Open Access Article
Open Access ArticleThe effect of ISR on OFMSW during acidogenic fermentation for the production of AD precursor: kinetics and synergies
Abdul F. Soomroa,
Zhe Nib,
Li Ying c and
Jianguo Liu
c and
Jianguo Liu *a
*a
aSchool of Environment, Tsinghua University, Beijing, 100084, China. E-mail: jgliu@tsinghua.edu.cn
bBeijing GeoEnviron Engineering & Technology, Inc, Beijing 100095, China
cKey Laboratory of Clean Energy of Liaoning, College of Energy and Environment, Shenyang Aerospace University, Shenyang 110136, China
First published on 10th June 2019
Abstract
Acidogenic fermentation of the organic fraction of municipal solid waste (OFMSW) and its components (food waste, paper waste) was studied in a batch percolator reactor without artificial pH adjustment. The effect of inoculum to substrate ratio on process performance, in terms of pH, hydrolysis and volatile fatty acid (VFA) production, has been investigated. The inoculum to substrate ratio (ISR) was varied from 0 to 0.36 VS/VS and at optimized conditions for fermentation of OFMSW, with ISR 0.23, pH, hydrolysis and acidification yield were 5.5, 625 mg sCOD per g BD VS and 408 mg g−1 BD VS respectively. Due to the uplift of pH from 4 to 5.5 because of addition of ISR, the VFA composition was dominated mostly by butyric, acetic acids and propionic acid. Kinetics regarding rate of hydrolysis and acidification were calculated and reported. A significant synergistic effect was noticed in the acidification and hydrolysis, which were 1.76 and 1.35 fold higher than individual components (paper waste and food waste) of OFMSW, respectively and approximately 70% of biodegradable solid carbon solubilized into the liquid carbon within a short retention time of 78 h.
1. Introduction
The organic fraction of municipal solid waste (OFMSW) is currently one of the major interests in the research community due to its high biodegradability (BD) and its potential for environmental pollution.1 Especially in developing countries, OFMSW is characterized by a wide range of easily biodegradable organic matter which includes 37–51% carbon and 80–90% volatile solids.2 Food waste (FW) and paper waste (PW) are two major components of OFMSW, comprising a total of ca. 90%.3 Simultaneously the nature of these wastes differ to a greater extent towards acidification. While PW contains a large amount of carbohydrate, it has a high C/N ratio which corresponds to the lack of basic raw materials for the production of amino acids for microorganisms.4 Conversely, food waste (FW) is nitrogen-rich due to the abundant availability of protein.5 Therefore, the supply of essential nitrogen element from FW can enhance the acidogenic fermentation of OFMSW. However, in municipal waste, PW and FW are always mixed.Moreover, MSW with high organic fraction is a potential resource, if treated under controlled conditions.6,7 From this perspective, volatile fatty acids (VFA), generated via anaerobic fermentation of OFMSW, is considered as an economical and feasible technology practice, since these VFAs have a broad range of practical applications which includes methane recovery from anaerobic digestion, biological nutrient removal (BNR) in modern municipal and industrial wastewater treatment plants (WWTP), polyhydroxyalkanoates (PHA) production.8–10 The composition and concentration of these VFAs are influenced by several factors, including hydraulic retention time (HRT), pH, initial organic load (IOL), inoculum to substrate ratio (ISR), C/N ratio, particle size and reactor modes.6,11–14
Most of the studies were reported for enhancement of VFA production through adjustment of pH by adding different chemicals. However, it is not economically viable at large-scale applications. Moreover, disposal of bioreactors effluent with high concentration of chemicals may lead to high cost and can have negative impacts on bacterial community.13 Some studies reported that VFAs could be efficiently produced without artificially controlling the pH.15,16 The pH can be elevated by applying different processing methods such as ultrasonication pretreatment,17 change in temperature 45–70 °C,18,19 ISR 0.2–1 (ref. 20) and mixed fermentation.21,22 Among all, ISR and mixed fermentation methods are recognized for their practical applicability at the large-scale for the separation of biodegradable dissolved organic matter as a renewable carbon sources for AD precursor.
ISR (microorganism) is the fundamental driver to accelerate the acidogenic process. The inoculum type and its quantity are significant factors that affect the hydrolysis and acidification pathways.23 Most of the research studies reported in the literature investigated the significance of ISR during acidogenic fermentation of FW with pH controlled by external means12,24 Furthermore, a wide range of ISR values were employed in the existing studies, however, it is quite complicated to compare different substrates and inoculum used. For example, an ISR 1 was suggested for the acidogenic fermentation of grass,25 0.02 for manure,26 0.13 for FW20 and 0.5 for mixed fermentation of excess sludge and FW.21 Several studies have been conducted for VFA production from sole FW.6,11,13,21,22,27–29 Sole PW was used for the production of methane30 and ethanol production at a controlled pH 5.31
However, particular organic components of OFMSW can significantly influence its behavior during anaerobic fermentation. To the best of our knowledge, no study has been conducted to compare the distinction in product characteristics from mixed OFMSW and its sole components (e.g. food waste and paper waste) during the acidogenic fermentation. The results will help to explain the effects of individual fraction of OFMSW on the acidification.
In this study, a series of ISR, namely 0.09, 0.23 and 0.36, were applied for VFAs fermentation of mixed OFMSW in percolator reactor, with respect to the comprehensive evolution of products characteristic and their kinetics. The novel aspect of this study was to investigate mixed- and mono-fermentation from OFMSW without pH control, as well as their synergistic effects, based on sole components, were evaluated for hydrolysis and acidogenic stages, respectively. The results obtained above were used to discover the possible pH mechanisms and relative parameters for enhancing acidogenic fermentation of OFMSW.
2. Materials and methods
2.1. Substrate & inoculum
A physical representative mixture of OFMSW was prepared on the basis of composition of the typical municipal solid waste (MSW) of China.3 The representative FW was prepared on the basis of the data reported by Xu et al.,32 which were mediated over the year of investigation. Before loading into the percolator, the synthetic OFMSW was manually shredded to maintain a uniform size of 40–50 mm (FW) and 5 × 20 mm (L × W) paper pieces before being mixed. Anaerobically digested sludge under mesophilic conditions was obtained from the Dongcun waste treatment plant, Beijing, China and after natural sedimentation for 15 days was used as inoculum. Additionally, the sludge was sieved through a 2 mm sieve for removal of large particles. Composition and ultimate analysis are shown in Tables 1 and 2.| Component | TS% | VS% | Dry COD | BD (VS%) |
|---|---|---|---|---|
| a Mixing on the basis of wet weight. | ||||
| FW | 94.52 ± 3.1 | 94.52 ± 2.9 | 1.33 ± 0.01 | 72.3 ± 4.12 |
| PW | 96.46 ± 2.1 | 79.66 ± 3.2 | 1.008 ± 0.053 | 60.2 ± 3.02 |
| OFMSW | 27.78 ± 1.1 | 87.3 ± 3.9 | 1.175 ± 0.04 | 69.37 ± 4.5 |
| AD sludge | 6.45 ± 0.4 | 40.3 ± 2.4 | 0.43 ± 0.06 | 15.2 ± 0.41 |
| Component/element | Ultimate analysis (%) | Component analysis (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Oxygen | Sulphur | TKN | Crude lipid | Crude protein | Crude carbohydrate | Crude fiber | |
| Food waste | 47.05 ± 0.26 | 6.58 ± 0.29 | 2.869 ± 0.2 | 37.72 ± 1.6 | 0.27 ± 0.015 | 3.09 ± 0.2 | 4.74 ± 0.13 | 19.33 ± 0.81 | 12.5 ± 0.41 | 58.43 ± 1.7 |
| Paper waste | 33.15 ± 0.5 | 4.89 ± 0.08 | 0 | 41.67 ± 0.1 | 0.042 ± 0.04 | 0 | 1.89 ± 0.07 | 0 | 0 | 76.3 ± 2.3 |
| OFMSW | 40.31 ± 0.31 | 5.76 ± 0.12 | 1.475 ± 0.1 | 39.64 ± 0.8 | 0.159 ± 0.005 | 1.67 ± 0.2 | 3.3 ± 0.04 | 9.6 ± 0.12 | 6.8 ± 0.041 | 68.2 ± 2.11 |
| AD sludge | 17.25 ± 0.07 | 2.24 ± 0.06 | 1.71 ± 0.014 | 18.72 ± 0.08 | 0.78 ± 0.015 | 2.33 ± 0.01 | 6.5 ± 0.05 | 14.56 ± 0.31 | 0 | 18.94 ± 2.6 |
2.2. Percolator reactor and experimental setup
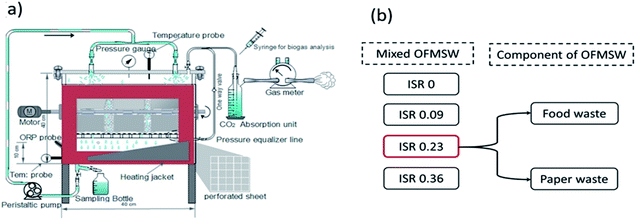
The laboratory scale percolation reactor consisting of a rectangular tank with a total volume of 30 L and working volume 20 L, as shown in Fig. 1, was used in the study.The reactor was manufactured by BAXH environment system Co. Ltd. Beijing. The reactor was designed, in accordance with the literature, with a surface to volume ratio of 2.5, to avoid side-effects at the reactor walls.33 The height of reactor was divided into top and bottom sections and were separated by a perforated sheet (diameter 0.5 mm and 15% perforation area) positioned 10 cm from the bottom of the reactor. While this arrangement facilitates to hold the solid substrate, a geotextile sheet was placed on the perforated sheet to avoid migration of solid particles into a bottom section of the reactor, thus creating a 5 L volume at the lower part of the reactor for temporary leachate collection and storage. The leachate was recirculated with the help of a peristaltic pump (WT600-2J) to the upper part of the reactor and is uniformly distributed at the surface by means of a sprinkler. Backwashing, to clear the blockages in the perforated sheet, was done by using a peristaltic pump (in reverse direction).
Two sets of laboratory-scale experiments were performed to determine separation of biodegradable organic carbon from OFMSW at liquid to solid ratio (L/S) 5(∼48 g VS L−1). Experimental setup is as shown in Fig. 1b. In the first set of experiments, three different ISR were used (0.09, 0.23 and 0.36) and compare with control (ISR 0) to determine the effect of ISR on hydrolysis and acidification for the optimization of process. In the second set of experiments, the acidogenic fermentation for sole components of OFMSW (PW and FW) at optimized ISR 0.23 to explore the synergistic effect.
All the experiments were done in duplicate, while the optimized batch was run in triplicate to ensure the authenticity of the results. The percolation reactor was operated with a hydraulic retention time (HRT) of 120 h at mesophilic temperature 35 ± 2.
2.3. Analytical methods
The concentrations of total solids (TS), volatile solids (VS), chemical oxygen demand (COD), nitrogen–ammonium (NH4+–N), pH, total kjeldahl nitrogen (TKN) were identified according to Standard Methods.34 Crude protein and lipids concentration were determined by TKN (multiplied by a factor of 6.25) and Soxhlet extraction method with petroleum ether used as organic solvent respectively. Biodegradability batch analyses was performed in accordance with the guidelines defined by Angelidaki.35 The elemental analysis (CHNOS) was performed with an elemental analyzer (Equipment CE 440; EAI USA). Dissolved organic carbon (DOC) was measured by using TOC analyzer (TOC-Vcph), Shimadzu, Kyoto, Japan.Volatile fatty acid (VFA) were measured using a gas chromatograph (GC-2014; Shimadzu, Japan) equipped with a capillary column (Stabilwax-DA, 30 m × 0.32 mm × 0.25 μm; Restek, Bellefonte, PA, USA) and flame ionization detector. A detailed description of the methodology to analyze VFAs is described in the literature elsewhere.32 The concentration of proteins and reducing sugars were measured by using Lowry–Folin with bovine serum albumin (BSA) as standard,36 Dinitrosalicylic acid method with glucose as a standard,37 respectively. The samples were centrifuged for 10 minutes at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and then were filtered (0.45 μm). To determine soluble COD, DOC, VFA, TAN, reducing sugar and protein, the same method as discussed above was adopted. The VFAs yield was calculated as the sum of the measured acetic, n-butyric, propionic, iso-valeric, n-valeric and iso-butyric acids. The conversion factors used for determination of the chemical oxygen demand (COD) of soluble organic materials were 1.07 for g COD g−1 acetic acid, 1.5 for g COD g−1 protein (assumed as (C4H6.1O1.2N)x), 2.04 for g COD g−1 valeric acid, 1.51 for g COD g−1 propionic acid, 1.82 for g COD g−1 butyric acid and 1.07 g COD g−1 reducing sugar (C6H12O6).
000 rpm and then were filtered (0.45 μm). To determine soluble COD, DOC, VFA, TAN, reducing sugar and protein, the same method as discussed above was adopted. The VFAs yield was calculated as the sum of the measured acetic, n-butyric, propionic, iso-valeric, n-valeric and iso-butyric acids. The conversion factors used for determination of the chemical oxygen demand (COD) of soluble organic materials were 1.07 for g COD g−1 acetic acid, 1.5 for g COD g−1 protein (assumed as (C4H6.1O1.2N)x), 2.04 for g COD g−1 valeric acid, 1.51 for g COD g−1 propionic acid, 1.82 for g COD g−1 butyric acid and 1.07 g COD g−1 reducing sugar (C6H12O6).
2.4. Analytical techniques
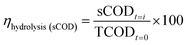 | (1) |
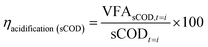 | (2) |
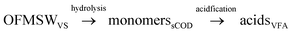 | (3) |
The relationship between substrate and product under the first order kinetics, it expressed in eqn (4) and (5).
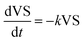 | (4) |
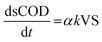 | (5) |
| sCOD = sCODi=0 + αVSi=0(1 − e−khti) | (6) |
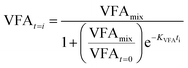 | (7) |
3. Results and discussion
3.1. Effect of ISR on process performance of acid fermentation
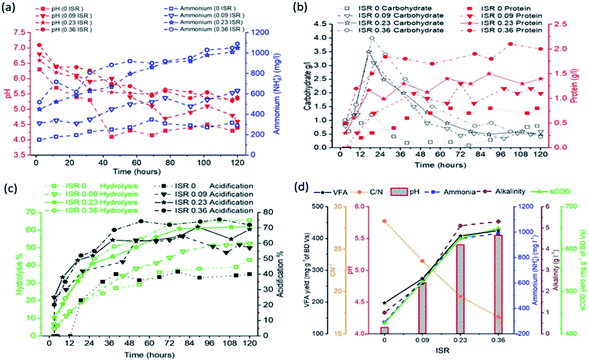
The stable performance of acid fermentation is influenced mainly by the accumulation of organic acids and decrease of pH. The positive effects of ISR addition on pH increase at the initial stage of fermentation were observed. In particular, an initial pH value of 6.4–7.2, when using ISR > 0.09. As the fermentation proceeded, the pH values of reactors with ISR 0.23 and 0.36 decreased slowly and maintained a relatively stable level of 5.5 throughout the experimental period. However, pH values in ISR 0.09 and control reactors were dropped to ∼4.0–4.5, which is below the optimal value for acid fermentation.12
The results regarding ammonia concentration are pointed out in Fig. 2a. Ammonia concentrations in ISR 0.23 and 0.36 reactors showed an increasing trend with time and reached to ∼1100 mg L−1 by the end of the experiment. In contrast, the initial NH4+–N concentrations in ISR 0 and 0.09 reactors were obviously lower, and increased slightly in the subsequent days of acid fermentation, with the final values of 300 and 600 mg L−1, respectively (Fig. 2d). These discrepancies of ammonia concentration among each reactor can be attributed to the higher protein content due to higher ISR (e.g., more inoculum addition) (Table 2). Furthermore, the obtained ammonia could provide alkalinity (Fig. 2d) and neutralize VFAs produced in acid fermentation,20 thus resulting in higher pH values in the fermentation liquor (∼5.5).
The soluble carbohydrate concentrations in all the reactors decreased rapidly after the initial peak at hours 18–24 (Fig. 2b).
However, it was observed that the maximum concentration of soluble carbohydrate was 4000 mg L−1 at ISR 0.36 which was almost 4-folds higher than that without inoculum. This result can be explained by accelerated hydrolysis achieved due to the application of high ISR. In contrast, the concentration of soluble protein increased gradually in all the reactors, implying the biological transformation of protein usually occurs after carbohydrate degradation and requires extended period.42
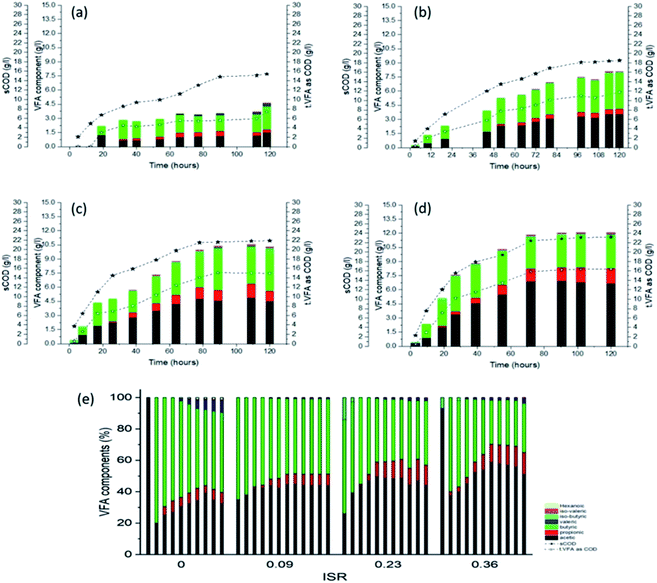 | ||
| Fig. 3 sCOD and VFA concentration and VFA composition under different ISR from OFMSW. (a) ISR 0, (b) ISR 0.09 (c) ISR 0.23 (d) ISR 0.36 and (e) overall compression of VFA compositions. | ||
With the progress in hydrolysis and acidification process, the concentrations of VFAs increased steadily in all reactors, ranging from 16 to 24 g COD L−1 by the end of the study. It should be noted that the increase in ISR enhanced the VFAs production. At ISR 0, it was observed that the VFAs production was ∼197 mg g−1 VS, which was the lowest yield in all ISR reactors.
The VFAs production from ISR 0.23 and 0.36 reactors reached 377 and 410 mg g−1 VS, respectively, which are ∼1.9 and ∼2.1 times higher compared with the control.
During the later phase of test, the concentrations of VFAs from ISR 0.23 and 0.36 reactors still maintained at relative and high level, possibly as the accumulation of fatty acids alters the system buffering condition leading to retardation of the rate of VFA production.
Butyric was dominating in all batches during the initial stage. Subsequently, butyrate-type fermentation was observed corresponding to the low ISR (e.g. 0 and 0.09), possibly due to pH effort. In contrast, it was observed that when ISR ≥ 0.23, butyrate type fermentation shifted to mixed-type and acetic-type fermentation which includes 42–53% of acidic acids and ∼14% of propionic. Hawkes et al.43 reported that, with the increase in the pH, butyric was converted to acidic and propionic acid and the authors attributed the change could be because of change in the dominant microbial species or change in the metabolic pathway within the same bacterial population. In the present study, the propionic acid accumulation was noticed at relatively low levels in ISR 0.23 and 0.36 reactors, which was suitable for the subsequent usage, such as methane production.
The comprehensive comparison of VFAs production in this study with the studies reported in literature is showed in Table 4. The positive effects of optimizing ISR addition, without pH adjustment, on acids production were satisfactory.
| Stage | Parameters | ISR 0 | ISR 0.09 | ISR 0.23 | ISR 0.36 |
|---|---|---|---|---|---|
| Hydrolysis | α | 0.307 ± 0.012 | 0.371 ± 0.023 | 0.4345 ± 0.041 | 0.456 ± 0.047 |
| Kh (h−1) | 0.019 ± 0.005 | 0.022 ± 0.0063 | 0.0366 ± 0.0071 | 0.0381 ± 0.0052 | |
| R2 | 0.976 | 0.998 | 0.992 | 0.997 | |
| SE | 0.003 | 0.0012 | 0.004 | 0.006 | |
| Acidogenesis | VFAmax (g L−1) | 7.395 ± 0.347 | 11.722 ± 0.417 | 13.53 ± 0.340 | 15.9 ± 0.573 |
| KVFA (h−1) | 0.0465 ± 0.0049 | 0.0494 ± 0.0057 | 0.0803 ± 0.0099 | 0.0790 ± 0.0113 | |
| R2 | 0.965 | 0.978 | 0.979 | 0.962 | |
| SE | 0.0012 | 0.002 | 0.008 | 0.0009 |
| Substrate | Seeding | Optimal condition | Reactor mode and capacity | Ac: % & Hy: % | VFA production | pH | Ref. |
|---|---|---|---|---|---|---|---|
| Temp* °C, OLR+ g VS L−1, ISR^, SRTx days & particle size† mm | |||||||
| a A.S.C = acidogenic seed culture. ND = not define (crushed by an electrical blender)†, FW = food waste, KW = kitchen waste, ES = excess sludge, AD = anaerobic digestion. WAS = waste activated sludge, PW = paper waste, Ac = acidification, C. Ac = carbon acidification, Hy = hydrolysis, SRT = solid retention time Adj = adjustable. OFMSWa derived from MBT and component doesn't show. OFMSWb derived from mixture of fruit, vegetable and kitchen waste. | |||||||
FW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ES ES |
WAS | 40*, 9+, 0.2^, 7x & ND† | Semi-conti. | Ac: 83% & Hy: 63% | 867 mg g−1 VS | 5.2 not fixed | 21 |
| FW | AD sludge | 35*, 11+, 8x & ND† | Batch, 4.5 L | Ac: 75% | 471 mg g−1 VS removal | 6 adj | 6 |
| FW | AD sludge | 35*, 10+, 0.5^ 10x | Batch, 0.5 L | Ac: 80% & Hy: 31% | ND | 5.5 adj | 11 |
| OFMSWa | AD sludge | 55*, 44.4+, 1.9x & 15 mm | Semi-conti., 10 L | Ac: 61% & Hy: 59.6% | ND | 5–5.5 adj | 46 |
| FW | AD sludge | 35*, 9+, 8x & ND† | Semi-conti., 2 L | Ac: 63% | 333 mg COD per g VS | 5.5 adj | 28 |
| OFMSWb | A.S.C | 35*, 28+, 80x & 4† | LBR, 5 L | Ac: 60% & Hy: 61% | 7 g L−1 | 5.5 adj | 26 |
| OFMSW | AD sludge | 37*, 48+, 0.23^ 3.25x & 40–50 mm | Percolator B, 20 L | Ac: 65% & Hy: 64% | 377.5 mg sCOD per g VS | 5.5 not fixed | Current study |
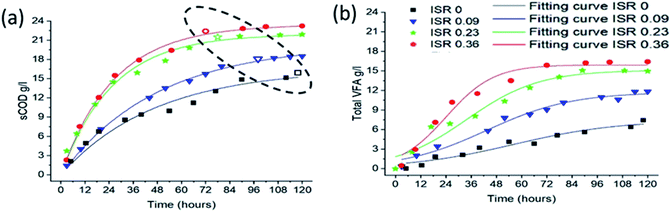 | ||
| Fig. 4 Kinetic analysis of different ISR during the hydrolysis and acidification process. (a) sCOD production under different ISR. (b) T VFA production under different ISR. | ||
The increase in ISR had a direct influence on the hydrolysis and acidification efficiencies: the highest hydrolysis and acidification efficiencies were 64% and 74%, respectively, at ISR 0.36, followed by ISR 0.23 (ηHy: 62 and ηAc: 70), ISR 0.09 (ηHy: 50 and ηAc: 60) and control ISR 0 (ηHy: 40 and ηAc: 40). Overall, hydrolysis rate as stipulated by the increasing production in sCOD from 0.019 to 0.038 per hour was observed as ISR was increased from 0 to 0.36. ISR 0.036 had a maximum value for α, Kh and VFAmax, which were 0.456, 0.0381 h−1 and 15.9 g L−1, receptively. These values were almost two-fold higher than the control (ISR 0). The hydrolysis rate constant (0.91 per day) obtained at the ISR 0.36 in this study was much higher than the reported value,44 which was due to high OLR used in this study.
However, it is worth to note that a slightly decrease of KVFA appeared with ISR 0.36 as compared to ISR 0.23, which might be attributed to quicker VFAs degradation with more inoculum addition. Similar result was also reported by Tomei et al.45 Hence, ISR 0.23 was chosen as optimal parameter in order to avoid the undesired loss of organic carbon in substrate.
3.2. Comparison of individual component and OFMSW for the process performance
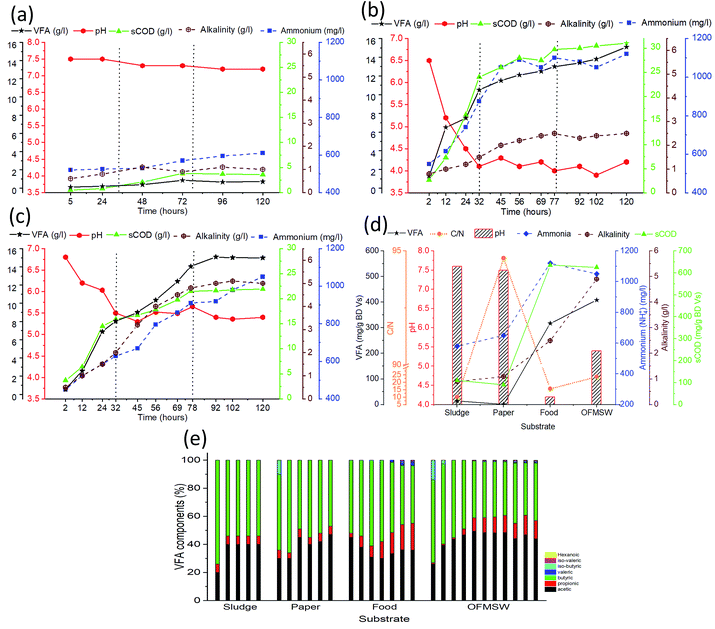
The pH of components of OFMSW (PW and FW) and mixed OFMSW were not adjusted artificially during fermentation process; they fluctuated in a range of 7.2–7.5, 4.1–6.5 and 5.4–6.5, respectively. Sole PW and FW fermented at ISR 0.23 and 120 HRT is shown in Fig. 5.The soluble products during the acidogenic fermentation of sole PW was observed to be lowest and, in contrast, the pH was observed to be highest as compared to FW and OFMSW. Due to the high C/N ratio, around 96, which imply that sufficient nitrogen is not available for the synthesis of bacteria to degrade the cellulosic natured substrate. This could be the possible reason for not recommending sole PW for acidification.30
Sole FW hydrolysis was noticed to rapidly increase up to 66% with highest production of sCOD 28 g L−1. On the other hand, pH rapidly dropped to reach the lowest value, 4.2, during the first 60 h. Acidification efficiency started from 40%, which was highest.
However, acidification efficiency was almost same throughout the experiments while the efficiency of OFMSW increased up to 70% at the end of experiments. It was noticed that the accumulation of carbohydrate and protein was 2 and 2.5 g L−1, respectively, which is ca. two-fold higher than the OFMSW. These results indicated that process of acidification and hydrolysis was suppressed due to the low pH 4.2, the organic acids freely pass through the cell membrane of microbes, consequently inhibited the overall acidogenic process.47 Similar results were reported by Jiang et al.5 and Wu et al.6,21
Acidogenic fermentation of mixed OFMSW (FW and PW at mixing ratio of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 by VS), during the initial phase of hydrolysis, soluble part of carbohydrate and protein from FW rapidly converted into VFA which caused a sudden drop in pH to 5.4 within 32 hours, which is 26% higher than sole FW. Consequently, the hydrolysis efficiency and acidification efficiency reached to 45% and 40%, respectively. Ammonia concentration showed contrasting values, i.e., 1050 mg L−1, 8.6% lower than FW and plausible reason could be the higher protein content in FW as compared to OFMSW as shown in Fig. 5 and Table 2. It can be concluded that ammonia is not the only parameter responsible for the pH stabilization in mixed fermentation.
45 by VS), during the initial phase of hydrolysis, soluble part of carbohydrate and protein from FW rapidly converted into VFA which caused a sudden drop in pH to 5.4 within 32 hours, which is 26% higher than sole FW. Consequently, the hydrolysis efficiency and acidification efficiency reached to 45% and 40%, respectively. Ammonia concentration showed contrasting values, i.e., 1050 mg L−1, 8.6% lower than FW and plausible reason could be the higher protein content in FW as compared to OFMSW as shown in Fig. 5 and Table 2. It can be concluded that ammonia is not the only parameter responsible for the pH stabilization in mixed fermentation.
After initial phase, PW would be hydrolyzed due to favorable pH conditions.48 Hydrolysis of PW provides two opportunities, (1) cellulose degrade into glucose, which is the most suitable substrates for VFA production. (2) It releases carbonates, a major additive in PW which accounts ca. 21%. Ultimately, in this case, alkalinity increased from 2 to 5 g L−1 which is two-fold higher than sole FW. In this way performance of mixed fermentation process was much better than the sole FW, especially after the initial phase31,49 shown in Fig. 5a, the pH of mixed fermentation was in the range of 5.3–5.5, is optimal for hydrolysis as well as acidification.21 The hydrolysis and acidification efficiency, and ammonia gradually increased from 40–64%, 50–70%, and 600–1050 mg L−1 respectively. The soluble carbohydrate and protein were stable due to their balanced conversion to VAF. During the course of time the overall pH was stable. The results demonstrated that fermentation of OFMSW concurrently accelerated the substrate acidification. It affects the composition of VFA and butyric acid was reduced from 60–40% in contrast to the acetic acid which increased from 26–41%, due to the pH uplift from 4.2 to 5.4 (sole FW to mixed OFMSW) recorded among all batches investigated in the present study and it might be due to plenty of soluble carbohydrate available in fermentation broth.21
| α = Experimental Yield/Theoretical Yield | (8) |
| Theoretical yield = yieldPaper × paper%BD + yieldFood × food%BD | (9) |
| Parameters | Paper | Food | OFMSW (theoretical) | OFMSW (experimental) | Synergistic effect | |
|---|---|---|---|---|---|---|
| COD | Biodegradability | 0.6 | 0.7 | 0.655 | 0.7 | 1.069 |
| sCOD (g−1 L) | 3.5 | 28.5 | 17.25 | 21.5 | 1.246 | |
| VFA (g−1 L) | 0.805 | 15.105 | 8.813 | 14.1 | 1.59 | |
| Carbon | Biodegradability | 0.55 | 0.65 | 0.605 | 0.65 | 1.074 |
| DOC (g−1 L) | 1.56 | 9.8 | 6.092 | 8.2 | 1.35 | |
Overall maximum synergistic effect was found on acidification (VFA) with 76% and 59% of carbon and COD, respectively. It also affected hydrolysis in 35% and 24.6% of carbon and COD respectively. A slight synergistic effect has been observed in biodegradability. There are two plausible possible reasons for synergies enhancing during hydrolysis and acidification; the first reason could be due to achieved pH (5.2–5.6) which is favorable for acidogenic fermentation (detail discuss in pH section).12 Same results were observed during the co-fermentation of food and excess sludge.21 At this pH, PW hydrolyzes into soluble carbohydrate; it was the preferred substrate by the microbes. It is firstly degraded to glucose, then to pyruvate. Pyruvate is an important intermediate product that is readily oxidized to acetyl-CoA, which can be further utilized to produce acetic and butyric by diverse enzymatic actions.52 Elliston et al.53 reported that the PW would be hydrolyzed under pH 5–5.5. The second reason might be microbial shift change due to the mixed fermentation, although no significant evidence was observed in the present study. It was suggested that bacteria, such as Clostridium the ocellus, may facilitate the extraction of cellulose from waste paper, which facilitates the degradation of cellulosic materials.54 Further research is required to determine the presence of cellulose-secreting microorganisms within the culture.
3.3. Carbon balance analysis
The carbon mass balance explains the electron flow through the anaerobic process12 and optimized conditions (ISR 0.23, results discussed in Section 3.2) were applied for PW, FW and OFMSW and shown in Fig. 6. In general, carbon represents the organic carbon from organic waste in the form of biodegradable portion of VS. It is reported that the organic carbon can be converted into a wide range of products during hydrolysis–acidification process of anaerobic digestion.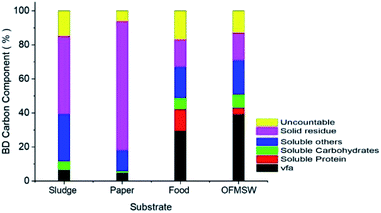 | ||
| Fig. 6 Percentage of different component of biodegradable carbon of mono and mixed fermentation, at HRT 120 h. | ||
Mainly it can be converted into three forms, (1) soluble liquid fraction, into VFAs, proteins, carbohydrate, lactic acid, and alcohols.6 (2) Gaseous fraction, carbon dioxide and methane,12 (3) solid fraction (solid residue).
The gaseous and solid fraction were measured at the start and end of the experiment, while the soluble liquid fraction was measured during the experiment. Gaseous fraction and suspended part of the liquid fraction was mathematically calculated by subtracting the soluble liquid and final solid residue from the initial carbon that is represented as “uncountable carbon.” The quantity of carbon in a soluble liquid fraction was quantified in terms of DOC. Furthermore, carbon distribution in a soluble protein, carbohydrate and VFA were measured according to the molecular weight of the chemical formula.15
Sole sludge effect was negligible because sludge has a low biodegradability around 15% as shown in Table 5. A major portion of carbon in sludge was accountable for solid residue and uncountable part of carbon. The carbon represented by solid residue and VFA were dominant in paper, food and OFMSW, respectively. Additionally, the solid residue mass in PW, protein, carbohydrate and uncountable mass in FW also showed greater percentages than in OFMSW and were 80%, 12%, 6% and 10% of the total carbon, respectively.
This data indicated that the fermentation substrates in PW and FW were not adequately utilized to produce VFAs. However, the carbon masses of protein, carbohydrate and uncountable carbon in FW are much higher than OFMSW, these parts of carbon might be converted into VFA and another soluble part of the carbon into OFMSW.
Biodegradable carbon represented by VFA in FW and OFMSW were 30% and 42%, respectively. Mixed fermentation has more than 70% of biodegradable solid carbon converted into liquid carbon, which is more than the sole component of OFMSW (FW and PW).
4. Conclusions
A novel, practical and cost-effective strategy for the converting biodegradable carbon of OFMSW into VFA via acidogenic fermentation without pH control has been proposed. The optimized VFA production by fermentation of OFMSW (FW + PW) at ISR 0.23 adjustment was attributed to favorable pH, 5.5–5.2, achieved, which subsequently enhanced the hydrolysis and acidification. The maximum synergies were observed during the acidification stage of fermentation, 1.76-fold greater as compared to individual substrate. Hydrolysis and acidification yields were observed to be 625 mg sCOD per g BD VS and 408 mg g−1 BD VS respectively. More than 80% of BD carbon was recovered from OFMSW within 78 h, out of which more than 70% was solubilized into leachate.An approximate 55% of dissolved biodegradable organic carbon was available in the form of VFA and major composition was acetic acid and butyric acid followed by propionic acid, which could be considered as the suitable substrate for methane production via acetotrophic methanogenesis metabolic pathway. Furthermore, the leachate contains soluble part of a protein, carbohydrate and the suspended carbon, which might be further hydrolyzed during methanogenesis to enhance methane production. Kinetics of hydrolysis and acidification rate was calculated and the kh values were noted to be 0.036 h−1 and 0.08 h−1, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the National Key Technology R&D Programs (No. 2014BAC02B02) and the State Natural Sciences Foundation of China (No. 51478240).References
- L. Mu, L. Zhang, K. Zhu, J. Ma and A. Li, Bioresour. Technol., 2018, 247, 103–115 CrossRef CAS PubMed.
- Y. Li, Y. Jin, A. Borrion, H. Li and J. Li, Bioresour. Technol., 2017, 243, 836–845 CrossRef CAS PubMed.
- H. Zhou, A. Meng, Y. Long, Q. Li and Y. Zhang, Renewable Sustainable Energy Rev., 2014, 36, 107–122 CrossRef CAS.
- P. L. Mc Carty, Public Works, 1964, 95, 123–126 CAS.
- C. J. Banks, M. Chesshire, S. Heaven and R. Arnold, Bioresour. Technol., 2011, 102, 612–620 CrossRef CAS PubMed.
- J. Jiang, Y. Zhang, K. Li, Q. Wang, C. Gong and M. Li, Bioresour. Technol., 2013, 143, 525–530 CrossRef CAS PubMed.
- L. Li, Y. Qin, Z. Kong, J. Wu, K. Kubota and Y. Y. Li, Sci. Total Environ., 2019, 652, 709–717 CrossRef PubMed.
- X. Zheng, W. Zhou, R. Wan, J. Luo, Y. Su, H. Huang and Y. Chen, Chem. Eng. J., 2018, 344, 556–564 CrossRef CAS.
- H. Chen, H. Meng, Z. Nie and M. Zhang, Bioresour. Technol., 2013, 128, 533–538 CrossRef CAS PubMed.
- G. Strazzera, F. Battista, N. H. Garcia, N. Frison and D. Bolzonella, J. Environ. Manage., 2018, 226, 278–288 CrossRef CAS PubMed.
- J. Garcia-Aguirre, E. Aymerich, J. González-Mtnez de Goñi and M. Esteban-Gutiérrez, Bioresour. Technol., 2017, 244, 1081–1088 CrossRef CAS PubMed.
- K. Wang, J. Yin, D. Shen and N. Li, Bioresour. Technol., 2014, 161, 395–401 CrossRef CAS PubMed.
- R. Slezak, J. Grzelak, L. Krzystek and S. Ledakowicz, Waste Manag., 2017, 68, 610–617 CrossRef CAS PubMed.
- H. J. Kim, S. H. Kim, Y. G. Choi, G. D. Kim and H. Chung, J. Chem. Technol. Biotechnol., 2006, 81, 974–980 CrossRef CAS.
- L. Feng, Y. Chen and X. Zheng, Environ. Sci. Technol., 2009, 43, 4373–4380 CrossRef CAS PubMed.
- R. Rajagopal, A. Ahamed and J.-Y. Wang, Bioresour. Technol., 2014, 167, 564–568 CrossRef CAS PubMed.
- W. Guo, Q. Wu, S. Yang, H. Luo, S. Peng and N. Ren, RSC Adv., 2014, 4, 53321–53326 RSC.
- M. He, Y. Sun, D. Zou, H. Yuan, B. Zhu, X. Li and Y. Pang, Procedia Environ. Sci., 2012, 16, 85–94 CrossRef CAS.
- J. Yu, Y. Zhao, H. Zhang, B. Hua, X. Yuan, W. Zhu, X. Wang and Z. Cui, Waste Manag., 2017, 59, 487–497 CrossRef CAS PubMed.
- S. Y. Xu, O. P. Karthikeyan, A. Selvam and J. W. C. Wong, Bioresour. Technol., 2012, 126, 425–430 CrossRef CAS PubMed.
- Q.-L. Wu, W.-Q. Guo, H.-S. Zheng, H.-C. Luo, X.-C. Feng, R.-L. Yin and N.-Q. Ren, Bioresour. Technol., 2016, 216, 653–660 CrossRef CAS PubMed.
- Q. Wu, W. Guo, S. Yang, H. Luo, S. Peng and N. Ren, RSC Adv., 2015, 5, 103876–103883 RSC.
- S. Dechrugsa, D. Kantachote and S. Chaiprapat, Bioresour. Technol., 2013, 146, 101–108 CrossRef CAS PubMed.
- W. Arras, A. Hussain, R. Hausler and S. R. Guiot, Waste Manag., 2019, 87, 279–287 CrossRef CAS PubMed.
- P. S. Jagadabhi, P. Kaparaju and J. Rintala, Bioresour. Technol., 2010, 101, 2818–2824 CrossRef CAS PubMed.
- E. Dogan, T. Dunaev, T. H. Erguder and G. N. Demirer, Chemosphere, 2009, 74, 797–803 CrossRef CAS PubMed.
- B. Zhang, L.-L. Zhang, S.-C. Zhang, H.-Z. Shi and W.-M. Cai, Environ. Technol., 2005, 26, 329–340 CrossRef CAS PubMed.
- S. J. Lim, B. J. Kim, C. M. Jeong, J. dal rae Choi, Y. H. Ahn and H. N. Chang, Bioresour. Technol., 2008, 99, 7866–7874 CrossRef CAS PubMed.
- N. Liu, J. Jiang, F. Yan, Y. Xu, M. Yang, Y. Gao, A. Aihemaiti and Q. Zou, RSC Adv., 2018, 8, 10457–10464 RSC.
- W. Li, M. A. H. Siddhu, F. R. Amin, Y. He, R. Zhang, G. Liu and C. Chen, Energy Convers. Manage., 2018, 156, 279–287 CrossRef CAS.
- H. Nishimura, L. Tan, Z.-Y. Sun, Y.-Q. Tang, K. Kida and S. Morimura, Waste Manag., 2016, 48, 644–651 CrossRef CAS PubMed.
- S. Xu, X. Kong, J. Liu, K. Zhao, G. Zhao and A. Bahdolla, Waste Manag., 2016, 58, 81–89 CrossRef CAS PubMed.
- A. Shewani, P. Horgue, S. Pommier, G. Debenest, X. Lefebvre, E. Gandon and E. Paul, Bioresour. Technol., 2015, 178, 209–216 CrossRef CAS PubMed.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association, Washington, DC, 20th edn, 2005 Search PubMed.
- I. Angelidaki, M. Alves, D. Bolzonella, L. Borzacconi, J. L. Campos, A. J. Guwy, S. Kalyuzhnyi, P. Jenicek and J. B. van Lier, Water Sci. Technol., 2009, 59, 927–934 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- L. Lin and X. Li, Chemosphere, 2018, 194, 692–700 CrossRef CAS PubMed.
- V. A. Vavilin, B. Fernandez, J. Palatsi and X. Flotats, Waste Manag., 2008, 28, 939–951 CrossRef CAS PubMed.
- Y. Mu, G. Wang and H.-Q. Yu, Bioresour. Technol., 2006, 97, 1302–1307 CrossRef CAS PubMed.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2009, 34, 3313–3323 CrossRef CAS.
- Y. Miron, G. Zeeman, J. B. van Lier and G. Lettinga, Water Res., 2000, 34, 1705–1713 CrossRef CAS.
- F. Hawkes, R. Dinsdale, D. Hawkes and I. Hussy, Int. J. Hydrogen Energy, 2002, 27, 1339–1347 CrossRef CAS.
- V. A. Vavilin, L. Y. Lokshina, J. P. Y. Jokela and J. A. Rintala, Bioresour. Technol., 2004, 94, 69–81 CrossRef CAS PubMed.
- M. C. Tomei, C. M. Braguglia and G. Mininni, Bioresour. Technol., 2008, 99, 6119–6126 CrossRef CAS PubMed.
- M. A. Romero Aguilar, L. A. Fdez-Güelfo, C. J. Álvarez-Gallego and L. I. Romero García, Chem. Eng. J., 2013, 219, 443–449 CrossRef CAS.
- T. Warnecke and R. T. Gill, Microb. Cell Fact., 2005, 4, 25 CrossRef PubMed.
- H. Shiratori, H. Ikeno, S. Ayame, N. Kataoka, A. Miya, K. Hosono, T. Beppu and K. Ueda, Appl. Environ. Microbiol., 2006, 72, 3702–3709 CrossRef CAS PubMed.
- E. Y. Park, A. Michinaka and N. Okuda, Biotechnol. Prog., 2001, 17, 379–382 CrossRef CAS PubMed.
- Y. Zhang, G. S. Caldwell, A. M. Zealand and P. J. Sallis, Biochem. Eng. J., 2019, 143, 91–100 CrossRef CAS.
- C. Rodriguez, A. Alaswad, Z. El-Hassan and A. G. Olabi, Energy, 2018, 154, 119–125 CrossRef CAS.
- Y. Chen, J. Luo, Y. Yan and L. Feng, Appl. Energy, 2013, 102, 1197–1204 CrossRef CAS.
- A. Elliston, S. R. A. Collins, C. B. Faulds, I. N. Roberts and K. W. Waldron, Appl. Biochem. Biotechnol., 2014, 172, 3621–3634 CrossRef CAS PubMed.
- M. Suto and F. Tomita, Induction and catabolite repression mechanisms of cellulase in fungi, 2001, vol. 92 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |