Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot regioselective C–H activation iodination–cyanation of 2,4-diarylquinazolines using malononitrile as a cyano source†
Ziqiao Yana,
Banlai Ouyangb,
Xunchun Maoa,
Wei Gaoa,
Zhihong Denga and
Yiyuan Peng *a
*a
aKey Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Province's Key Laboratory of Green Chemistry, Jiangxi Normal University, Nanchang, Jiangxi 330022, China. E-mail: yypeng@jxnu.edu.cn
bDepartment of Chemistry, Nanchang Normal University, Nanchang 330032, China
First published on 11th June 2019
Abstract
A one-pot cyanation of 2,4-arylquinazoline with NIS and malononitrile has been developed. The one-pot reaction includes two steps. The Rh-catalyzed selective C–H activation/iodization of 2,4-diarylquinazoline with NIS, and then Cu-catalyzed cyanation of the corresponding iodinated intermediate with malononitrile to selectively give 2-(2-cyanoaryl)-4-arylquinazolines or 2-(2,6-dicyanoaryl)-4-arylquinazolines in good to excellent yields.
Introduction
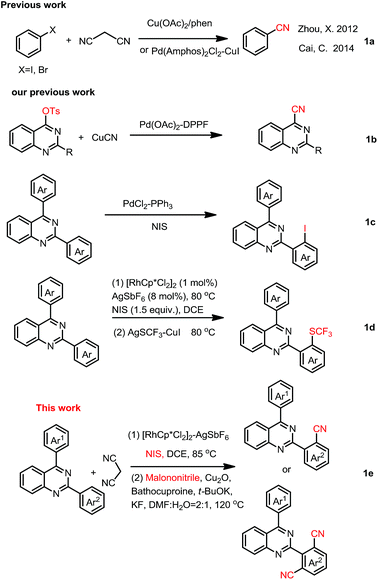
Aromatic nitriles have broad applications in agrochemicals, pharmaceuticals and materials science. The nitrile moiety also serves as a pivotal precursor for a multitude of conversions into a great number of other functional groups, such as hydrolysis into carboxyl or amide, reduction into aldehydes or amines, cycloaddition into heterocycles, etc.1 Consequently, a number of methods have been developed to introduce a cyanogen group to an aromatic ring. Various catalytic systems and cyanating agents have been established. Of these transformations, transition-metal-catalyzed cyanation of aryl halides2a or aromatic C–H bond activation/cyanation was an elegant route to arylnitriles.2b Cu(CN)2,3 Zn(CN)2,4 NaCN and KCN,5 CuSCN,6 cyanogen halides,7 TMSCN,8 NaN3,9 and K4[Fe(CN)6]10 have been used as cyanation agents to introduce CN into organic molecules. Organic cyanide sources and their combined cyano-groups, such as CH3NO2,11 acetone cyanohydrin,12 N-cyano-N-phenyl-p-toluenesulfonamide (NCTS),13 aryl(cyano)iodonium triflates,14 amine/DMSO,15 DMF,16 formamide,16f,16g ethyl cyanoacetate,17 ethyl(ethoxymethylene)cyanoacetate,18 benzyl cyanide,19 tert-butyl nitrite (TBN),20 isocyanides,21 and acetonitrile22 have been used in cyanation for more solubility in organic solvents.Recent, malononitrile, being more inexpensive, readily industry available, less toxic, stable and easy-to-handle, had been used as an alternative organic cyano-group source.23 Although attempts have been made to utilize malononitrile as the cyano-group source in several aryl halogens cyanation reactions (Scheme 1a),23 there is no report about utilizing it as a cyano-group source in one-pot C–H bond activation/cyanation.
On the other hand, quinazoline core structure exhibits a wide range of important potential biological activities.24,25 For example, Erlotinib and Gefitinib are well-known lung cancer drugs.26 Prazosin is used for curing high blood pressure27 and Trimetrexate has been used in the treatment of pneumocystis pneumonia28 (Fig. 1). Thus tremendous efforts have been devoted to develop new synthetic methods for the construction of diverse quinazoline architectures and evaluate their bioactivities.29 In the past few years, our group has focused extensively on the development methods for the synthesis of quinazoline skeletons and the late-stage functionalization of the quinazoline core with a wish to construct a quinazoline-based molecular library for bioactivity assay.30
However, the construction of quinazoline core coupling-nitrile moiety had been rarely reported. In 2013, we reported palladium catalyzed cyanation of quinazoline-4-tosylates with CuCN for access to quinazoline-4-nitriles (Scheme 1b).31 In 2017, we disclosed quinazoline-directed selective ortho-iodination of 2,4-di-arylquinazolines for the synthesis of 2-(2-iodoaryl)-4-arylquinazolines (Scheme 1c).32 Very recently, iodination of 2,4-diarylquinazoline with NIS catalyzed by rhodium, and then trifluoromethylthiolation of the corresponding iodides for the synthesis of SCF3-substituted 2,4-diarylquinazolines was reported in our group (Scheme 1d).33 Base on these, we here reported selective synthesis of 2-(2-cyanophenyl)-4-phenylquinazolines and 2-(2,4-dicyanophenyl)-4-phenylquinazolines by one-pot C–H activation iodination/cyanation using malononitrile as a cyano-group source (Scheme 1e).
Results and discussion
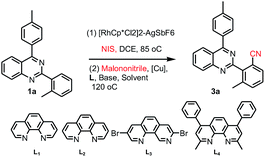
Our interest in this particular on the regioselective C–H activation/cyanation of 2,4-diarylquinazolines originated from our recent studies that [RhCp*Cl2]2/AgSbF6 catalyzed iodination of 2,4-arylquinazoline.33 Here, we first optimize the reaction conditions of one-pot C–H iodination/cyanation of 2,4-phenylquinazoline. To avoid produce mixture of mono- and bis-functionalization products, 2-(o-methyl)phenyl-4-(p-methyl)phenyl quinazoline 1a, which one of ortho position was blocked by a methyl, was chosen as the model substrate for the optimization of the one-pot cyanation conditions. According to our previous work,33 the standard iodination protocol was carried out by using 1a (1.0 eq.), NIS (N-iodosuccinimide, 1.5 eq.), [RhCp*Cl2]2 (1.0 mol%) and AgSbF6 (8.0 mol%) in DCE at 85 °C for 30 min. When the iodination reaction was finished, the DCE solvent was removed under reduced pressure, and the mixture of malononitrile (2.0 eq.), catalyst-system Cu2O (10 mol%)–L (20 mol%), t-BuOK (2.0 eq.) and DMF (2.0 mL) as the solvent were added and then reacted at 120 °C. To our delight, the target product 3a was isolated in a yield of 25% (Table 1, entry 1). Various of bases, such as K2CO3, KOH, Cs2CO3, and NaOH, were screened, and t-BuOK was found to be the best one (entries 1–5). Different copper salts were then examined, and Cu2O was proved to be the best selected, which resulted in a yield of 58% (entries 6–12). The results of solvents tested indicated that trace amount of product was detected in H2O or acetone (not listed in Table 1); low yield of 47% and 49% were obtained in DMAC or DMSO (entries 13 and 14). Additive KF found to be beneficial to the reaction, and gave a yield up to 63% (entry 15). Among the ligands used, L4 (Bathocuproine) was found to be more facilitating to the reaction than the others (entries 16–18). Excitingly, adding 50 v% of water in DMF can improve the yield to 84% (entries 19–21) and short the reaction time from 18 h to 3 h. Finally, when NBS (N-bromosuccinimide) was used as the reactant instead of NIS under the standard reaction conditions, only trace amount of the desired product was detected.| Entry | [Cu] | L | Base | Solvent | Yieldc |
|---|---|---|---|---|---|
| a Optimized conditions are denoted in bold.b Reaction conditions: 1a (0.1 mmol), NIS (1.5 eq.). [RhCp*Cl2]2 (1.0 mol%)–AgSbF6 (8 mol%), malononitrile (2.0 eq.) [Cu] (10 mol%)–ligand (20 mol%), base (2.0 eq.), in solvent (2.0 mL) at 120 °C.c Isolated yield.d NIS was replaced by NBS. | |||||
| 1 | Cu(OAc)2 | L1 | t-BuOK | DMF | 25 |
| 2 | Cu(OAc)2 | L1 | NaOH | DMF | 16 |
| 3 | Cu(OAc)2 | L1 | K2CO3 | DMF | 21 |
| 4 | Cu(OAc)2 | L1 | KOH | DMF | 15 |
| 5 | Cu(OAc)2 | L1 | Cs2CO3 | DMF | 18 |
| 6 | CuCl | L1 | t-BuOK | DMF | 32 |
| 7 | CuCO3 | L1 | t-BuOK | DMF | 24 |
| 8 | Cu(TFA)2 | L1 | t-BuOK | DMF | 26 |
| 9 | CuBr | L1 | t-BuOK | DMF | 48 |
| 10 | CuSO4 | L1 | t-BuOK | DMF | 45 |
| 11 | Cu2O | L1 | t-BuOK | DMF | 58 |
| 12 | CuI | L1 | t-BuOK | DMF | 44 |
| 13 | Cu2O | L1 | t-BuOK | DMAC | 47 |
| 14 | Cu2O | L1 | t-BuOK | DMSO | 49 |
| 15 | Cu2O | L1 | t-BuOK–KF | DMF | 63 |
| 16 | Cu2O | L2 | t-BuOK–KF | DMF | 33 |
| 17 | Cu2O | L3 | t-BuOK–KF | DMF | 57 |
| 18 | Cu2O | L4 | t-BuOK–KF | DMF | 69 |
| 19 | Cu2O | L4 | t-BuOK–KF | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 20 | Cu2O | L4 | t-BuOK–KF | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
71 |
| 21 | Cu2O | L4 | t-BuOK–KF | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
75 |
| 22d | Cu2O | L4 | t-BuOK–KF | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
Trace |
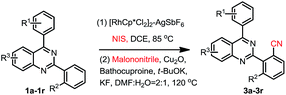
We then started to investigate the scope and the generality of this reaction under optimized conditions [NIS (1.5 eq.), [RhCp*Cl2]2 (1.0%), AgSbF6 (8.0 mol%), malononitrile (2.0 eq.), Cu2O (10 mol%), Bathocuproine (20 mol%), t-BuOK (2.0 eq.), KF (2.0 eq.) in the solvent of DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2
H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at 120 °C for 3.5 hours]. The results are listed in Table 2.
