polychlorinated biphenyl concentrations and sources along an urban-rural gradient in the Yellow River Delta, J. Environ. Sci., 2012, 24, 1655–1661 CrossRef CAS.
- O. Faroon and P. Ruiz, Toxicological profile for polychlorinated biphenyls (pcbs) (addendum), US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry ATSDR, 2011 Search PubMed.
- V. A. Venkatesha, S. Venkataraman, E. H. Sarsour, A. L. Kalen, G. R. Buettner, L. W. Robertson, H. J. Lehmler and P. C. Goswami, Catalase ameliorates polychlorinated biphenyl-induced cytotoxicity in nonmalignant human breast epithelial cells, Free Radicals Biol. Med., 2008, 45, 1094–1102 CrossRef CAS PubMed.
- G. Krishnamoorthy, P. Venkataraman, A. Arunkumar, R. C. Vignesh, M. M. Aruldhas and J. Arunakaran, Ameliorative effect of vitamins (α-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm, Reprod. Toxicol., 2007, 23, 239–245 CrossRef CAS PubMed.
- J. Pincemail, K. Bonjean, K. Cayeux and J. O. Defraigne, Mécanismes physiologiques de la défense antioxydante, Nutr. Clin. Metab., 2002, 16, 233–239 CrossRef CAS.
- B. Wahlang, J. T. Perkins, M. C. Petriello, J. B. Hoffman, A. J. Stromberg and B. Hennig, A compromised liver alters polychlorinated biphenyl-mediated toxicity, Toxicology, 2017, 380, 11–22 CrossRef CAS PubMed.
- R. Haag, G. Scheu, L. Warngard and R. Fransson-Steen, Analysis of rat Gronlund M., Conolly liver foci growth with a quantitative two-cell model after treatment with 2,4,5,3',4'-pentachlorobiphenyl, Toxicol. Sci., 2000, 57(1), 32–42 CrossRef PubMed.
- G. Ludewig, L. Lehmann, H. Esch and L. W. Robertson, Metabolic activation of PCBs to carcinogens in vivo – a review, Environ. Toxicol. Pharmacol., 2008, 25(2), 241–246 CrossRef CAS PubMed.
- C. Maddox, B. Wang, P. A. Kirby, K. Wang and G. Ludewig, Mutagenicity of 3-methylcholanthrene, Pcb3, and 4-Oh-Pcb3 in the lung of transgenic bigblue rats, Environ. Toxicol. Pharmacol., 2008, 25(2), 260–266 CrossRef CAS.
- G. H. Park, J. H. Park, H. M. Song, H. J. Eo, M. K. Kim, J. W. Lee, M. H. Lee, K. H. Cho, J. R. Lee, H. J. Cho and J. B. Jeong, Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells, BMC Complement, BMC Complementary Altern. Med., 2014, 14, 8 CrossRef PubMed.
- M. Thomson, R. Corbin and L. Leung, Effects of ginger for nausea and vomiting in early pregnancy: a metal-analysis, J. Am. Board Fam. Med., 2014, 27(1), 115–122 CrossRef PubMed.
- H. Y. Young, Y. L. Luo, H. Y. Cheng, W. C. Hsieh, J. C. Liao and W. H. Peng, Analgesic and anti-inflammatory activities of [6]-gingerol, J. Ethnopharmacol., 2005, 96(1–2), 207–210 CrossRef CAS PubMed.
- Y. Masuda, H. Kikuzaki, M. Hisamoto and N. Nakatani, Antioxidant properties of gingerol related compounds from ginger, BioFactors, 2004, 21(1–4), 293–296 CrossRef CAS.
- S. H. Lee, M. Cekanova and S. J. Baek, Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells, Mol. Carcinog., 2008, 47(3), 197–208 CrossRef CAS.
- S. K. Verma, M. Singh, P. Jain and A. Bordia, Protective effect of ginger, Zingiber officinale Rosc. on experimental atherosclerosis in rabbits, Indian J. Exp. Biol., 2004, 42(7), 736–738 CAS.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–153 CAS.
