Open Access Article
Open Access ArticlePreparation of disperse inks for direct inkjet printing of non-pretreated polyester fabrics
Chengyong Gao ,
Tieling Xing
,
Tieling Xing ,
Xueni Hou and
Guoqiang Chen*
,
Xueni Hou and
Guoqiang Chen*
National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215000, China. E-mail: chenguojiang@suda.edu.cn
First published on 25th June 2019
Abstract
The preparation of disperse inks for direct inkjet printing was carried out using disperse dye, dispersant (TD-1109, hydrophilic polyacrylic acid block copolymer), wetting agent (diethylene glycol and ethylene glycol), water-based polymers (polyvinyl alcohol (PVA) or waterborne polyester), deionized water, etc. The suitability of disperse inks for polyester fabrics was assessed via measurement of conductivity, pH, particle size surface tension and viscosity. The theological behavior of disperse inks was evaluated by rotational viscometry; the disperse inks had the characteristics of a Newtonian fluid. The inkjet printability of disperse inks was investigated by monitoring droplet formation dynamics, where the viscosity and surface tension of disperse ink were 2.78 mPa s and 33.28 mN m−1, respectively. The disperse ink has excellent ejection performance. The printing accuracy of disperse inks was evaluated by inkjet printing quality of lines on untreated polyester fabrics. The fastness properties of printed polyester fabrics were also evaluated and they presented excellent color fastness. Compared with different printed polyester fabrics, the polyester fabrics printed with disperse ink made from water-based polymers have high color strength (K/S) and saturation (C*) values.
Introduction
Inkjet printing on textiles can give subtle, delicate images on fabrics using a high-resolution device and printing system without the need for masks. Now, direct inkjet printing is one of the fastest growing printing technologies in the textile industry1–4 due to it having more advantages compared with flat-screen printing and transfer inkjet printing, such as environmental protection, quick proofing, quick response times, mass customization and less manpower and time.5–9Accurate printing and color quality are extremely important for direct inkjet printing due to ink diffusion on the textile.3,9 Direct inkjet printing of disperse inks must be carefully formulated to yield specific properties enabling optimum droplet formulation within the fine nozzles of a printing head for application at high speed. Such properties are dependent on the optimal particle size distribution, surface tension, viscosity and the physical fluid properties of the ink.10,11 Water-based disperse inkjet inks offer environmental advantages over their nonaqueous-based counterparts through reducing the emission of volatile organic compounds in solvent-based formulations.1 The disperse inks typically contain 30–80 wt% deionized water as a component of the total mass of the ink, with a water-miscible organic solvent such as a wetting agent (e.g. alcohols, 5–25 wt%), colorant (e.g. disperse dye, the content range of disperse dye is 0.3–15 wt% in the ink), and dispersant (2–5 wt%), which acts as dispersant in the case of disperse dyes in aqueous medium.1,2,9,10,12,13
Most textile substrates can be digitally printed, including polyester, cotton, silk and nylon.9,14–16 Among them, polyester fabric is considered to be one of the most widely applied synthetic fibers for inkjet printing due to its advantages, such as high production, superior mold and insect resistance, higher strength and excellent resilience.9 Beautiful designs are possible on polyester textiles, which can be used as curtains and sheets, automotive upholstery, flags and banners, T-shirts, promotional material, etc. In order to keep the beautiful and distinct patterns on the textile substrates, inks designed for inkjet printing on textile materials need to satisfy various fastness criteria, namely washing, rubbing, light and sublimation color fastness.1,2 Before direct inkjet printing, polyester fabrics are usually pretreated to improve the printing accuracy and color strength of the fabric. ZhaoY. et al.9 pretreated polyester fabrics using a cationic modification agent (B-1), sodium alginate (SA), and polyvinyl alcohol (PVA) to improve the printing accuracy. B-Cyclodextrin and citric acid were also used to modify the polyester fabric for inkjet printing and the printed fabric exhibited excellent color strength and printing accuracy.17 However, the printed fabric has to be washed after inkjet printing, producing lots of sewage, which causes ecological environmental pollution and affect human health and quality of life.
In the process of direct inkjet printing, in order to control the printing effect, the ink droplets are accurately inkjeted in the ideal area by adjusting the viscosity of the disperse ink, and the viscosity of the disperse ink is regulated via the diffusion of the ink on the untreated polyester fabrics. The fixation of disperse dyes on untreated polyester fabric is the key to constructing a printing system for direct inkjet washing-free disperse ink for untreated polyester fabrics.
Experimental section
Materials
Disperse magenta 896 (M) was provided by Zhejiang Wanfeng Chemical Co., Ltd (Zhejiang, China). Disperse yellow 54 (Y) and disperse blue 359 (B) were provided by Shanghai Anruoqi Chemical Co., Ltd (Shanghai, China). Dispersing agent (TD-1109, hydrophilic polyacrylic acid block copolymer) was supplied by Shanghai Jiuyu Chemical Co., Ltd (Shanghai, China). Polyvinyl alcohol (1788 low-viscosity) and triethanolamine (TEOA, pH regulator) were supplied by Macklin Biochemical Co., Ltd (Shanghai, China). Waterborne polyester was provided by Yangzhou Xinghui Fiber Material Co., Ltd (Yangzhou, China). Diethylene glycol (DEG, wetting agent) was purchased from Alfa Aesar Co., Ltd (Shanghai, China). Ethylene glycol (EG, wetting agent) was supplied by Sinopharm Chemical Beijing Reagent Co., Ltd (Beijing, China). Organosilicon defoamer was bought from Shanghai BBI Co., Ltd (Shanghai, China).Preparation of disperse dye paste
Disperse dyes (16.7 wt%), dispersing agent (TD-1109, 9.2 wt%), diethylene glycol (16.7 wt%) and deionized water (57.4 wt%) were accurately weighed and added into an agate mortar with 100 g of 0.4–0.6 mm diameter zirconia beads. Grinding was performed using a planetary ball mill (NETZSCH Group, Germany) at a 800 rpm for 3 h.Formulation of disperse ink for direct inkjet printing
The ink formulation on a weight basis is given in Table 1. Disperse dye, dispersing agent (TD-1109), DEG, water-based polymers (PVA or waterborne polyester), EG, organosilicon defoamer, TEOA and deionized water were mixed under stirring at 1000 rpm at 25 °C until a homogeneous dispersion was obtained, and then filtered with a 0.22 μm filter membrane by vacuum pump.| Sample | Disperse dye (wt%) | TD-1109 (wt%) | DEG (wt%) | Water-based polymers (wt%) | EG (wt%) | Organosilicon defoamer (wt%) | TEOA (wt%) | Water (wt%) |
|---|---|---|---|---|---|---|---|---|
| a Water-based polymers (a: polyvinyl alcohol; b: waterborne polyester). | ||||||||
| 1-1 | 5.01 | 2.76 | 5.01 | 0.0 | 22.5 | 0.5 | 0.05 | 64.17 |
| 1-2a | 5.01 | 2.76 | 5.01 | 0.5 | 17.5 | 0.5 | 0.05 | 68.67 |
| 1-3a | 5.01 | 2.76 | 5.01 | 0.25 | 20.0 | 0.5 | 0.05 | 66.42 |
| 1-4a | 5.01 | 2.76 | 5.01 | 1.0 | 12.5 | 0.5 | 0.05 | 73.17 |
| 1-5a | 5.01 | 2.76 | 5.01 | 1.5 | 7.5 | 0.5 | 0.05 | 77.67 |
| 1-2b | 5.01 | 2.76 | 5.01 | 0.5 | 17.5 | 0.5 | 0.05 | 68.67 |
| 1-3b | 5.01 | 2.76 | 5.01 | 0.25 | 20.0 | 0.5 | 0.05 | 66.42 |
| 1-4b | 5.01 | 2.76 | 5.01 | 1.0 | 12.5 | 0.5 | 0.05 | 73.17 |
| 1-5b | 5.01 | 2.76 | 5.01 | 1.5 | 7.5 | 0.5 | 0.05 | 77.67 |
| 1-6b | 5.01 | 2.76 | 5.01 | 2.0 | 2.5 | 0.5 | 0.05 | 82.17 |
Preparation of polyester fabrics
Polyester fabrics (Twill, 217 g m−2) were washed with a commercial detergent, rinsed with clean water, and dried in an ambient atmosphere. The polyester fabrics were ironed until a smooth surface was obtained. In order to print textile substrates, it is necessary to assure polyester fabric stability in printer feed rollers. Therefore, a polyester fabric sample was held on a piece of A4 printing plastic sheet with conventional adhesive tape.Digital printing
Pattern printing was performed using an Epson digital printer R230 (Seiko Epson Corporation, Japan) at 20 °C. The line (linewidth, 353 μm) on the fabric was printed in warp and filling directions to evaluate the printing quality and color fastness, respectively. The printed polyester fabrics were subsequently thermo fixed by treatment in a hot air oven at 110 °C for 30 s and post-treatment at 180 °C for 1 min.Characterization
The Z-average size of the disperse ink was determined by a nanoparticle size and Zeta potential analyzer (Nano-ZS90, Malvern Instruments Co., Ltd, UK) at 25 °C. The surface tensions of the samples were measured using an automatic surface tension meter (DCAT21, KINO Instruments Co., Ltd., USA) fitted with a De Nouy small platinum ring at 20 °C. The viscosity of the disperse ink was measured using a rotational viscometer (Rhel lab QC, Anton Paar Instruments Co., Ltd., CHN) at 20 °C with a speed at 60 rpm. The relationship between the ink viscosity and temperature was tested in the range of 20–40 °C with speed of 60 rpm and heating rate of 1 °C min−1. The relationship between the ink viscosity and shear rate was observed in the range of 10–1000 s−1 at 20 °C. The pH of the ink was measured by pH meter (PHS-3C, Shanghai Instrument Science Instrument Co., Ltd, CHN). The conductivity of the ink was detected by conductivity meter (ST3100C, OHAUS Instrument Co., Ltd, CNH).A jetxpert analytical instrument (JX-BT, 220 v, 50 Hz, Suzhou Sigmatek Trading Co., Ltd, China) was used to monitor the jetting behavior of the disperse ink at 20 °C. The inkjet printing system consisted of a jetting driver (piezoelectric type, a waveform was kept), a print head (EPSON DX5, 8 nozzle replaceable cartridge producing approximately 5 pL drop volume) and a charge-coupled device camera (CCD; with an inter frame time of 1 μs). The jetxpert analytical instrument coupled with a drop volume measurement module was implemented to determine characteristics such as drop shape, drop velocity, drop volume, ligament length and drop trajectory.
The printing accuracy of the disperse inks was evaluated from the super depth of a field microscope under the conditions of 150× magnification (SDFM) (Keyence vhx-1000, Japan Kenshi Co., Ltd.). The morphology of the sample was observed by field emission scanning electron microscope (FESEM) (TM-3030, Hitachi, Co., Ltd, Japan). The reflectance values of the printed polyester fabrics were measured using an Ultra Scan HunterLab K/S (USA) under D65 illumination. The spectrum scan range was 350–700 nm, and the samples were folded four times and four measurements were performed each time. Color fastness upon washing at 60 °C was tested in accordance with ISO 20105-C03:1992. Color fastness to crocking was examined according to AATCC Test Method 8-2007 using an Y571 crocking fastness tester, provided by Electron Instrument Co., Ltd, Laizhou, China.
Results and discussion
Physical properties of the disperse inks
Fig. 1(a) represents the morphology of original particle sizes of disperse dye. It can be seen that the original particles of disperse dye were obviously big and agglomerated particles. According to Fig. 1(b), the average particle size of disperse dye was about 150–180 nm, and the particle size was distributed evenly with no aggregation, which was beneficial to the preparation of the ink. The reason for this is that polymer dispersant (TD-1109) is insensitive to environmental temperature, pH and impurity ions in the system and has a good dispersion effect. The dispersion system relies on multiple anchoring groups on the polymer segment to closely absorb onto the surface of disperse dye, with its long solvated chains fully stretched in the liquid phase. The steric hindrance effect allows the dispersion system to maintain high stability.18,19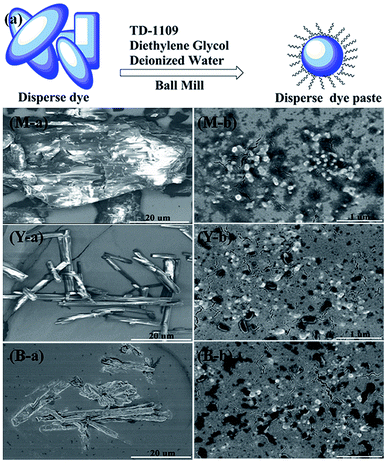 | ||
| Fig. 1 Grinding mechanism of disperse dye paste (a), M-a, Y-a, B-a (original disperse dyes) and M-b, Y-b, B-b (grinding disperse dyes). | ||
The suitability of the disperse inks for polyester fabrics was evaluated via measurement of the viscosity, surface tension, conductivity, pH and particle size,2,11,20–22 the measurements of which are shown in Table 2. The viscosity of the disperse inks was in the range of 1.2–3.2 mPa s. It is well known that more additive (hydroxyl groups in their chemical structure) in the disperse ink help to increase the interaction forces (van der Waals forces, hydrogen bonds) with water and reduce the amount of free water in the system.3 The viscosity of disperse inks increased with an increase in the content of either PVA or DEG. However, the viscosity of the disperse ink with waterborne polyester decreased with a decrease in the EG content. The surface tensions of the disperse inks remained relatively constant at 32 mN m−1, for the concentration of dispersant (TD-1109) to reach the critical micelle concentration (CMC). The pH values of the disperse inks were at around 8.2, mainly influenced by TEOA. The conductivity is related to the salt content of the disperse ink. If the salt content is too high, salt crystals will precipitate in the disperse ink and it may block the nozzle during printing. The Z-average size inks were lower than 200 nm. Typical inkjet ink should have a surface tension of 21–50 mN m−1, a viscosity of 1–25 mPa s,2,21–23 a pH in the range of 6–9, a conductivity of around 500 mS cm−1, and a Z-average size of between 100 and 250 nm.2,10,23 From Table 2, it can be seen that all of the properties measured are within the operational range for disperse inkjet ink.
| Sample | Viscosity (mPa s) | Conductivity (mS cm−1) | pH | Surface tension (mN m−1) | Z-Average size (nm) |
|---|---|---|---|---|---|
| CI disperse magenta 896 | |||||
| 1-1 | 2.60 | 0.58 | 8.28 | 31.79 | 153.6 |
| 1-2a | 2.33 | 0.58 | 8.30 | 31.09 | 157.9 |
| 1-3a | 2.78 | 0.57 | 8.23 | 33.28 | 166.7 |
| 1-4a | 3.05 | 0.58 | 8.14 | 32.46 | 146.5 |
| 1-5a | 3.24 | 0.58 | 8.20 | 32.22 | 161.7 |
| 1-2b | 1.98 | 0.58 | 8.13 | 32.23 | 145.4 |
| 1-3b | 2.13 | 0.58 | 8.17 | 32.45 | 148.2 |
| 1-4b | 1.72 | 0.58 | 8.15 | 32.82 | 147.6 |
| 1-5b | 1.45 | 0.57 | 8.21 | 32.32 | 147.2 |
| 1-6b | 1.25 | 0.58 | 8.19 | 32.95 | 149.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CI disperse yellow 54 | |||||
| 1-1 | 2.42 | 0.56 | 8.02 | 31.89 | 186.1 |
| 1-2a | 2.25 | 0.58 | 8.15 | 31.69 | 185.4 |
| 1-3a | 2.81 | 0.56 | 8.31 | 32.28 | 175.1 |
| 1-4a | 3.15 | 0.58 | 8.23 | 31.86 | 171.5 |
| 1-5a | 3.24 | 0.57 | 8.19 | 32.22 | 182.8 |
| 1-2b | 2.13 | 0.56 | 8.08 | 31.73 | 184.9 |
| 1-3b | 2.29 | 0.58 | 8.26 | 31.85 | 183.0 |
| 1-4b | 1.80 | 0.59 | 8.31 | 32.32 | 183.2 |
| 1-5b | 1.55 | 0.58 | 8.17 | 32.52 | 179.8 |
| 1-6b | 1.32 | 0.58 | 8.26 | 32.15 | 182.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CI disperse blue 359 | |||||
| 1-1 | 2.54 | 0.58 | 8.14 | 32.29 | 185.4 |
| 1-2a | 2.45 | 0.57 | 8.21 | 31.89 | 175.1 |
| 1-3a | 2.82 | 0.58 | 8.17 | 32.48 | 171.5 |
| 1-4a | 3.08 | 0.57 | 8.18 | 32.49 | 182.8 |
| 1-5a | 3.17 | 0.58 | 8.25 | 32.32 | 177.5 |
| 1-2b | 2.16 | 0.56 | 8.30 | 32.33 | 181.3 |
| 1-3b | 2.23 | 0.58 | 8.26 | 31.85 | 187.5 |
| 1-4b | 1.75 | 0.57 | 8.18 | 31.89 | 181.2 |
| 1-5b | 1.52 | 0.57 | 8.28 | 32.22 | 183.3 |
| 1-6b | 1.28 | 0.58 | 8.23 | 32.45 | 179.2 |
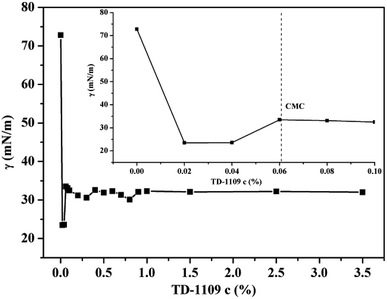
As Table 2 indicates the surface tensions of the disperse inks, which are approximately 32 mN m−1. The surface tension of the disperse ink is mainly controlled by dispersant (TD-1109), the concentration of which in the disperse ink was 2.76 wt%. Fig. 2 shows that with an increase in the dispersant concentration, the surface tension of the TD-1109 solution gradually reduced. When the concentration reached the critical micelle concentration (CMC, 0.06%), the surface tension of the solution tended to be steady (32 mN m−1).
Fig. 3(a) shows that the viscosity of the above ink increased gradually with an increase in the shear rate (10–100 s−1), which presents the characteristics of a dilatant fluid.24 When the shear rate reaches 100 s−1, the viscosity tends to be stable. Fig. 3(b) shows the rheological properties of the disperse inks, and the shear-rate increases almost linearly with the shear stress. Table 3 gives the data of the linear fitting, which predicts the ink to be a Newtonian fluid (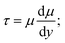 τ, shear stress; μ, viscosity;
τ, shear stress; μ, viscosity; 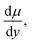 shearing deformation).25 The intercept was caused by the ink concentration, particle and temperature, and so on.
shearing deformation).25 The intercept was caused by the ink concentration, particle and temperature, and so on.
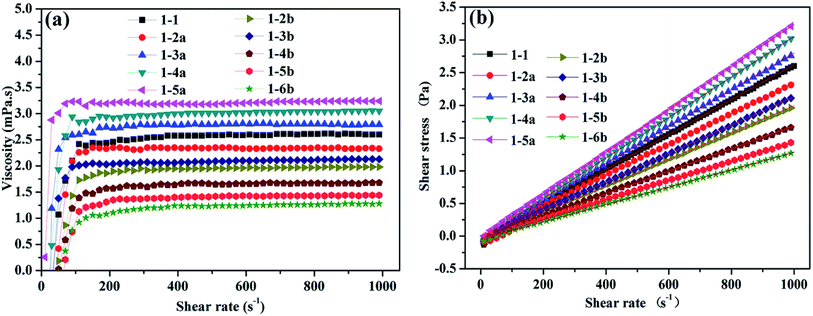 | ||
| Fig. 3 (a) The correlation between disperse ink shear rate and viscosity and (b) shear rate and stress. | ||
| Sample | Fitting equation | R2 |
|---|---|---|
| 1-1 | y = 0.00267x − 0.0453 | 0.9997 |
| 1-2a | y = 0.00238x − 0.0287 | 0.9989 |
| 1-3a | y = 0.00282x − 0.0200 | 0.9998 |
| 1-4a | y = 0.00309x − 0.0402 | 0.9998 |
| 1-5a | y = 0.00325x − 0.0171 | 0.9999 |
| 1-2b | y = 0.00204x − 0.0501 | 0.9989 |
| 1-3b | y = 0.00216x − 0.0343 | 0.999 |
| 1-4b | y = 0.00173x − 0.0477 | 0.9984 |
| 1-5b | y = 0.0015x − 0.0513 | 0.9985 |
| 1-6b | y = 0.0033x − 0.0511 | 0.9993 |
It is well known that the viscosity of Newtonian fluids is only affected by temperature. It can be observed from Fig. 4 that the temperature has a great influence on the viscosity and shear stress of the disperse ink, respectively. The viscosity of the disperse inks decreases gradually with an increase in the temperature. When the temperature of the ink is 20 °C, the viscosity of the disperse ink (1-1, 1-2a-1-5a) is in the range of 2.2–3.2 mPa s, which is related to the addition of PVA and EG. However, the viscosity of the disperse ink (1-2b-1-6b) is less than 1-2a-1-5a. The reason for this is that the viscosity of PVA (10 wt% aqueous solution, 20 °C, 0.637 Pa s) is higher than that of waterborne polyester (10 wt% aqueous solution, 20 °C, 0.0023 Pa s).
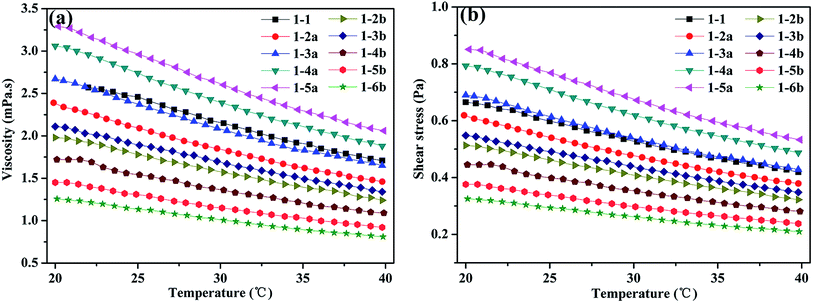 | ||
| Fig. 4 Relationship between the (a) viscosity and temperature and (b) shear stress and temperature of disperse ink. | ||
Disperse inks for inkjet processing
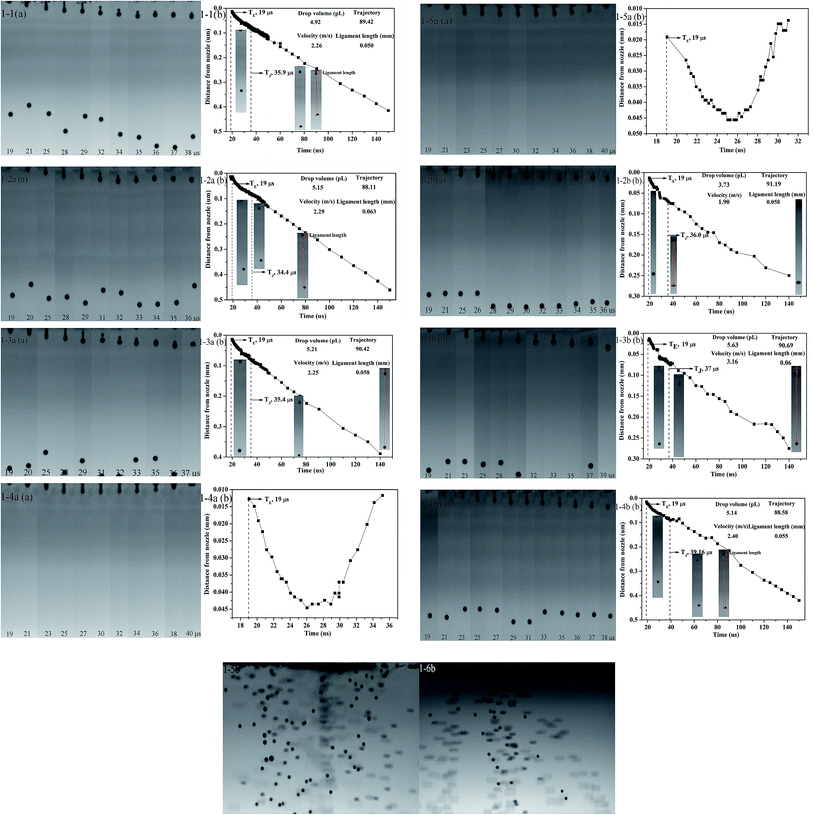
Indeed, in piezoelectric inkjet printing processing, the ink droplet is ejected thanks to an electromechanical impulse induced by a piezoelectric element at a given voltage. The droplet is jetted only if its kinetic energy overcomes interactions linked to surface energy and viscous forces through a nozzle that is typically 21.5 or 20–50 μm in diameter.11,26 The injection process and attached ligament, measured from high speed images captured at different times after the initial ejection, are present in Fig. 5. Fig. 5 (1-1, 1-2a, 1-3a, 1-2b, 1-3b, 1-4b) shows the first two drops of ink ejected and the spheres dropped. The inkjet printing process can be divided into the follow five steps. Disperse ink is extruded out of a nozzle and filament with the meniscus bulge formed at an elapsed time 0–19 μs (I, TE). The traveling velocity difference between the droplet head and the filament stretches the droplet filament until an elapsed time of about 20–31 μs (II). The filament tail is ruptured from the nozzle tip at about 28–33 μs (III, TR). Finally, the primary ink drop collides with a transient satellite at an elapsed time of around 33–37 μs (IV, TJ), and then locates onto the substrate (V). The ejected drop should have a straight trajectory, a high ink drop velocity, and appropriate volume. Fig. 5 (1-1, 1-2a, 1-3a, 1-4b) also shows the ink droplet volume (5 ± 0.2 pL), where the volumes of the ink drops (1-2b, 1-3b) are 3.73 and 5.63 pL, the velocities of the inks (1-1, 1-2a, 1-3a, 1-4b) are approximately 2.3 ± 0.1 m s−1, the velocities of the ink drops (1-2b, 1-4b) were 1.90 and 3.16 m s−1, the trajectories of the ink drops were about 90 and the ligament lengths of the inks were approximately 0.055 ± 0.005 mm. The volumes of the ink drops (1-2b, 1-3b) were different from the other inks, which might be affected by viscosity and surface tension. The distance between the separated ink drop and the nozzle is the separation length. The ink drop (1-4a, 1-5a) only leaks out of the nozzle, and the inkjet discharge properties are very poor, suggesting that PVA, with a high molecular weight and concentration, can easily cause clogging problems due to entanglement and fluidity decline.20 The inks (1-5b, 1-6b) with adequate surface tension but inadequate viscosity are shown in Fig. 5 (1-5b and 1-6b). It can be seen that the unstable ink drops from nozzles with mist generation, indicating that it is necessary to adjust not only the surface tension but also the viscosity for stable ink droplet formation.20 According to the experiments, the viscosity of the ink (1-5b, 1-6b) is low. The disperse inkjet ink has an appropriate ejection speed, volume, and trajectory to help improve the print quality of the printing patterns. For contrast purposes, different disperse inks are shown in Fig. 5, where it can be seen that the trajectories of the inks (1-2a, 1-2b, 1-4b) show deviation and lead to the droplets that have deviated from the desired location. Ink drops that are too slow or fast (1-2b, 1-3b) can also affect the accuracy of ink drop landing.Printing pattern measurements
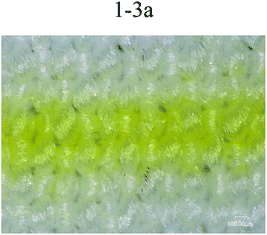
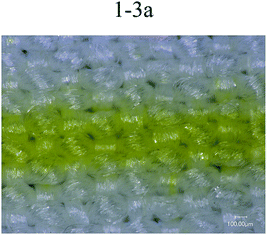
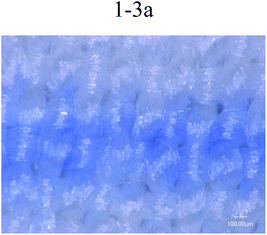
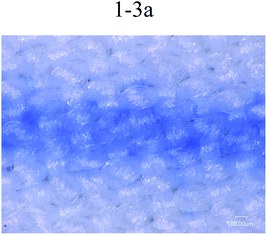
Fig. 6 shows printing patterns of disperse magenta inks. The line was set to the same linewidth (353 μm) and printed with different formulations of disperse inks. The linewidths (Fig. 6 1-2a (443 μm), 1-2b (507 μm), 1-3b (451 μm), 1-4b (437 μm)) were much wider than this range (353 μm), the edge contour of the lines became blurred and the linewidth increased due to the inappropriate trajectory or velocity of droplets deviating from the desired location. Despite this, the disperse ink (1-1, 456 μm) has a suitable trajectory and velocity of droplets and the viscosity of the ink (1-1) is relatively low, leading to diffusion of the ink along the interlacing directions of the yarn and bad sharpness of the inkjet printing. From 1-3a in Fig. 6, it can be seen that the PVA-containing disperse ink not only has a smaller linewidth but also shows a better regular edge sharpness, with the results showing that the presence of PVA helps the ink stick to the surface of the polyester fabrics and reduces diffusion. Therefore, from the experiment results, disperse magenta ink (1-3a, 370 μm) was identified to be the optimal ink.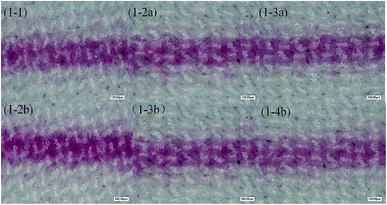 | ||
| Fig. 6 Printing lines in the filling direction for different disperse magenta inks (1-1, 1-2a, 1-3a, 1-2b, 1-3b and 1-4b). | ||
The line was printed with different formulations of disperse ink, printed in the warp or filling directions. As could be seen from Table 4, overall, the outline of the line in the filling direction is better than in the warp direction. The edge of the printed line in the warp direction is hazy, the reason for this being that the ink drops fall on these filling yarns and the ink then wicks in the filling yarn direction, which is transverse to the printing direction.26
The K/S values, which are regarded as expressions of color strength on the printing polyester fabrics, were measured. As can be seen in Table 5, the disperse inks (1-2a, 1-3a, 1-2b, 1-3b, 1-4b) have a deeper color (higher K/S value), darker shade (lower L* value) and higher chroma (C* value) than the disperse ink (1-1). The experimental data shows that the addition of water-based polymers results in a deeper color (higher K/S value), darker shade (lower L* value) and higher chroma (C* value) than the disperse ink (1-1) without water-based polymers. The experimental data thus shows that the addition of water-based polymers can improve the color strength of printed polyester fabrics.
| Sample | K/S | L* | C* |
|---|---|---|---|
| a L* values for the printed samples were higher than those of the dyed samples due to the lighter shades produced by printing. | |||
| CI disperse magenta 896 | |||
| 1-1 | 2.04 | 63.24 | 47.89 |
| 1-2a | 2.37 | 61.82 | 48.65 |
| 1-3a | 2.26 | 61.99 | 48.03 |
| 1-2b | 2.54 | 60.46 | 48.99 |
| 1-3b | 2.17 | 62.69 | 48.64 |
| 1-4b | 2.28 | 61.86 | 48.16 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CI disperse yellow 54 | |||
| 1-1 | 2.85 | 92.35 | 58.45 |
| 1-2a | 2.78 | 92.27 | 58.58 |
| 1-3a | 3.86 | 91.53 | 64.67 |
| 1-2b | 3.88 | 91.43 | 64.97 |
| 1-3b | 3.76 | 90.84 | 64.34 |
| 1-4b | 3.81 | 91.37 | 63.84 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CI disperse blue 359 | |||
| 1-1 | 2.38 | 61.54 | 28.86 |
| 1-2a | 2.43 | 60.93 | 29.18 |
| 1-3a | 3.18 | 57.41 | 33.54 |
| 1-2b | 3.26 | 56.71 | 33.12 |
| 1-3b | 3.29 | 56.62 | 32.92 |
| 1-4b | 3.09 | 57.22 | 33.32 |
The printed samples were subjected to washing, and dry and wet rubbing tests (Table 6). The colour fastness of sample 1-1 was 4. The colour fastness value upon washing at 60 °C was 5 for samples 1-2a, 1-3a, 1-2b, 1-3b and 1-4b. The colour fastness to dry and wet rubbing was 4-5 or 5 for printed samples 1-2a, 1-3a, 1-2b, 1-3b and 1-4b. The reason for this is that disperse dyes diffuse, migrate and fix in the polymer film-polyester fiber layer, which can improve the color fastness and color fastness of the printed fabrics (Fig. 7(a)), and there is no color loss without washing after printing.
| Sample | Washing fastness | Rubbing fastness | |
|---|---|---|---|
| Dry | Wet | ||
| CI disperse magenta 896 | |||
| 1-1 | 4 | 4 | 4 |
| 1-2a | 5 | 5 | 4-5 |
| 1-3a | 5 | 5 | 5 |
| 1-2b | 5 | 5 | 5 |
| 1-3b | 5 | 5 | 5 |
| 1-4b | 5 | 5 | 5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CI disperse yellow 54 | |||
| 1-1 | 4 | 4-5 | 4 |
| 1-2a | 5 | 5 | 4-5 |
| 1-3a | 5 | 5 | 4-5 |
| 1-2b | 5 | 5 | 5 |
| 1-3b | 5 | 5 | 5 |
| 1-4b | 5 | 5 | 5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CI disperse blue 359 | |||
| 1-1 | 4 | 4-5 | 4-5 |
| 1-2a | 5 | 5 | 5 |
| 1-3a | 5 | 5 | 5 |
| 1-2b | 5 | 5 | 5 |
| 1-3b | 5 | 5 | 5 |
| 1-4b | 5 | 5 | 5 |
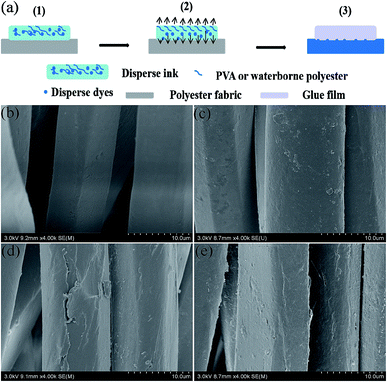 | ||
| Fig. 7 Fixation mechanism of disperse dyes (a), SEM micrographs of the unprinted (b) and printed PET fabrics ((c), 1-1; (d), 1-3a; (e), 1-3b). | ||
The surface morphologies of the unprinted and printed polyester fabrics were observed by scanning electron microscopy (Fig. 7). Fig. 7(b) shows that the surface of the unprinted polyester fabrics is smooth. From Fig. 7(c), it can be seen that there is some disperse dye on the surface of the polyester fabrics. Fig. 7(d and e) show that film and dye particles formed on the surface of the polyester fabrics, and that the film is beneficial to the fastness of the disperse ink because disperse dye was embedded on the surface of the polyester fabric by PVA or waterborne polyester.
Conclusions
Disperse ink for direct inkjet printing was successfully prepared via the addition of water-based polymers and was used to inkjet print ink for polyester fabric applications. The results show that disperse dye, surfactant, wetting agent and water-based polymers are important factors that influence the rheological properties and inkjet printability of the disperse ink. The trajectory and velocity of the droplets effects the linewidth and edge contour of the disperse ink. The viscosity of the disperse ink also influences the diffusion of the ink along the interlacing directions of the polyester fabrics and the printing accuracy. The lines were printed in the warp or filling directions, and the results showed that overall the outline of the lines in the filling direction was better than in the warp direction. In the presence of water-based polymers, the disperse dye was embedded and fixed in the polyester fabrics, forming a layer film on the surface of the polyester fabrics, which is advantageous for the improvement of color fastness. Finally, the optimal formula of the disperse ink was found to be sample 1-3a. Meanwhile, there was no color lost without washing after printing, making it an economical and environmentally friendly inkjet dyeing technology.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFB0309800), the Applied Basic Research program of “Textile Vision Science” (J201605), the Six Talent Peaks Project of Jiangsu Province (JNHB-066) and Suzhou Integration, the Development Strategy of Famous City and University Project (2016-06) and the Innovation plan for postgraduates in Jiangsu Province (SJKY19-2293).References
- E. K. Karanikas and E. G. Tsatsaroni, J. Appl. Polym. Sci., 2012, 125, 3396–3403 CrossRef CAS.
- C. T. Kosolia, E. G. Tsatsaroni and N. F. Nikolaidis, Color. Technol., 2011, 127, 357–364 CAS.
- M. Li, L. Zhang and D. Wang, Color. Technol., 2017, 133, 374–484 Search PubMed.
- X. I. Wang, W. W. Carr and D. G. Bucknall, Int. J. Multiphase Flow, 2012, 38, 17–26 CrossRef.
- T. R. Tuladhar and M. R. Mackley, J. Non-Newtonian Fluid Mech., 2008, 148, 97–108 CrossRef CAS.
- M. Fryberg, Color. Technol., 2010, 35, 1–30 Search PubMed.
- I. Holme, Textiles Magazine, 2006, vol. 31, pp. 11–16 Search PubMed.
- Y. Zhang, V. Cheung and S. Westland, Color. Technol., 2010, 125, 29–35 Search PubMed.
- Y. Zhao, M. Li and L. Zhang, Text. Res. J., 2017, 89, 121–132 Search PubMed.
- V. Karanikas, N. Nikolaidis and E. Tsatsaroni, Text. Res. J., 2013, 83, 450–461 CrossRef.
- D. Jang, D. Kim and J. Moon, Langmuir, 2009, 25, 2629–2635 CrossRef CAS PubMed.
- H. R. Kang, J. Imaging Sci. Technol., 1991, 35, 189–194 CAS.
- C. T. Kosolia and E. G. Tsatsaroni, J. Appl. Polym. Sci., 2010, 116, 1422–1427 CAS.
- N. H. Momin, R. Padhye and A. Khatri, J. Appl. Polym. Sci., 2010, 119, 2495–2501 CrossRef.
- A. Janasak, C. Koopipat and H. Noguchi, J. Imaging Sci. Technol., 2007, 51, 127–140 CrossRef CAS.
- Y. Yang, V. Naarani and V. Thillainayagam, J. Imaging Sci. Technol., 2006, 50, 181–186 CrossRef CAS.
- L. Chen, C. Wang and A. Tian, Surf. Interface Anal., 2012, 44, 1324–1330 CrossRef CAS.
- J. Schmitz, H. Frommelius and U. Pegelow, Prog. Org. Coat., 1999, 35, 191–196 CrossRef CAS.
- E. Reuter, S. Silber and C. Psiorz, Prog. Org. Coat., 1999, 37, 161–167 CrossRef CAS.
- J. Y. Park, Y. Hirata and K. Hamada, Dyes Pigm., 2012, 95, 502–511 CrossRef CAS.
- S. Daplyn and L. Lin, Pigm. Resin Technol., 2003, 32, 307–318 CrossRef CAS.
- I. Holme, Textiles Magazine, 2004, vol. 31, pp. 8–13 Search PubMed.
- S. Leelajariyakul, H. Noguchi and S. Kiatkamjornwong, Prog. Org. Coat., 2008, 62, 145–161 CrossRef CAS.
- S. W. Churchill and R. U. Churchill, Rheol. Acta, 1975, 14, 404–409 CrossRef.
- P. C. F. Møller, J. Mewis and D. Bonn, Soft Matter, 2006, 2, 274–283 RSC.
- P. Heungsup and W. C. Wallace, Text. Res. J., 2006, 76, 720–728 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |