Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology
Annalisa Dalmoroab,
Sabrina Bochicchioa,
Gaetano Lamberti ac,
Paolo Bertoncind,
Barbara Janssense and
Anna Angela Barba
ac,
Paolo Bertoncind,
Barbara Janssense and
Anna Angela Barba *ab
*ab
aEng4Life Srl, Spin-off Accademico, Via Fiorentino, 32, 83100 Avellino, Italy
bDipartimento di Farmacia, Università degli Studi di Salerno, via Giovanni Paolo II, Fisciano, 132 84084, SA, Italy. E-mail: aabarba@unisa.it
cDipartimento di Ingegneria Industriale, Università degli Studi di Salerno, via Giovanni Paolo II Fisciano, 132, 84084, SA, Italy
dDipartimento di Scienze della Vita – Centro Microscopia Elettronica, Università degli Studi di Trieste, Via Fleming 31, A/B, 34127 Trieste, Italy
eDepartment of Pharmaceutics, University of Gent, Sint-Pietersnieuwstraat 25, 9000, Belgium
First published on 25th June 2019
Abstract
Micronutrients administration by fortification of staple and complementary foods is a followed strategy to fight malnutrition and micronutrient deficiencies and related pathologies. There is a great industrial interest in preparation of formulations for joint administration of vitamin D3 and vitamin K2 for providing bone support, promoting heart health and helping boost immunity. To respond to this topic, in this work, uncoated nanoliposomes loaded with vitamin D3 and K2 were successfully prepared, by using a novel, high-yield and semi continuous technique based on simil-microfluidic principles. By the same technique, to promote and to enhance mucoadhesiveness and stability of the produced liposomal structures, chitosan was tested as covering material. By this way polymer–lipid hybrid nanoparticles, encapsulating vitamin D3 and vitamin K2, with improved features in terms of stability, loading and mucoadhesiveness were produced for potential nutraceutical and pharmaceutical applications.
1. Introduction
It is proven by numerous researches that micronutrients intake is related to long term health, cognition, healthy development and aging. Health care investigations have shown that inadequate micronutrient status is an issue in industrialized countries as well as in low-income countries where billions of people still suffer from malnutrition and micronutrient deficiencies.1 To prevent and/or treat micronutrients deficiency several strategies are currently adopted, such as fortification of staple and complementary foods, provision of supplements.2 For these latter purposes encapsulation of micronutrients is, under production point of view, the main approach to ensure suitable dosing, loaded molecules stability and bioavailability.Vitamins are a class of micronutrients that play a significant role in human growth. The major part cannot be synthetized in the human body or is formed in very little amount. Hence, the importance to provide vitamins in adequate quantities through a diet of fortified food and/or supplements suitably produced by delivery systems.3,4 Indeed, in their naked form, vitamins are highly susceptible to degradation and possess poor bioavailability, thus it is essential to wrap vitamins in protective materials in order to prevent their deterioration during both food processes and their uptake in the organism,5 i.e. to enhance their solubility, stability and targeting profile.6 The main methods used for encapsulating vitamins are emulsions, solid–lipid nanoparticles, surfactant systems and polymer/lipid encapsulation. The latter includes liposome encapsulation which has recently drawn great interest due to its ability of prolonging shelf life and improving the bioavailability of vitamins and of a wide variety of hydrophilic and hydrophobic molecules such as peptides, proteins, Nucleic Acid Based Drugs (NABDs), which are useful for pharmaceutical, cosmetic, biochemical and nutraceutical purposes.7–10 Liposomes are closed vesicular structures constituted by one or more phospholipid bilayers surrounding an aqueous core. They are highly biocompatible and biodegradable drug delivery systems, which, due to their low intrinsic toxicity and immunogenicity and their capability to incorporate hydrophilic and hydrophobic drugs, are the ideal candidates in the controlled release of many kinds of active ingredients. The similarity of their structure to that of cell membranes helps the penetration and overcoming of biological barriers to cellular and tissue uptake of entrapped nutrients.11 Despite their several advantages, sometimes they need further stabilization to avoid degradation or aggregation into biological fluids as well as in stocking conditions.
Polymer coating is a promising way to modify the surface properties of liposomes in order to improve their applicability.12–14 Among useful polymers, chitosan is a polysaccharide widely used in industry due to its biocompatibility, non-toxic, biodegradable and fungistatic properties, furthermore thanks to its net positive charge it could efficiently interact with negatively charged liposomes due to electrostatic interactions.15,16 The coating of liposomes with chitosan has been found to increase their stability, to provide them with mucoadhesive properties, to extend their blood-circulation time, and to decrease the leakage of loaded active principles.15,17–19 The point is that the many techniques that have been used so far for an efficacious superficial polymeric coating of nanoliposomes. Currently most applied methods from industries or studied at research level, operate discontinuously on small volumes and require long preparation times. These methodologies can present drastic conditions such as high/low temperatures and pressures or the use of organic solvents that could remain in trace amounts in the final products. For example, in the work of12 nanoliposomes containing vitamin E were prepared by organic solvent evaporation technique followed by sonication. Subsequently, for the coating process, a chitosan solution was added under stirring condition to obtain chitosan-coated nano-size liposomes. Besides being a slow and discontinuous process, by means of this bulk techniques it is not possible to obtain a control on the liposomes covering process, thus particles are often characterized by a non-uniform polymeric surface.12 The chitosan layer distributed unevenly on the surface of the vesicles leads to a greater propensity to aggregate with the consequent loss of stability. Moreover, the surface portion of the liposomes that is not covered is more subject to degradation with a reduced retention time of the active molecule encapsulated in biological fluids or in storage conditions. Mady and Darwish20 used similar conditions, i.e. organic solvent evaporation followed by a dropwise chitosan solution – discontinuous bulk techniques, in order to prove that appropriate combinations of the liposomal and chitosan characteristics may produce stable liposomes with specific features. Shin and collaborators used the ethanol injection method for liposomes production, which, although is a rapid process, has the limits imposed by the syringe device volume that make it, once again, a discontinuous technique providing the continuous handling of the prepared suspensions. Finally, the drop-wise chitosan covering method was used in same work16 with the same disadvantages seen before.
In this work attention is focused on lipophilic vitamins K and D encapsulation to produce, with a new manufacturing approach characterized by massive and continuous production, stable nanoliposomal chitosan-covered additives as micronutrients delivery model for nutraceutical purposes.
Vitamin D, and in particular the D3 form or cholecalciferol, is able to adjust the in vivo metabolization of calcium and phosphorus against osteoporosis, and its deficiency provokes an increased risk of many diseases, such as osteoporosis, some cancers, type 1 diabetes, cardiovascular diseases.21 Vitamin K is required by body for both synthesizing some proteins which are prerequisites for blood coagulation and controlling binding of calcium in bones and other tissues, thus a poor vitamin K status is associated with an increased risk of osteoporotic bone fractures.22 The most widely used vitamin K form for supplementation is vitamin K2 and more specifically menaquinone-4, more used in trials with bone outcomes, and menaquinone-7, more used in trials with cardiovascular outcomes.23 Current evidence reveals that joint supplementation of vitamins D3 and K2 might be more effective than the consumption of either alone in supporting healthy absorption of calcium by D3 action and directing calcium to the bones by K2 action, preventing it from depositing on the arteries and joints. Thus, vitamin D3 and K2 combination provides bone support, promotes heart health and helps boost immunity.23–25
Few investigations about vitamin D3 encapsulation in nano systems were found in literature: nanoparticles of whey protein isolate,26 nanoparticles of polymers, such as polylactic acid,27 solid lipid nanoparticles,28 liposomes.5,29–31 Vitamin K2 encapsulation was found only in very few patents,32 but there was no trace in literature about K2 encapsulation in liposomes. On the contrary, from the industrial view point there are several commercial products containing vitamin D3 and K2 combinations based on liposomal technology (see as examples: Nanonutra, CureSupport, SANUS-q, LipoLife, Doctor's Formulas, QuickSilver Scientific, DesBio, NOW, Protocol, PuraThrive, Clinicians, BioCeuticals, Advanced Therapeutic Medicinals, Actinovo, VitOrtho, Liposol Elivera, Lippomix, LipoPharmacy, GreenLeaves Vitamins, TIB, GoEnergetics, Anatis, Vitasomal, Sanasis – all liposomal products without polymer coatings), attesting the great industrial interest for such a formulation.
Starting from this scenery, this work presents the production of nanoliposomal carriers loaded with D3 and K2 vitamins and coated by chitosan layer to improve features such as load, encapsulation, stability and mucoadhesiveness in order to obtain enhanced nanolipid carriers for vitamin micronutrient delivery. To this aim the novel tested simil-microfluidic technique33,34,35, characterized by high production yield, continuous regime and mild operative conditions, was adopted both to produce nanoliposomal carriers and to cover the nanostructures with chitosan obtaining, at last, polymer–lipid hybrid delivery systems.
2. Experimental
2.1 Materials
L-α-Phosphatidylcholine (PC) from soybean, type II-S, 14–23% choline basis (CAS no. 8002-43-5), cholesterol (CHOL) (CAS no. 57-88-5), chitosan (CHIT) with medium molecular weight (190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–310
000–310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and 75% degree of deacetylation (DD) (CAS no. 9012-76-4; supplier specification product available on http://www.sigmaaldric.com, cod. 448877), ethanol of analytical grade (CAS no. 64-17-5), glacial acetic acid (CAS no. 64-19-7), vitamin D3 or cholecalciferol (D3) (CAS no. 67-97-0), vitamin K2 (K2) (CAS no. 863-61-6), Triton X-100 (CAS no. 9002-93-1), and mucin from porcine stomach type III, bound sialic acid 0.5–1.5%, partially purified powder (CAS no. 84082-64-4), were purchased from Sigma Aldrich (Milan, Italy).
000 Da) and 75% degree of deacetylation (DD) (CAS no. 9012-76-4; supplier specification product available on http://www.sigmaaldric.com, cod. 448877), ethanol of analytical grade (CAS no. 64-17-5), glacial acetic acid (CAS no. 64-19-7), vitamin D3 or cholecalciferol (D3) (CAS no. 67-97-0), vitamin K2 (K2) (CAS no. 863-61-6), Triton X-100 (CAS no. 9002-93-1), and mucin from porcine stomach type III, bound sialic acid 0.5–1.5%, partially purified powder (CAS no. 84082-64-4), were purchased from Sigma Aldrich (Milan, Italy).
2.2. Methods
Different chitosan concentrations, i.e. 0.0025%, 0.005%, 0.00625%, 0.0075%, 0.01% w/v, in a 0.5% (v/v) acetic acid solution, were tested in order to check the best coverage of unloaded, D3 and K2 liposomes. Briefly, the previously prepared suspension of nanometric unilamellar liposomes and the chitosan solution were pushed in the simil-microfluidic set-up at the same flow rate (25 mL min−1) to produce a suspension of chitosan-coated liposomes, subjected first to stirring for 1 h, then to characterization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v with distilled water and were deposited about 10 μL on a carbon support film on copper specimen grid mesh 200 (Electron Microscopy Sciences). Then, after 5 min, samples were negatively stained with 1% w/v of uranyl acetate solution for 5 min, drying the exceeded water, and finally were completely dried at room temperature.
10 v/v with distilled water and were deposited about 10 μL on a carbon support film on copper specimen grid mesh 200 (Electron Microscopy Sciences). Then, after 5 min, samples were negatively stained with 1% w/v of uranyl acetate solution for 5 min, drying the exceeded water, and finally were completely dried at room temperature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (118
000 rpm (118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 × g), under vacuum at 4 °C (to avoid liposomes overheating during centrifugation, used centrifuge: Beckman Optima L-90K centrifuge with SW 55 Ti rotor, Beckman Instruments, Palo Alto, CA, USA), with the aim to separate the supernatant, containing the unencapsulated vitamin, from the precipitated liposomes (pellet). After centrifugation the supernatant was thus removed by aspiration with a Gilson pipette and substituted by a detergent for destroying liposomes and detect the encapsulated vitamin. In particular, for vitamin D3-loaded liposomes, the pellet was treated with 3 mL of ethanol, instead for vitamin K2-loaded ones, the pellet was treated with 3 mL of Triton X-100 at 1% (v/v). Triton X-100 was not used for lysing D3-loaded liposomes because it absorbs in the same range as vitamin D3 and disturbs in this way the UV-VIS quantification. The pellet was left to incubate for approximately 30 minutes and afterwards sonicated for one minute at 100% amplitude (VCX 130 PB Ultrasonic Processors, 130 W, frequency 20 kHz, Sonics & Materials Inc., CT, USA). Both the supernatant and pellet of each sample were submitted to UV-VIS spectrophotometric analysis (Lambda 35, PerkinElmer, Monza, Italy) by investigating an absorption spectrum from 200 nm to 400 nm, and inside it choosing the maximal wavelength of absorbance at 270 nm for vitamin D3 and 329 nm for vitamin K2. The encapsulation efficiency (EE, %) was consequently defined as the percentage ratio between the amount of vitamin (D3 or K2) detected in the pellet, thus encapsulated in liposomes (for both chitosan-coated and uncoated liposomes) and the amount of vitamin initially included in the formulation (i.e. inserted in the lipophilic solution), as expressed by the following equation:
443 × g), under vacuum at 4 °C (to avoid liposomes overheating during centrifugation, used centrifuge: Beckman Optima L-90K centrifuge with SW 55 Ti rotor, Beckman Instruments, Palo Alto, CA, USA), with the aim to separate the supernatant, containing the unencapsulated vitamin, from the precipitated liposomes (pellet). After centrifugation the supernatant was thus removed by aspiration with a Gilson pipette and substituted by a detergent for destroying liposomes and detect the encapsulated vitamin. In particular, for vitamin D3-loaded liposomes, the pellet was treated with 3 mL of ethanol, instead for vitamin K2-loaded ones, the pellet was treated with 3 mL of Triton X-100 at 1% (v/v). Triton X-100 was not used for lysing D3-loaded liposomes because it absorbs in the same range as vitamin D3 and disturbs in this way the UV-VIS quantification. The pellet was left to incubate for approximately 30 minutes and afterwards sonicated for one minute at 100% amplitude (VCX 130 PB Ultrasonic Processors, 130 W, frequency 20 kHz, Sonics & Materials Inc., CT, USA). Both the supernatant and pellet of each sample were submitted to UV-VIS spectrophotometric analysis (Lambda 35, PerkinElmer, Monza, Italy) by investigating an absorption spectrum from 200 nm to 400 nm, and inside it choosing the maximal wavelength of absorbance at 270 nm for vitamin D3 and 329 nm for vitamin K2. The encapsulation efficiency (EE, %) was consequently defined as the percentage ratio between the amount of vitamin (D3 or K2) detected in the pellet, thus encapsulated in liposomes (for both chitosan-coated and uncoated liposomes) and the amount of vitamin initially included in the formulation (i.e. inserted in the lipophilic solution), as expressed by the following equation:
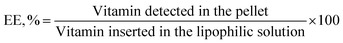 | (1) |
Moreover, the sum of vitamin detected in the pellet, i.e. encapsulated in liposomes, and vitamin found in the supernatant, i.e. unencapsulated, was compared with the initial amount of vitamin used for the formulation in order to verify that it was not degraded by the process.
Vitamin loading percentage was determined as the percentage ratio between the amount of vitamin encapsulated in liposomes and the total amount of components included in the formulations, i.e. PC, CHOL, vitamin (D3 or K2), and CHIT for coated liposomes, calculated using the following equation:
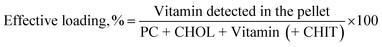 | (2) |
The theoretical loading has as numerator in eqn (2) the vitamin inserted in the lipophilic solution.
All the kinds of measurements were performed in triplicate and all the results were expressed as average values with the corresponding standard deviations, SD.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and left to incubate at room temperature (23 °C) for 2 h. Afterwards, 2 mL of the liposome/mucin solution were centrifuged for 60 min at a relative centrifugal force of 118
1, v/v) and left to incubate at room temperature (23 °C) for 2 h. Afterwards, 2 mL of the liposome/mucin solution were centrifuged for 60 min at a relative centrifugal force of 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 × g and 4 °C (Beckman Optima L-90K centrifuge with SW 55 Ti rotor, Beckman Instruments, Palo Alto, CA, USA) in order to remove the supernatant. Mucin not absorbed by the pellet, thus kept free in the supernatant itself, was detected by UV spectrophotometry (Lambda 35, PerkinElmer, Monza, Italy) at 384 nm. The sample mucoadhesiveness was defined as Mucin Binding Efficiency (MB eff., %), calculated as the percentage ratio between the mucin bounded to liposomes, expressed as the difference between the initial mucin concentration, C0, in the liposome/mucin solution (200 μg mL−1), and the free mucin detected in the supernatant, CS, and the initial mucin concentration C0, as visible by the following equation:
443 × g and 4 °C (Beckman Optima L-90K centrifuge with SW 55 Ti rotor, Beckman Instruments, Palo Alto, CA, USA) in order to remove the supernatant. Mucin not absorbed by the pellet, thus kept free in the supernatant itself, was detected by UV spectrophotometry (Lambda 35, PerkinElmer, Monza, Italy) at 384 nm. The sample mucoadhesiveness was defined as Mucin Binding Efficiency (MB eff., %), calculated as the percentage ratio between the mucin bounded to liposomes, expressed as the difference between the initial mucin concentration, C0, in the liposome/mucin solution (200 μg mL−1), and the free mucin detected in the supernatant, CS, and the initial mucin concentration C0, as visible by the following equation:
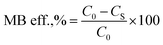 | (3) |
All the determinations were performed in triplicate and the results were expressed as average values with standard deviation, SD.
3. Results
3.1 Properties of uncoated nanoliposomes
As expected, lipid unilamellar vesicles produced by the simil-microfluidic technique have shown nanodimensions with a reduced PDI (Table 1). D3-loaded nanoliposomes had size properties similar to unloaded ones, unlike K2-loaded nanoliposomes. As visible in Table 1, numerical size and Z-average were about 90 nm and 250 nm, respectively, for both unloaded and D3-loaded nanoliposomes. On the contrary, starting from roughly 90 nm in size for unloaded nanoliposomes, size and PDI values for K2-loaded nanoliposomes were higher, 150 nm and 290 nm, respectively. Looking at the differences in structure of vitamin D3 (MW 384.33 amu) and vitamin K2 (MW 444.7 amu), the aliphatic side chain of isoprenoid residues gives to vitamin K2 a more stretched structure whereas vitamin D3 is more compact. This could lead to a different incorporation of vitamin K2 in the nanoliposome's lipid bilayer. In effect, according to ref. 29, vitamin D3 into the lipid bilayer is able to interchange with phospholipid membrane, causing disordered structures, lower melting temperatures and decrease in the membrane fluidity,36 compared to that of unloaded liposomes.30 found that vitamin D3 is incorporated into the phosphatidylcholine bilayer and intercalated between the hydrocarbon chains of phospholipid molecules, thereby disturbing the gel–liquid crystalline phase transition.37 proved that vitamin K1 (with a similar structure to vitamin K2), in opposition to D3 behaviour, had a limited miscibility with phosphatidylcholine. In particular, the methyl substituents of the phytanoyl chain of K1 prevent its accommodation to the all-trans configurations adopted by the chains of phosphatidylcholine, thus the incorporation of K1 into the gel phase is thermodynamically unfavourable. In effect, at high K1 concentrations, new phase rich in K1 was detected.| Properties | Unloaded nanoliposomes | D3-loaded nanoliposomes | K2-loaded nanoliposomes |
|---|---|---|---|
| EE% ± SD | — | 88.4 ± 2.5 | 94.7 ± 0.8 |
| Numerical size (nm) ± SD | 88.3 ± 19.0 | 87.4 ± 17.3 | 145 ± 32.7 |
| Z-Average (nm) ± SD | 252.8 ± 2.1 | 247.8 ± 1.2 | 289.0 ± 5.6 |
| PDI ± SD | 0.38 ± 0.02 | 0.40 ± 0.07 | 0.32 ± 0.02 |
| Zeta potential (mV) ± SD | −35.4 ± 0.8 | −38.5 ± 1.6 | −36.2 ± 0.3 |
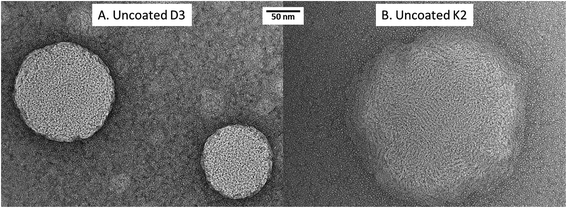
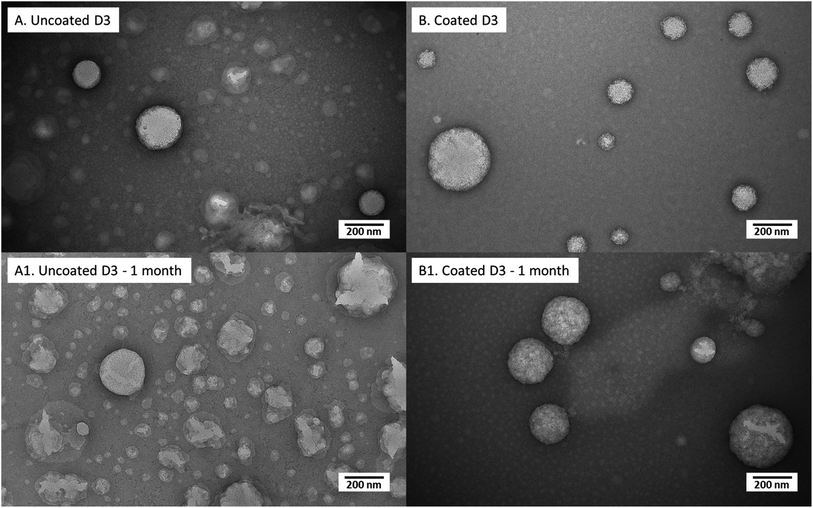
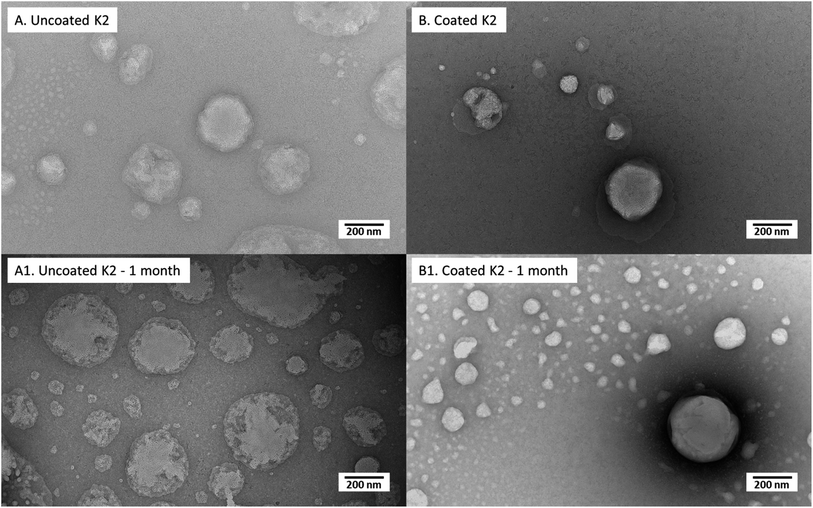
This different structure between vitamin D3 and vitamin K2 loaded nanoliposomes was also visible in TEM pictures (Fig. 1A and B): larger nanoparticles and a more irregular structure were observed for K2-loaded nanoliposomes (Fig. 1B) than D3 ones (Fig. 1A).
For both vitamin D3 and vitamin K2-loaded nanoliposomes, preliminary experiments proved that increasing the vitamin concentration up to 590 μg mL−1 led to much more acceptable values regarding the encapsulation efficiency. In particular, EE was of 88.4% and 94.7%, for vitamin D3 and vitamin K2, respectively (Table 1), values comparable to those reported for similar systems (about 95% for K1-loaded liposomes in ref. 37, and 86% for D3-loaded liposomes in ref. 28). However, increasing the vitamin concentrations to even higher levels did not brought to higher EEs.28 At some point, the increase in vitamin concentration lead to a decrease in EE due to reaching the maximum encapsulation ability of the liposomal structures. Concentrations added beyond the encapsulation ability of the liposomes resulted in free, unencapsulated vitamin molecules.28 Moreover, the fact that the K2 encapsulation was higher than D3 was probably due to the more hydrophobic character of K2 (water solubility in mg l−1: 2.70 × 10−7 for K2, 2.22 × 10−5 for D3).
3.2 Chitosan coverage of liposomes
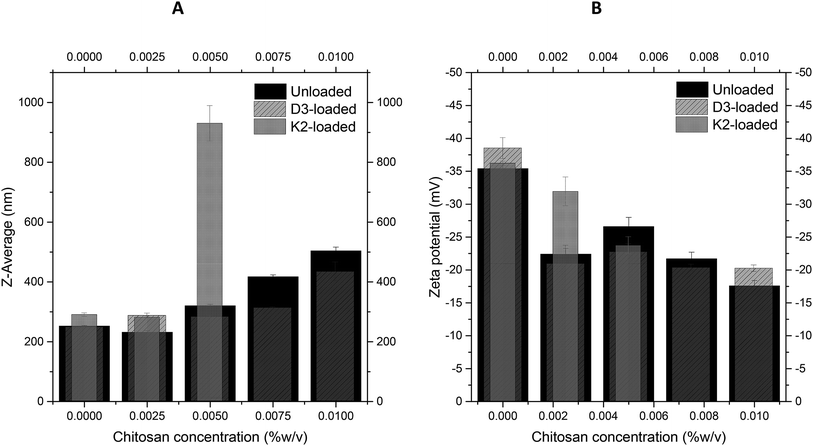
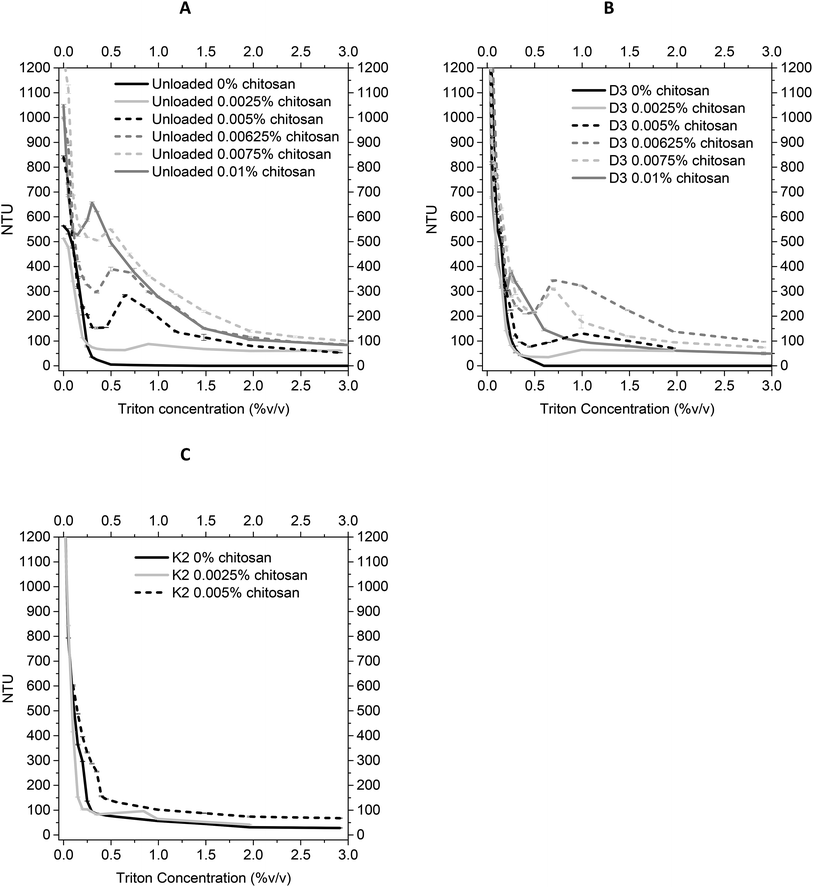
As described in Section 2.2.1, the unloaded, vitamin D3 and vitamin K2 loaded nanoliposomes were coated by using different concentrations of chitosan, varying from 0.0025% to 0.01% (higher concentrations gave aggregation phenomena). For the unloaded and vitamin D3-loaded nanoliposomes, no problems were detected during the coating process. However, the coating of vitamin K2-loaded nanoliposomes, performed with concentrations higher than 0.005% of chitosan, showed visual aggregation, and were thus not suited to work with. The mechanism behind the aggregation of the particles is strongly associated with the concentration of chitosan added to the liposomal suspension. In literature is found that, in order to achieve stable and fully coated liposomes, a chitosan concentration within a specific range must be added to the system. This range reaches from a minimum concentration to the saturation concentration, required to fully cover the liposome's surface. Any excess amount of chitosan, which is not adsorbed on the liposome's surface, will generate an attractive force promoting the aggregation of the particles.34,38 The fact that this aggregation did not occur with the unloaded and vitamin D3 nanoliposomes, suggests that vitamin K2 might interact in a different way with chitosan on the surface of the vesicle, due to the previously detailed differences in structure between vitamin D3 and vitamin K2, which lead not only to a different incorporation of vitamin K2 in the liposome's lipid bilayer, but also induce interaction with chitosan causing a different coverage of the surface.| Chitosan concentration (% w/v) | Numerical size (nm) ± SD | Z-Average (nm) ± SD | PDI ± SD | Zeta potential (mV) ± SD |
|---|---|---|---|---|
| 0 | 88.3 ± 19.0 | 252.8 ± 2.1 | 0.38 ± 0.02 | −35.4 ± 0.8 |
| 0.0025 | 85.8 ± 39.6 | 232.2 ± 4.8 | 0.29 ± 0.02 | −22.4 ± 0.9 |
| 0.005 | 114 ± 32.3 | 320.6 ± 4.2 | 0.36 ± 0.05 | −26.6 ± 1.4 |
| 0.00625 | 94.6 ± 35.7 | 322.5 ± 2.2 | 0.28 ± 0.07 | −18.7 ± 2.0 |
| 0.0075 | 100 ± 30.8 | 417.4 ± 6.2 | 0.29 ± 0.02 | −21.7 ± 1.0 |
| 0.01 | 257 ± 37.6 | 504.4 ± 12.1 | 0.34 ± 0.03 | −17.6 ± 0.8 |
3.3 Properties of polymer–lipid hybrid delivery system
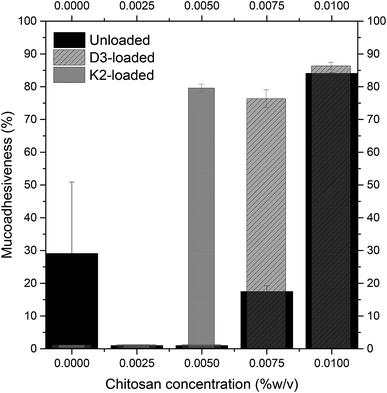
From previous results about the behaviour of size and zeta potential, mucoadhesive properties, and the turbidimetry measurements of the lipid hybrid delivery systems suspensions in function of the chitosan coating degree, 0.01% w/v and 0.005% w/v chitosan concentrations were chosen for covering D3-loaded and K2-loaded nanoliposomes, respectively.| D3 nanoliposomes | D3 chitosan-coated nanoliposomes (0.01% w/v) | K2 nanoliposomes | K2 chitosan-coated nanoliposomes (0.005% w/v) | |
|---|---|---|---|---|
| EE (%) ± SD | 88.4 ± 2.5 | 98.3 ± 0.5 | 94.7 ± 0.8 | 98.2 ± 0.6 |
| Theoretical loading (%) | 10.4 | 10.2 | 10.4 | 10.3 |
| Effective loading (%) | 9.2 ± 0.3 | 10.0 ± 0.0 | 9.8 ± 0.1 | 10.1 ± 0.1 |
D3-loaded nanoliposomes kept intact their structure also after chitosan coverage (Fig. 5); in effect both uncoated (Fig. 5A) and coated ones (Fig. 5B) showed a spherical shape. Moreover, the chitosan layer surrounding liposomes, clearly visualized in TEM photos of a previous work35 in unloaded liposomes, here was not evident perhaps for the perturbation of liposomal structure by D3 insertion, causing a thinner coating thickness (not visible in photo), which could be the reason of the lower stability (with respect to unloaded liposomes) during the previously discussed turbidimetry tests. After 1 month of storage, the uncoated nanoliposomes (Fig. 6A1) showed signs of some disaggregation of the external layer, as confirmed by the increment of Z-average size (from 344 nm at time 0 to 503 nm after 1 month) and of PDI (Table 4). However, it was only a superficial effect because Z-potential was kept unchanged, as well as the encapsulation efficiency (stable at around 88%). Looking at the 0.01% (w/v) coated D3 nanoliposomes stored for 1 month in Fig. 6B1, it is evident that their morphology seems unchanged with respect to freshly prepared liposomes. This behaviour is confirmed by all other properties, i.e. numerical size, Z-average, Z-potential, PDI, encapsulation efficiency and mucoadhesiveness, which remained unchanged (P > 0.05) (Table 4). Thus, also in storage conditions, the chitosan coverage confirmed to impart stability to liposomes, in agreement with previous studies demonstrating that polymers forming a layer around liposomes reduced the oxidation of the lipids and prevented the leakage of drugs.46,47
| Uncoated D3-nanoliposomes | Chitosan-coated D3-nanoliposomes | |||
|---|---|---|---|---|
| Time | t = 0 | t = 1 | t = 0 | t = 1 |
| Size (nm) ± SD | 87.4 ± 17.3 | 117.0 ± 42.6 | 235.1 ± 29.3 | 211.1 ± 76.6 |
| Z-Average (nm) ±S D | 344.0 ± 61.9 | 503.0 ± 10.9 | 434.0 ± 31.5 | 393.9 ± 11.7 |
| PDI ± SD | 0.40 ± 0.07 | 0.50 ± 0.03 | 0.25 ± 0.05 | 0.29 ± 0.04 |
| Zeta potential (mV) ± SD | −38.5 ± 1.6 | −37.2 ± 2.1 | −20.2 ± 0.5 | −18.9 ± 1.5 |
| EE (%) ± SD | 88.4 ± 2.5 | 87.3 ± 0.7 | 98.3 ± 0.5 | 98.4 ± 0.1 |
| Mucoadhesivity ± SD | 0.0 ± 0.0 | 3.2 ± 7.7 | 86.3 ± 1.1 | 88.5 ± 3.5 |
| Uncoated K2-nanoliposomes | Chitosan-coated K2-nanoliposomes | |||
|---|---|---|---|---|
| Time | t = 0 | t = 1 | t = 0 | t = 1 |
| Size (nm) ± SD | 144.8 ± 32.7 | 95.6 ± 33.0 | 278.5 ± 105.0 | 324.7 ± 122.0 |
| Z-Average (nm) ± SD | 289.1 ± 5.6 | 322.9 ± 6.85 | 930.1 ± 59.5 | 726.2 ± 35.0 |
| PDI ± SD | 0.31 ± 0.02 | 0.37 ± 0.03 | 0.71 ± 0.09 | 0.50 ± 0.09 |
| Zeta potential (mV) ± SD | −36.2 ± 0.3 | −36.6 ± 3.0 | −23.7 ± 1.3 | −21.1 ± 0.6 |
| EE (%) ± SD | 94.7 ± 0.8 | 93.8 ± 0.4 | 98.2 ± 0.6 | 95.2 ± 0.2 |
| Mucoadhesivity ± SD | 0.0 ± 0.0 | 4.0 ± 5.4 | 79.5 ± 1.2 | 77.8 ± 0.4 |
Similarly, to D3-loaded nanoliposomes, the morphology (Fig. 6) and properties (Table 4) of uncoated and 0.005% (w/v) coated vitamin K2 nanoliposomes right after preparation and after 1 month of storage were compared. The TEM images of just produced samples (Fig. 6A) showed spherical particles with some superficial defects due to vitamin K2 presence. The behaviour of uncoated K2-loaded nanoliposomes after 1 month of storage showed no significant change in number size, but an increase in Z-average and PDI (P < 0.05) (Table 4), confirmed by a kind of swelling of liposome matrix visible in TEM image (Fig. 6A1). However, the zeta potential was not affected, together with the encapsulation efficiency and the mucoadhesiveness, which both remained unchanged (P > 0.05) (Table 4). The 0.005% (w/v) chitosan coated K2 nanoliposomes showed irregular coating of liposomes surface with more aggregates (Fig. 6B), explaining the high Z-average (930 nm) and PDI (0.7) values (Table 4). The storage for 1 month did not cause change in number size over time (P > 0.05), as well as for the encapsulation efficiency and the mucoadhesiveness. However, the Z-average and PDI decreased, as well as a little bit the zeta potential (P < 0.05) (Table 4). This is an indication of the stabilization of the suspension over time, becoming more monodisperse, as confirmed by TEM image (Fig. 6B1), which shows more regular structures compared to the freshly prepared sample (Fig. 6B). Moreover, by comparing images of uncoated (Fig. 6A1) and coated (Fig. 6B1) K2-loaded nanoliposomes, appearing uneven and compact, respectively, it is evident that also in this case chitosan acts as protection against degradation over time.
4. Conclusions
Nanoliposomal carriers and chitosan coated nanoliposomes, encapsulating vitamin D3 and vitamin K2, were both successfully produced by the simil-microfluidic technique, with the advantages of massive production, operating at environmental conditions and continuously.Investigation on uncoated nanoliposomes showed high encapsulation efficiencies, especially for vitamin K2 (EE: 95%) due to its more hydrophobic character. Uncoated K2 an D3 loaded nanoliposomes have been shown poor mucoadhesive characteristics thus chitosan coating was performed to overcome this issue. The coverage efficacy was proven to be dependent on chitosan concentrations and on kind of enwrapped vitamin in the liposomal structure. In this study, the best coverage was obtained with 0.01% w/v chitosan for unloaded and D3-loaded liposomes, and with 0.005% per K2-loaded liposomes. Moreover, the best chitosan coverage for each liposomal formulation has led an increase of the entrapment efficiency from 88% to 98% for D3-loaded liposomes and from 95% to 98% for K2-loaded ones. This enhancement is occurred, reasonably, due to the fact that during the coating process, chitosan covers the surface of the liposomes and fills the gaps in the hydrophobic bilayer.
After 1 month of storage, the uncoated nanoliposomes (both of D3 and K2) showed signs of some disaggregation of the external layer (as confirmed by TEM photographs and by increments of Z-average and PDI) but it has been ascertained that it was only a superficial effect because Z-potential kept unchanged, as well as the encapsulation efficiency. After the same period of storage, the 0.01% w/v coated D3-loaded liposomes and the 0.005% w/v coated K2-nanoliposomes assured better stability than the uncoated structures. Coated nanoliposomes morphology and all other properties (i.e. numerical size, Z-average, Z-potential, PDI, encapsulation efficiency and mucoadhesiveness), indeed, remained unchanged with respect to freshly prepared ones.
At last, it can be concluded that by the novel developed technique and the optimized chitosan coverage, very stable and mucoadhesive polymer–lipid hybrid nanoparticles, encapsulating vitamin D3 and vitamin K2, are produced as micronutrients delivery systems for potential nutraceutical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Part of the present work has been done within the funded project “Campania Oncoterapie” – POR FESR 2014–2020 – D. D. n. 4 22/01/2019. The authors thank the Centro di Microscopia Elettronica – University of Trieste – Italy.References
- B. Hoeft, P. Weber and M. Eggersdorfer, Micronutrients - a global perspective on intake, health benefits and economics, Int. J. Vitam. Nutr. Res., 2012, 82(5), 316–320 CrossRef CAS PubMed.
- C. M. Chaparro and K. G. Dewey, Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings, Matern. Child Nutr., 2010, 6(Suppl 1), 1–69 CrossRef PubMed.
- N. Gueli, W. Verrusio, A. Linguanti, F. Di Maio, A. Martinez and B. Marigliano, et al. Vitamin D: drug of the future. A new therapeutic approach, Arch. Gerontol. Geriatr., 2012, 54(1), 222–227 CrossRef CAS PubMed.
- I. Katouzian and S. M. Jafari, Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins, Trends Food Sci. Technol., 2016, 53, 34–48 CrossRef CAS.
- S. Bochicchio, A. A. Barba, G. Grassi and G. Lamberti, Vitamin delivery: carriers based on nanoliposomes produced via ultrasonic irradiation, LWT--Food Sci. Technol., 2016, 69, 9–16 CrossRef CAS.
- M. C. Braithwaite, P. Kumar, Y. E. Choonara, L. C. du Toit, L. K. Tomar and C. Tyagi, et al. A novel multi-tiered experimental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solubility and stability, Int. J. Pharm., 2017, 532(1), 90–104 CrossRef CAS PubMed.
- S. Bochicchio, A. Dalmoro, A. Angela Barba, G. Grassi and G. Lamberti, Liposomes as siRNA delivery vectors, Curr. Drug Metab., 2014, 15(9), 882–892 CrossRef CAS PubMed.
- S. Bochicchio, M. Sala, A. Spensiero, M. C. Scala, I. M. Gomez-Monterrey and G. Lamberti, et al. On the design of tailored liposomes for KRX29 peptide delivery, New J. Chem., 2017, 41(19), 11280–11290 RSC.
- S. Bochicchio, B. Dapas, I. Russo, C. Ciacci, O. Piazza and S. De Smedt, et al. In vitro and ex vivo delivery of tailored siRNA-nanoliposomes for E2F1 silencing as a potential therapy for colorectal cancer, Int. J. Pharm., 2017, 525(2), 377–387 CrossRef CAS PubMed.
- H. Singh, A. Thompson, W. Liu and M. Corredig, Liposomes as food ingredients and nutraceutical delivery systems, in Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals, ed. N. Garti and D. J. McClements, Woodhead Publishing, 2012, pp. 287–318 Search PubMed.
- A. Barba, S. Bochicchio, G. Lamberti and A. Dalmoro, Ultrasonic energy in liposome production: process modelling and size calculation, Soft Matter, 2014, 10(15), 2574–2581 RSC.
- N. Liu and H.-J. Park, Chitosan-coated nanoliposome as vitamin E carrier, J. Microencapsulation, 2009, 26(3), 235–242 CrossRef CAS PubMed.
- C. Caddeo, O. Díez-Sales, R. Pons, C. Carbone, G. Ennas and G. Puglisi, et al. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin, J. Colloid Interface Sci., 2016, 461, 69–78 CrossRef CAS PubMed.
- T. Klemetsrud, A.-L. Kjøniksen, M. Hiorth, J. Jacobsen and G. Smistad, Polymer coated liposomes for use in the oral cavity – a study of the in vitro toxicity, effect on cell permeability and interaction with mucin, J. Liposome Res., 2018, 28(1), 62–73 CrossRef CAS PubMed.
- Z. Jiao, X. Wang, Y. Yin, J. Xia and Y. Mei, Preparation and evaluation of a chitosan-coated antioxidant liposome containing vitamin C and folic acid, J. Microencapsulation, 2018, 1–9 Search PubMed.
- G. H. Shin, S. K. Chung, J. T. Kim, H. J. Joung and H. J. Park, Preparation of chitosan-coated nanoliposomes for improving the mucoadhesive property of curcumin using the ethanol injection method, J. Agric. Food Chem., 2013, 61(46), 11119–11126 CrossRef CAS PubMed.
- M. Hasan, G. B. Messaoud, F. Michaux, A. Tamayol, C. Kahn and N. Belhaj, et al. Chitosan-coated liposomes encapsulating curcumin: study of lipid–polysaccharide interactions and nanovesicle behavior, RSC Adv., 2016, 6(51), 45290–45304 RSC.
- M. Wang, M. Liu, T. Xie, B.-F. Zhang and X.-L. Gao, Chitosan-modified cholesterol-free liposomes for improving the oral bioavailability of progesterone, Colloids Surf., B, 2017, 159, 580–585 CrossRef CAS PubMed.
- H. Refai, D. Hassan and R. Abdelmonem, Development and characterization of polymer-coated liposomes for vaginal delivery of sildenafil citrate, Drug Delivery, 2017, 24(1), 278–288 CrossRef CAS PubMed.
- M. M. Mady and M. M. Darwish, Effect of chitosan coating on the characteristics of DPPC liposomes, J. Adv. Res., 2010, 1(3), 187–191 CrossRef.
- A. A. Barba, A. Dalmoro, M. d'Amore and G. Lamberti, Liposoluble vitamin encapsulation in shell–core microparticles produced by ultrasonic atomization and microwave stabilization, LWT--Food Sci. Technol., 2015, 64(1), 149–156 CrossRef CAS.
- C. Vermeer, K.-S. G. Jie and M. H. J. Knapen, Role of Vitamin K in Bone Metabolism, Annu. Rev. Nutr., 1995, 15(1), 1–21 CrossRef CAS PubMed.
- A. J. Van Ballegooijen, S. Pilz, A. Tomaschitz, M. R. Grübler and N. Verheyen, The synergistic interplay between vitamins D and K for bone and cardiovascular health: a narrative review, Int. J. Endocrinol., 2017, 2017, 7454376 Search PubMed.
- G. K. Schwalfenberg, Vitamins K1 and K2: the emerging group of vitamins required for human health, J. Nutr. Metab., 2017, 2017, 6254836 Search PubMed.
- S. H. Je, N.-S. Joo, B.-h. Choi, K.-M. Kim, B.-T. Kim and S.-B. Park, et al. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old, J. Korean Med. Sci., 2011, 26(8), 1093–1098 CrossRef CAS PubMed.
- A. Abbasi, Z. Emam-Djomeh, M. A. E. Mousavi and D. Davoodi, Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate, Food Chem., 2014, 143, 379–383 CrossRef CAS PubMed.
- E. Almouazen, S. Bourgeois, L. P. Jordheim, H. Fessi and S. Briançon, Nano-encapsulation of Vitamin D3 Active Metabolites for Application in Chemotherapy: Formulation Study and In Vitro Evaluation, Pharm. Res., 2013, 30(4), 1137–1146 CrossRef CAS PubMed.
- S. J. Park, C. V. Garcia, G. H. Shin and J. T. Kim, Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3, Food Chem., 2017, 225, 213–219 CrossRef CAS PubMed.
- M. Mohammadi, B. Ghanbarzadeh and H. Hamishehkar, Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification, Adv. Pharm. Bull., 2014, 4(Suppl 2), 569 Search PubMed.
- K. Merz and B. Sternberg, Incorporation of vitamin D3-derivatives in liposomes of different lipid types, J. Drug Targeting, 1994, 2(5), 411–417 CrossRef CAS PubMed.
- V. Kirilenko and G. Gregoriadis, Fat soluble vitamins in liposomes: studies on incorporation efficiency and bile salt induced vesicle disintegration, J. Drug Targeting, 1993, 1(4), 361–368 CrossRef CAS PubMed.
- D. Neuls and M. Neves, Calcium supplements, US20060223730A1, 2009.
- S. Bochicchio, A. Dalmoro, F. Recupido, G. Lamberti and A. Barba, Nanoliposomes production by a protocol based on a simil-microfluidic approach, Advances in Bionanomaterials, Springer, 2017 Search PubMed.
- A. Dalmoro, S. Bochicchio, S. F. Nasibullin, P. Bertoncin, G. Lamberti and A. A. Barba, et al. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems, Eur. J. Pharm. Sci., 2018, 121, 16–28 CrossRef CAS PubMed.
- S. Bochicchio, A. Dalmoro, P. Bertoncin, G. Lamberti, R. I. Moustafine and A. A. Barba, Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: conventional and next-generation approaches, RSC Adv., 2018, 8(60), 34614–34624 RSC.
- A. Elgavish, J. Rifkind and B. Sacktor, In vitro effects of vitamin D3 on the phospholipids of isolated renal brush border membranes, J. Membr. Biol., 1983, 72(1–2), 85–91 CrossRef CAS PubMed.
- A. Ortiz, J. Villalaln and J. C. Gòmez-Fernàndez, The interaction of vitamin K1 with phospholipid vesicles, Biochim. Biophys. Acta, Biomembr., 1986, 863, 185–192 CrossRef CAS.
- C. Laye, D. J. McClements and J. Weiss, Formation of Biopolymer-Coated Liposomes by Electrostatic Deposition of Chitosan, J. Food Sci., 2008, 73(5), N7–N15 CrossRef CAS PubMed.
- S. Pistone, M. Rykke, G. Smistad and M. Hiorth, Polysaccharide-coated liposomal formulations for dental targeting, Int. J. Pharm., 2017, 516(1), 106–115 CrossRef CAS PubMed.
- J. Filipović-Grčić, N. Škalko-Basnet and I. Jalšienjak, Mucoadhesive chitosan-coated liposomes: characteristics and stability, J. Microencapsulation, 2001, 18(1), 3–12 CrossRef PubMed.
- H. Takeuchi, Y. Matsui, H. Yamamoto and Y. Kawashima, Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats, J. Controlled Release, 2003, 86(2), 235–242 CrossRef CAS PubMed.
- A. Haeri, S. Sadeghian, S. Rabbani, M. S. Anvari, S. Ghassemi and F. Radfar, et al. Effective attenuation of vascular restenosis following local delivery of chitosan decorated sirolimus liposomes, Carbohydr. Polym., 2017, 157, 1461–1469 CrossRef CAS PubMed.
- M. M. Mady, M. M. Darwish, S. Khalil and W. M. Khalil, Biophysical studies on chitosan-coated liposomes, Eur. Biophys. J., 2009, 38(8), 1127–1133 CrossRef CAS PubMed.
- C. Tan, J. Xue, K. Eric, B. Feng, X. Zhang and S. Xia, Dual effects of chitosan decoration on the liposomal membrane physicochemical properties as affected by chitosan concentration and molecular conformation, J. Agric. Food Chem., 2013, 61(28), 6901–6910 CrossRef CAS PubMed.
- P. R. Karn, Z. Vanić, I. Pepić and N. Škalko-Basnet, Mucoadhesive liposomal delivery systems: the choice of coating material, Drug Dev. Ind. Pharm., 2011, 37(4), 482–488 CrossRef CAS PubMed.
- L. Li, Y. Zhang, S. Han, Z. Qu, J. Zhao and Y. Chen, et al. Penetration enhancement of lidocaine hydrochloride by a novel chitosan coated elastic liposome for transdermal drug delivery, J. Biomed. Nanotechnol., 2011, 7(5), 704–713 CrossRef CAS PubMed.
- T. X. Nguyen, L. Huang, L. Liu, A. M. E. Abdalla, M. Gauthier and G. Yang, Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride, J. Mater. Chem. B, 2014, 2(41), 7149–7159 RSC.
| This journal is © The Royal Society of Chemistry 2019 |