1, at 120 °C for 3.5 hours]. The results are listed in Table 2.
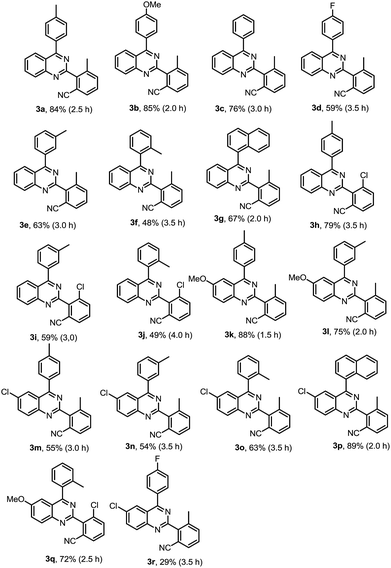
As shown in Table 2, a variety of 2-(o-canyophenyl)-4-arylquinazolines were generated under the standard experimental conditions. In general, the electron-donating R1, R2, R3 group substituent fascinated the reactions, and gave higher yields than that of electron-withdrawing ones. For examples, when R2 is a methyl, and R1 is p-methyl or p-methoxyl, the corresponding products 3a and 3b were obtained in excellent of yields of 84% and 85%. The reaction of electron-withdrawing fluorinated substrate 1d gave good yield of 59%. The substituent group at the meta or ortho position of 4-phenyl, due to the steric hindrance, finished the corresponding products 3e, 3f and 3g in a yield of 63%, 48% and 67%, respectively. When R2 is chloro, the reactions gave the corresponding products 3h–3j in good yields 79–49%.
Subsequently, the effects of substituent on the phenyl moiety of quinazoline mother ring were then examined. Pleasingly, methoxy and chloro functionalities were all tolerated, providing the desired products 3k–3q in good to excellent yields. 3r was obtained in a low yield due to double electron-withdrawing substituent effect.
As we seen, when R2 is H, the reaction may produce mono-cyanation and bis-cyanation selectivity. In our previous work, exclusively generate mono-iodination on the 2-aryl group was obtained catalyzed by PbCl2–PPh3, and then cyanation of the corresponding 2-(2-iodoaryl)quinazolines using conventional procedure1e could selective giving 2-(2-mono-nitrile)quinazolines. Thus, here we focus our attention on selective to produce bis-cyanation quinazolines in one-pot reaction. The results are listed in Table 3.
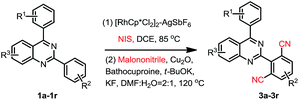
When 2,4-di-(p-methyl)phenylquinazoline 1aa was selected as the model substrate, and the corresponding regents were adding double of the standard protocol above, the corresponding bis-cyanation product 4a was obtained in a yield of 65% (Table 3). The electron-donating R1, R2, R3 group substituent also fascinating the reactions, and gave higher yields (4a, 4b, 4f and 4i) than that of electron-withdrawing ones (4d, 4e, 4g and 4h). Interestingly, dicyano-compounds were obtained in reasonable yields (4j and 4k) when m-substituted of 2,4-diarylquinazolines were used.
In order to further explore the substrate scope, 2-phenylpyridine was then investigated under the standard conditions, however, the corresponding product 4l was obtained in 23% yield. This result again disclosed that the directing properties of diazine in quinazoline are distinctive from that of pyridine.
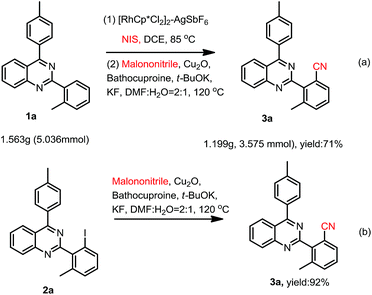
To demonstrate the potential synthetic utility of this transformation, a gram-scale reaction of 2-(o-methyl)phenyl-4-(p-methyl)phenylquinazoline (1a) was carried out. As shown in Scheme 2a, the cyanation product (3a) was isolated in an 71% yield.
To further understand the reaction mechanism, a controlled experiment was conducted. When 2-(2-iodo-6-methylphenyl)-4-(p-methyl)phenylquinazoline (2a) was used as the reactant under the standard reaction conditions in Scheme 2b, the cyanation product (3a) was isolated in an 92% yield.
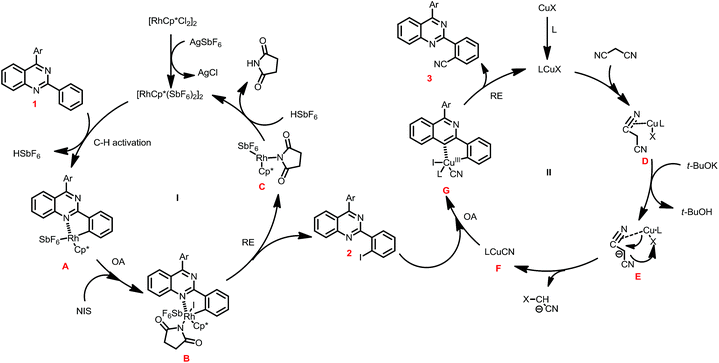
In view of the above results, a plausible mechanism was disclosed in Scheme 3. The one-pot iodination/cyanation reaction include two catalytic cycles. In the first catalytic cycle I, the reaction of [Cp*RhCl2]2 and AgSbF6 forms [Cp*Rh(SbF6)2]2, which reacted with 1 through C–H activation to give five-membered rhodacycle A, and then was oxidation adduction with NIS to provide the Rh(IV) intermediate B, followed reductive eliminated to give iodide intermediate product 2 along with the Rh(III) C, which was acidized to regenerate catalyst [Cp*Rh(SbF6)2]2 and finish catalytic cycle I.
In the second catalytic cycle II, the ligand was incorporated with CuIX to form the catalyst LCuX, and then incorporate with malononitrile to provide the complex D, which was reacted with t-BuOK to give intermediate E, and then undergoes transmetalation to generate the active species LCuICN. The LCuICN subjected to oxidative adduction with 2 to form five-membered cyclic Cu(III) G, and followed reductive eliminated to give product 3, and release the LCuIX catalyst and finish the catalytic cycle.
In summary, we here have developed methods for one-pot process for selective the synthesis of 2-(2-cyano)aryl-4-aryiquinazolines or 2-(2,6-dicyano)aryl-4-arylquinazolines. The one-pot reaction include two steps, the Rh-catalyzed selective C–H activation iodization of 2,4-diarylquinazoline with NIS, and then Cu-catalyzed cyanation of the corresponding iodide intermediate with malononitrile to give 2-(o-cyanoaryl)-4-arylquinazolines or 2-(2,6-dicyano)aryl-4-arylquinazolines in good to excellent yields.
Experimental section
Unless otherwise noted, commercial reagents were purchased from Aldrich, Alfa, or other commercial suppliers. All solvents were dried and distilled according to standard procedures before use. Reactions were conducted in standard techniques on vacuum line. Analytical thin-layer chromatography (TLC) was performed using glass plates pre-coated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Flash column chromatography was performed using silica gel (60 Å pore size, 32–63 μm, standard grade). Organic solutions were concentrated on rotary evaporators at ∼20 torr (house vacuum) at 25–35 °C. Nuclear magnetic resonance (NMR) spectra are recorded in parts per million (ppm) from internal standard tetramethylsilane (TMS) on the δ scale.General procedure for preparation of 2-(o-cyanoaryl)-4-arylquinazoline 3 (3a as an example)
A mixture of 2,4-phenylquinazolines 1a (0.2 mmol), NIS (0.3 mmol, 1.5 eq.), [RhCp*Cl2]2 (1.0 mol%), AgSbF6 (8.0 mol%) in DCE (2.0 mL) was stirred at 85 °C, until 1a was completed consumed (detected by TLC). The solvent was removed under reduced pressure. A mixture of malononitrile (2.0 eq.), Cu2O (10 mol%), Bathocuproin (20 mol%), t-BuOK (2.0 eq.), KF (2.0 eq.) and DMF and water (3.0 mL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added and stirred at 120 °C for 3 h. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature. The solvent was evaporated, residue was diluted with EtOAc (10 mL), washed with H2O (10 mL), dried by anhydrous Na2SO4. Evaporation of the solvent followed purification by column chromatograph over silica gel provided the corresponding product 3a.
1) was added and stirred at 120 °C for 3 h. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature. The solvent was evaporated, residue was diluted with EtOAc (10 mL), washed with H2O (10 mL), dried by anhydrous Na2SO4. Evaporation of the solvent followed purification by column chromatograph over silica gel provided the corresponding product 3a.
General procedure for preparation of 2-(o-dicyanoaryl)-4-arylquinazoline 4 (4a as an example)
A mixture of 2,4-phenylquinazolines 1aa (0.2 mmol), NIS (0.6 mmol. 3.0 eq.), [RhCp*Cl2]2 (2.0 mol%), AgSbF6 (16 mol%) and in DCE (2.0 mL) was stirred at 85 °C for 0.5 h, until 1a was completed consumed. The solvent was removed under reduced pressure. A mixture of malononitrile (4.0 eq.), Cu2O (20 mol%), Bathocuproin (40 mol%), t-BuOK (4.0 eq.), KF (4.0 eq.) in DMF and water (3.0 mL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred at 120 °C. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature. The solvent was evaporated, residue was diluted with EtOAc (10 mL), washed with H2O (10 mL), dried by anhydrous Na2SO4. Evaporation of the solvent followed purification by column chromatograph over silica gel provided the corresponding product 4a.
1) was stirred at 120 °C. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature. The solvent was evaporated, residue was diluted with EtOAc (10 mL), washed with H2O (10 mL), dried by anhydrous Na2SO4. Evaporation of the solvent followed purification by column chromatograph over silica gel provided the corresponding product 4a.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from National Natural Science Foundation of China (no. 21762020), Jiangxi Provincial Department of Science and Technology (no. 20171BAB203006), and key laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Normal University (no. KLFS-KF-201702) is gratefully acknowledged.Notes and references
- (a) A. J. Fatiadi in Preparation and Synthetic Applications of Cyano Compounds, ed. S. Patai and Z. Rappoport, Wiley, New York, 1983, vol. 2, pp. 1057–1303 Search PubMed; (b) Z. Rappoport, The Chemistry of the Cyano Group, Interscience, New York, 1970 Search PubMed; (c) R. C. Larock, Comprehensive Organic Transformations, VCH, New York, 1989 Search PubMed; (d) J. X. Qiao, X. Cheng, D. P. Modi, K. A. Rossi, J. M. Luettgen, R. M. Knabb, P. K. Jadhav and R. R. Wexler, Bioorg. Med. Chem. Lett., 2005, 15, 29–35 CrossRef CAS PubMed; (e) M. Tobisu and N. Chatani, Chem. Soc. Rev., 2008, 37, 300–307 RSC.
- (a) P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049–5067 RSC; (b) Y. Y. Ping, Q. P. Ding and Y. Y. Peng, ACS Catal., 2016, 6, 5989–6005 CrossRef CAS For recent review, see: .
- G. P. Ellis and T. M. Romney-Alexander, Chem. Rev., 1987, 87, 779–797 CrossRef CAS.
- (a) P. E. Maligres, M. S. Waters, F. Fleitz and D. Askin, Tetrahedron Lett., 1999, 40, 8193–8195 CrossRef CAS; (b) R. Chidambaram, Tetrahedron Lett., 2004, 45, 1441–1444 CrossRef CAS; (c) R. S. Jensen, A. S. Gajare, K. Toyota, M. Yoshifuji and F. Ozawa, Tetrahedron Lett., 2005, 46, 8645–8647 CrossRef CAS; (d) A. Littke, M. Soumeillant, R. F. Kaltenbach, R. J. Cherney, C. M. Tarby and S. Kiau, Org. Lett., 2007, 9, 1711–1714 CrossRef CAS PubMed.
- (a) K. Takagi, T. Okamoto, Y. Sakakibara and S. Oka, Chem. Lett., 1973, 471–474 CrossRef CAS; (b) B. A. Anderson, E. C. Bell, F. O. Ginah, N. K. Harn, L. M. Pagh and J. P. Wepsiec, J. Org. Chem., 1998, 63, 8224–8228 CrossRef CAS; (c) M. Sundermeier, A. Zapf, M. Beller and J. Sans, Tetrahedron Lett., 2001, 42, 6707–6710 CrossRef CAS; (d) A. Zanon, J. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 2890–2891 CrossRef PubMed; (e) R. K. Arvela and N. E. Leadbeater, J. Org. Chem., 2003, 68, 9122–9125 CrossRef CAS PubMed; (f) A. V. Ushkov and V. V. Grushin, J. Am. Chem. Soc., 2011, 133, 10999–11005 CrossRef CAS PubMed.
- G. Y. Zhang, J. T. Yu, M. L. Hu and J. Cheng, J. Org. Chem., 2013, 78, 2710–2714 CrossRef CAS PubMed.
- (a) P. H. Gore, F. S. Kamounah and A. Y. Miri, Tetrahedron, 1979, 35, 2927–2929 CrossRef CAS; (b) M. Murai, R. Hatano, S. Kitabata and K. Ohe, Chem. Commun., 2011, 2375–2377 RSC; (c) K. Okamoto, M. Watanabe, M. Murai, R. Hatano and K. Ohe, Chem. Commun., 2012, 3127–3129 RSC.
- (a) N. Chatani and T. Hanafusa, J. Org. Chem., 1986, 51, 4714–4716 CrossRef CAS; (b) M. Sundermeier, S. Mutyala, A. Zapf, A. Spannenberg and M. Beller, J. Organomet. Chem., 2003, 684, 50–55 CrossRef CAS.
- (a) W. Zhou, L. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2009, 48, 7094–7097 CrossRef CAS PubMed; (b) W. Zhou, J. Xu, L. Zhang and N. Jiao, Org. Lett., 2010, 12, 2888–2891 CrossRef CAS PubMed.
- (a) T. Schareina, A. Zapf and M. Beller, Chem. Commun., 2004, 1388–1389 RSC; (b) T. Schareina, A. Zapf, W. Mägerlein, N. Müller and M. Beller, Tetrahedron Lett., 2007, 48, 1087–1090 CrossRef CAS; (c) Y. Ren, W. Wang, S. Zhao, X. Tian, J. Wang, W. Yin and L. Cheng, Tetrahedron Lett., 2009, 50, 4595–4597 CrossRef CAS; (d) C. DeBlase and N. E. Leadbeater, Tetrahedron, 2010, 66, 1098–1101 CrossRef CAS; (e) L. Liu, J. Li, J. Xu and J. T. Sun, Tetrahedron Lett., 2012, 53, 6954–6956 CrossRef CAS; (f) T. Chatterjee, R. Dey and B. C. Ranu, J. Org. Chem., 2014, 79, 5875–5879 CrossRef CAS PubMed.
- X. Chen, X. S. Hao, C. E. Goodhue and J. Q. Yu, J. Am. Chem. Soc., 2006, 128, 6790–6791 CrossRef CAS PubMed.
- (a) M. Sundermeier, A. Zapf and M. Beller, Angew. Chem., Int. Ed., 2003, 42, 1661–1664 CrossRef CAS PubMed; (b) M. Sundermeier, A. Zapf, S. Mutyala, W. Baumann, J. Sans, S. Weiss and M. Beller, Chem.–Eur. J., 2003, 9, 1828–1836 CrossRef CAS PubMed; (c) H. J. Cristau, A. Ouali, J. F. Spindler and M. Taillefer, Chem.–Eur. J., 2005, 11, 2483–2492 CrossRef CAS PubMed; (d) T. Schareina, A. Zapf, A. Cotté, M. Gotta and M. Beller, Adv. Synth. Catal., 2011, 353, 777–780 CrossRef CAS.
- (a) J. Cui, J. Song, Q. Liu, H. Liu and Y. H. Dong, Chem.–Asian J., 2018, 13, 482–495 CrossRef CAS PubMed and references therein.; (b) Y. Yang, Y. Zhang and J. Wang, Org. Lett., 2011, 13, 5608–5611 CrossRef CAS PubMed; (c) P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 519–522 CrossRef CAS PubMed.
- (a) T. Dohi, K. Morimoto, Y. Kiyono, H. Tohma and Y. Kita, Org. Lett., 2005, 7, 537–540 CrossRef CAS PubMed; (b) Z. Shu, W. Ji, X. Wang, Y. Zhou, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2014, 53, 2186–2189 CrossRef CAS PubMed.
- (a) X. Ren, J. Chen, F. Chen and J. Cheng, Chem. Commun., 2011, 6725–6727 RSC; (b) K. Zheng, B. Liu, S. Chen and F. Chen, Tetrahedron Lett., 2013, 54, 5250–5252 CrossRef CAS.
- (a) J. Kim and S. Chang, J. Am. Chem. Soc., 2010, 132, 10272–10274 CrossRef CAS PubMed; (b) G. Zhang, X. Ren, J. Chen, M. Hu and J. Cheng, Org. Lett., 2011, 13, 5004–5007 CrossRef CAS PubMed; (c) J. Kim, J. Choi, K. Shin and S. Chang, J. Am. Chem. Soc., 2012, 134, 2528–2531 CrossRef CAS PubMed; (d) A. B. Pawara and S. Chang, Chem. Commun., 2014, 448–450 RSC; (e) S. Ding and N. Jiao, J. Am. Chem. Soc., 2011, 133, 12374–12377 CrossRef CAS PubMed; (f) D. N. Sawant, Y. S. Wagh, P. J. Tambade, K. D. Bhatte and B. M. Bhanage, Adv. Synth. Catal., 2011, 353, 781–787 CrossRef CAS; (g) A. B. Khemnar, D. N. Sawant and B. M. Bhanage, Tetrahedron Lett., 2013, 54, 2682–2684 CrossRef CAS.
- (a) X. J. Wang and S. L. Zhang, New J. Chem., 2017, 41, 14826–14830 RSC; (b) S. Zheng, C. Yu and Z. Shen, Org. Lett., 2012, 14, 3644–3647 CrossRef CAS PubMed.
- Z. L. Li, K. K. Sun and C. Cai, Org. Chem. Front., 2018, 5, 1848–1853 RSC.
- (a) Q. Wen, J. Jin, Y. Mei, P. Lu and Y. Wang, Eur. J. Org. Chem., 2013, 4032–4036 CrossRef CAS; (b) Q. Wen, J. Jin, B. Hu, P. Lu and Y. Wang, RSC Adv., 2012, 2, 6167–6169 RSC; (c) J. Jin, Q. Wen, P. Lu and Y. Wang, Chem. Commun., 2012, 9933–9935 RSC.
- (a) B. Lücke, K. V. Narayana, A. Martin and K. Jähnisch, Adv. Synth. Catal., 2004, 346, 1407–1424 CrossRef; (b) Z. Shu, Y. Ye, Y. Deng, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2013, 52, 10573–10576 CrossRef CAS PubMed; (c) D. Tsuchiya, Y. Kawagoe, K. Moriyama and H. Togo, Org. Lett., 2013, 15, 4194–4197 CrossRef CAS PubMed.
- X. Jiang, J.-M. Wang, Y. Zhang, Z. Chen, Y.-M. Zhu and S.-J. Ji, Tetrahedron, 2015, 71, 4883–4887 CrossRef CAS.
- (a) M. D. Zhao, W. Zhang and Z. M. Shen, J. Org. Chem., 2015, 80, 8868–8873 CrossRef CAS PubMed and references therein.; (b) Y. M. Zhu, L. Y. Li and Z. M. Shen, Chem.–Eur. J., 2015, 21, 13246–13252 CrossRef CAS PubMed.
- (a) G. P. Lu, M. J. Bu and C. Cai, Synlett, 2014, 25, 547–550 CrossRef CAS; (b) Z. Q. Jiang, Q. Huang, S. Chen, L. S. Long and X. G. Zhou, Adv. Synth. Catal., 2012, 354, 589–592 CrossRef CAS.
- For recent review, see: X. F. Shang, S. L. Morris-Natschke, Y. Q. Liu, X. Guo, X. S. Xu, M. Goto and J. C. Li, Med. Res. Rev., 2017, 1–54 Search PubMed.
- For selected examples, see: (a) S. G. Li, K. B. Wang, C. Gong, Y. Bao, N. B. Qin, D. H. Li and Z. L. Li, Bioorg. Med. Chem. Lett., 2018, 28, 103–106 CrossRef CAS PubMed; (b) Z. Haghighijoo, Q. Firuzi, B. Hemmateenejad, S. Emami, N. Edraki and R. Miri, Bioorg. Chem., 2017, 74, 126–133 CrossRef CAS PubMed; (c) L. J. Wilso, Org. Lett., 2001, 3, 585–588 CrossRef; (d) W. Szczepankiewicz, P. Wagner, M. Danicki and J. Suwiński, Tetrahedron Lett., 2003, 44, 2015–2017 CrossRef CAS; (e) D. S. Yoon, Y. Han, T. M. Stark, J. C. Haber, B. T. Gregg and S. B. Stankovich, Org. Lett., 2004, 6, 4775–4778 CrossRef CAS PubMed; (f) Y. Z. Yan, Y. H. Zhang, C. T. Feng, Z. G. Zha and Z. Y. Wang, Angew. Chem., Int. Ed., 2012, 51, 8077–8081 CrossRef CAS PubMed; (g) X. S. Fan, B. Li, S. H. Guo, Y. Y. Wang and X. Y. Zhang, Chem.–Asian J., 2014, 9, 739–743 CrossRef CAS PubMed; (h) D. Zhao, T. Wang and J. X. Li, Chem. Commun., 2014, 50, 6471–6474 RSC.
- R. Gundla, R. Kazemi, R. Sanam, R. Muttineni, J. A. R. P. Sarma, R. Dayam and N. Neamati, J. Med. Chem., 2008, 51, 3367–3377 CrossRef CAS PubMed.
- S. F. Campbell, M. J. Davey, J. D. Hardstone, B. N. Lewis and M. J. Palmer, J. Med. Chem., 1987, 30, 49–57 CrossRef CAS PubMed.
- C. E. S. Short, Y. C. Gilleece, M. J. Fisher and D. R. Churchill, AIDS, 2009, 23, 1287–1290 CrossRef CAS PubMed.
- (a) D. N. Garad, A. B. Viveki and S. B. Mhaske, J. Org. Chem., 2017, 82, 6366–6372 CrossRef CAS PubMed; (b) C. Milite, E. Barresi, E. Pozzo, B. Costa, M. Vivian, A. Porta, A. Messere, G. Sbardella, F. Settimo, E. Novellino, S. Coscoati, S. Castellano, S. Talian and C. Martini, J. Med. Chem., 2017, 60, 7897–7909 CrossRef CAS PubMed; (c) M. Hrast, K. Rozman, M. Jukic, D. Patin, S. Gobec and M. Sova, Bioorg. Med. Chem. Lett., 2007, 27, 3529–3533 CrossRef PubMed; (d) A. Ward, L. L. Dong, J. M. Harris, K. K. Khanna, F. Al-Eje, D. P. Fairlie, A. P. Wiegmans and L. G. Liu, Bioorg. Med. Chem. Lett., 2007, 27, 3096–3100 CrossRef PubMed; (e) H. A. Abuelizz, R. E. Dib, M. Marzouk, E. H. Anouar, Y. A. Maklad, H. N. Attia and R. A1-Salahi, Molecules, 2017, 22, 1094–1107 CrossRef PubMed; (f) G. F. Smith and M. D. Altman, Bioorg. Med. Chem. Lett., 2007, 27, 2721–2726 CrossRef PubMed.
- (a) Y. Y. Peng, G. Y. S. Qiu, Q. Yang, J. J. Yuan and Z. H. Deng, Synthesis, 2012, 44, 1237–1246 CrossRef CAS; (b) H. Lu, Q. Yang, Y. R. Zhou, Y. Q. Guo, Z. H. Deng, Q. P. Ding and Y. Y. Peng, Org. Biomol. Chem., 2014, 12, 758–764 RSC; (c) M. k. Wei, W. M. Chai, R. Wang, Q. Yang, Z. H. Deng and Y. Y. Peng, Bioorg. Med. Chem., 2017, 25, 1303–1308 CrossRef CAS PubMed; (d) Y. Zhao, W. S. Liu, Q. Li, Q. Yang, W. B. Chai, M. J. Zeng, R. H. Li and Y. Y. Peng, Bull. Environ. Contam. Toxicol., 2015, 94, 376–381 CrossRef CAS PubMed.
- X. Zhao, Y. R. Zhou, Q. Yang, Y. P. Xie, Q. P. Ding, Z. H. Deng, M. Zhang, J. S. Xu and Y. Y. Peng, Synthesis, 2013, 45, 3245–3250 CrossRef CAS.
- L. D. Hu, H. F. Xu, Q. Yang, Z. H. Deng, C.-Y. Yu and Y. Y. Peng, J. Organomet. Chem., 2017, 843, 20–25 CrossRef CAS.
- (a) W. Gao, Q. P. Ding, J. J. Yuan, X. C. Mao and Y. Y. Peng, Chin. J. Chem., 2017, 35, 1717–1725 CrossRef CAS; (b) W. Gao, C. Gong, L. C. Xu, R. Gan and W. Y. Huang, Faming Zhuan Li Shen Qing, CN106632086, 2017; (c) W. Gao, Y. Y. Peng and Q. P. Ding, Faming Zhuan Li Shen Qing, CN106632087, 2017.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, 1H and 13C NMR spectra of compounds 3 and 4. See DOI: 10.1039/c9ra02979f |
| This journal is © The Royal Society of Chemistry 2019 |