- A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker and N. Vidal, Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds, Food Chem., 2006, 97(4), 654–660 CrossRef CAS.
- D. Heimler, P. Vignolini, M. G. Dini, F. Vincieri and A. Rmani, Antiradical activity and polyphenol composition of local Brassicaceae edible varieties, Food Chem., 2006, 99(3), 464–469 CrossRef CAS.
- I. Grzegorczyk, A. Matkowski and H. Wysokinska, Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L, Food Chem., 2007, 104, 536–541 CrossRef CAS.
- Y. H. Chu, C. L. Chang and H. F. Hsu, Flavonoid content of several vegetables and their antioxidant activity, J. Sci. Food Agric., 2000, 80, 561–566 CrossRef CAS.
- M. A. Shalaby and A. R. Hamowieh, Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats, Food Chem. Toxicol., 2010, 48, 2920–2924 CrossRef CAS PubMed.
- R. Roos, P. L. Andersson, K. Halldin, H. Hakansson, E. Westerholm, T. Hamers, G. Hamscher, P. Heikkinen, M. Korkalainen, H. A. Leslie, M. Niittynen, S. Sankari, H.-J. Schmitz, L. T. M. Van der Ven, M. Viluksela and D. Schrenka, Hepatic effects of a highly purified 2,2′,3,4,4′,5,5′-heptachlorbiphenyl (PCB 180) in male and female rats, Toxicology, 2011, 284, 42–53 CrossRef CAS.
- K. Yagi, A simple fluorometric assay for lipoperoxide in blood plasma, Biochem. Med., 1976, 15, 212–216 CrossRef CAS PubMed.
- H. Aebi, Catalase in vitro, Methods Enzymology, ed. H. U. Bergneyer, 1984, vol. 105, pp. 121–126 Search PubMed.
- I. Durak, Z. Yurtarslani, O. Canbolat and O. Akyol, A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction, Clin. Chim. Acta, 1993, 214, 103–104 CrossRef CAS.
- L. Flohe and W. A. Gunzler, Assays of glutathione peroxidase, Methods Enzymol., 1984, 105, 114–121 CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275 CAS.
- M. Gabe, Techniques Histologiques, Masson et Cie, Paris, 1968 Search PubMed.
- I. Lai, Y. Chai, D. Simmons, G. Luthe, M. C. Coleman, D. Spitz, W. M. Haschek, G. Ludewig and L. W. Robertson, Acute toxicity of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status, Environ. Int., 2009, 36(8), 918–923 CrossRef PubMed.
- C. Pereira and C. V. Rao, Combined and individual administration of diethyl phthalate and polychlorinated biphenyls and its toxicity in female Wistar rats, Environ. Toxicol. Pharmacol., 2006, 21, 93–102 CrossRef CAS PubMed.
- Y. Wang, C. Lu, Z. Sheng, G. Liu, Z. Fu, B. Zhu and S. Peng, Enhanced hepatotoxicity induced by repeated exposure to polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin in combination in male rats, J. Environ. Sci., 2011, 23(1), 119–124 CrossRef CAS.
- A. S. El-Sharaky, A. A. Newairy, M. A. Kamel and S. M. Eweda, Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats, Food Chem. Toxicol., 2009, 47(7), 1584–1590 CrossRef CAS PubMed.
- K. Mallikarjuna, C. P. Sahitya, R. K. Sathyavelu and W. Rajendra, Ethanol toxicity: rehabilitation of hepatic antioxidant defense system with dietary ginger, Fitoterapia, 2008, 79, 174–178 CrossRef CAS PubMed.
- T. A. Ajith, U. Hema and M. S. Aswathy, Zingiber officinale Roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status, Food Chem. Toxicol., 2007, 45, 2267–2272 CrossRef CAS PubMed.
- O. K. Yemitan and M. C. Izegbu, Protective Effects of Zingiber officinale (Zingiberaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats, Phytother. Res., 2006, 20, 997–1002 CrossRef PubMed.
- Z. Shirdel, R. Mirbadalzadeh and H. Madani, Antidiabetic and antilipidemic effect of ginger in alloxan monohydrate diabetic rats in comparison with glibenclamide, Iran. J. Diabetes Lipid Disord., 2009, 9, 7–15 Search PubMed.
- A. Kalaiselvi, G. AadhinathReddy and V. Ramalingam, Ameliorating effect of ginger extract (Zingiber officinale roscoe) on liver marker enzymes, lipid profile in aluminium chloride induced male rats, Int. J. Pharm. Sci. Drug Res., 2015, 7(1), 52–58 CAS.
- G. H. Heeba and M. I. Abd-Elghany, Effect of combined administration of ginger (Zingiber officinale Roscoe) and atorvastatin on the liver of rats, Phytomedicine, 2010, 17(14), 1076–1081 CrossRef CAS PubMed.
- T. Arablou, N. Aryaeian, M. Valizadeh, A. Hosseini and M. Djalali, The effect of ginger consumption on some cardiovascular risk factors in patients with type 2 diabetes mellitus, Razi J. Med. Sci., 2014, 21(118), 1–12 Search PubMed.
- T. Arablou, N. Aryaeian, M. Valizadeh, F. Sharifi, A. Hosseini and M. Djalali, The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus, Int. J. Food Sci. Nutr., 2014, 65(4), 6 CrossRef PubMed.
- H. Gao, T. Guan, C. Li, G. Zuo, J. Yamahara, J. Wang and Y. Li, Treatment with ginger ameliorates fructose-induced fatty liver and hypertriglyceridemia in rats: modulation of the hepatic carbohydrate response element-binding protein-mediated pathway, J. Evidence-Based Complementary Altern. Med., 2012 DOI:10.1155/2012/570948.
- K. R. Shanmugam, K. Mallikarjuna, N. Kesireddy and K. S. Reddy, Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats, Food Chem. Toxicol., 2011, 49, 893–897 CrossRef CAS PubMed.
- T. P. Twaroski, M. L. O'Brien, N. Larmonier, H. P. Glauert and L. W. Rorbertson, Polychlorinated biphenyls-induced effects of metabolic enzymes, AP-1 binding, vitamin E, and oxidative stress in the rat liver, Toxicol. Appl. Pharmacol., 2001, 171, 85–93 CrossRef CAS PubMed.
- D. V. Rajkumar and M. N. A. Rao, Dihydro-gingerone and isoeugenol as inhibitors of lipid peroxidation and as free radical scavengers, Biochem. Pharmacol., 1993, 46, 2067–2072 CrossRef.
- R. S. Ahmed, V. Seth, S. T. Pasha and B. D. Banerjee, Influence of dietary ginger (Zingiber officinale Roscoe) on oxidative stress induced by malathion in rats, Food Chem. Toxicol., 2000, 38, 443–450 CrossRef CAS.
- P. Venkataraman, M. Sridhar, S. Dhanammal, M. R. Vijayababu, N. Srinivasan and J. Arunakaran, Antioxidant role of zinc in PCB (Aroclor 1254) exposed ventral prostate of albino rats, J. Nutr. Biochem., 2004, 15, 608–613 CrossRef CAS PubMed.
- W. S. Chang, Y. H. Chang, F. J. Lu and H. C. Chiang, Inhibitory effects of phenolic on xanthine oxidase, Anticancer Res., 1994, 14, 501–506 CAS.
- S. A. Gabr, A. H. Alghadir and G. A. Ghoniem, Biological activities of ginger against cadmium-induced renal toxicity, Saudi J. Biol. Sci., 2019, 26, 382–389 CrossRef CAS PubMed.
- C. O. Okoli, P. A. Akah, S. V. Nwafor, A. I. Anisiobi, I. N. Ibegbunam and O. Erojikwe, Antiinflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams, J. Ethnopharmacol., 2007, 109(2), 219–225 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |





