Open Access Article
Open Access ArticleRuxSe@MoS2 hybrid as a highly efficient electrocatalyst toward hydrogen evolution reaction†
Qi Chenab,
Kefeng Wang *b,
Jingjing Qinb,
Songzhu Wangb,
Wei Weib,
Jingge Wangc,
Qi Shend,
Peng Qub and
Daosheng Liu*a
*b,
Jingjing Qinb,
Songzhu Wangb,
Wei Weib,
Jingge Wangc,
Qi Shend,
Peng Qub and
Daosheng Liu*a
aCollege of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001, Liaoning, China. E-mail: dsliu05@126.com
bHenan Engineering Center of New Energy Battery Materials, Henan Key Laboratory of Biomolecular Recognition and Sensing, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, Henan, China. E-mail: wangkf2007@163.com
cSchool of Physics and Engineering, Henan University of Science and Technology, Luoyang 471023, China
dCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China
First published on 1st May 2019
Abstract
Alkaline hydrogen evolution reaction (HER) requires highly efficient and stable catalytic materials, the engineering of which needs overall consideration of the water dissociation process as well as the intermediate hydrogen adsorption process. Herein, a RuxSe@MoS2 hybrid catalyst was synthesized by the decoration of MoS2 with RuxSe nanoparticles through a two-step hydrothermal reaction. Due to the bifunctionality mechanism in which Ru promotes the water dissociation and the nearby Se atoms, unsaturated Mo and/or S atoms act as active sites for the intermediate hydrogen adsorption, the hybrid catalyst exhibits an exceptional HER performance in basic media with a rather low overpotential of 45 mV at a current density of 10 mA cm−2 and a small Tafel slope of 42.9 mV dec−1. The synergetic effect between RuxSe and MoS2 not only enables more catalytically active sites, but also increases the inherent conductivity of the hybrid catalyst, leading to more favorable HER kinetics under both alkaline and acidic conditions. As a result, RuxSe@MoS2 also demonstrates an enhanced catalytic activity toward HER in 0.5 M H2SO4 in comparison with pure RuxSe and MoS2, which requires an overpotential of 120 mV to deliver a 10 mA cm−2 current density and gives a Tafel slope of 72.2 mV dec−1. In addition, the hybrid electrocatalyst also exhibits superior electrochemical stability during the long-term HER process in both acidic media and alkaline media.
1. Introduction
The gradual depletion of traditional fossil fuels forces us to search for renewable energies such as solar energy, wind energy, hydrogen energy, etc. Among all these renewable energies, hydrogen energy is deemed as the potential alternative to fossil fuels in future due to its various favorable features including carbon-free footprint, high mass-energy density and simple production methodologies. Water electrolysis coupling with renewable energies is considered as one of the most appealing approaches for large-scale hydrogen generation.1 Due to the sluggish reaction kinetics at the electrode interface, an overpotential is required to drive the HER and the oxygen evolution reaction (OER).2 Platinum-based materials and Ru, Ir-based materials are the state-of-the-art HER and OER catalysts, respectively.3,4 Unfortunately, their scarcity, unaffordable high cost and inferior durability greatly impede the wide application of such catalysts in industrial water-splitting technology. Therefore, it is of paramount importance to develop low-cost and highly efficient electrocatalysts with abundant sources. Relentless efforts have been devoted to exploring highly efficient electrocatalysts with abundant and low-cost transition metal-based materials.3,5–8 However, the electrocatalytic activity of these earth-abundant materials is still far from satisfactory compared with that of Pt-based catalysts. Combining the abundant transition metal-based materials with a low dosage of noble metal could be a promising approach to design highly efficient and low-cost electrocatalysts for HER.MoS2 is considered as the most promising alternative to Pt for electrocatalytic hydrogen evolution reaction due to its low cost, abundant source as well as the excellent electrochemical stability. However, the catalytic activity of MoS2 is greatly limited by the insufficient active sites and inherent poor conductivity. To address these issues, various strategies have been explored to boost the catalytic performance of MoS2-based materials in acidic electrolytes, including morphology and phase control,9–16 defect engineering,17–19 regulating the electronic structures by heteroatom doping,20–25 engineering heterostructures with conducting carbonaceous materials and other transition metal-based materials.26–32 Through these intrinsic and extrinsic modifications, the activities of some MoS2-based composites in acidic electrolytes have been approaching those of Pt-based materials. However, due to the high energy barrier to initiate water dissociation for MoS2, the HER kinetics in alkaline media is sluggish. In order to surmount this obstacle to accelerate the HER process in alkaline media, water dissociation promoters have been integrated for designing heterostructures.29,33–35
Among all the precious metals, Ru with a price 1/25 that of Pt possesses the best water dissociation ability. In this regard, PtRu demonstrates the best electrocatalytic performance for methanol oxidation reaction. The superior catalytic performance is due to the synergetic effect in which Ru promotes the water electro-oxidation to supply oxygen-containing species and Pt serves as active sites for the oxidation of poisonous CO.36,37 However, as the adsorption of oxygenated species on Ru is too strong, Ru has demonstrated the relative lower HER activities under both acidic and basic conditions when compared with other noble metals such as Ir and Pt.38 By forming phosphides (RuP or RuP2) or integration with transition-metal compound and N and/or P-doped carbon materials, the electronic structure of Ru would be well tuned, leading to less strong adsorption of oxygenated species. As a result, RuPx alone or encapsulated in carbon nanomaterials,39–45 carbonaceous materials-supported Ru nanoparticles or nanoclusters,46–55 RuS2/S-doped graphene composite,56 NiCoP@Ru,57 Ru/MoS2,58 Ru/Cu-doped RuO2,59 Ru/CoP,60 et al. have been investigated as highly efficient HER electrocatalysts in alkaline electrolytes. Nevertheless, little attention has been paid to the potential application of ruthenium selenides (RuxSe), though other metal selenides have been widely utilized in catalysis.61–64 As Se centres in several transition metal selenides have been proved to possess favorable free energies for hydrogen adsorption,65,66 RuxSe is expected to be a potential highly efficient HER catalyst in alkaline electrolytes in which Ru could facilitate the water cleavage process to generate hydrogen intermediates. In a more recent study, we have demonstrated that Ru0.33Se nanoparticles-decorated TiO2 nanotube arrays (Ru0.33Se@TNA) could be utilized as a highly efficient alkaline HER catalyst with an overpotential of only 57 mV to deliver a current density of 10 mA cm−2 and a Tafel slope of 50 mV dec−1. More importantly, the current density of Ru0.33Se@TNA exceeds that of 20% Pt/C catalyst at higher overpotentials.67 However, the active sites for the intermediate hydrogen adsorption in RuxSe is limited and the free energies on Se sites are far less favorable than those on the Mo-edge sites in MoS2. Furthermore, MoS2 nanosheets with an ultrahigh specific area enable the engineering of hybrid nanomaterials with other materials. A large variety of materials have been integrated with MoS2 nanosheets to fabricate hybrid catalysts for water electrolysis, and the resultant hybrids usually exhibit enhanced catalytic activities owing to the synergetic effect between the building blocks.32,34,35,68 In this regard, a hybrid catalyst combining MoS2 and RuxSe would demonstrate an impressive electrocatalytic performance toward HER in alkaline electrolytes owing to the bifunctionality mechanism in which Ru promotes the water dissociation and the nearby Se atoms, unsaturated Mo and/or S atoms act as active sites for the intermediate hydrogen adsorption.
In the present work, RuxSe@MoS2 hybrid catalyst was fabricated by integrating RuxSe nanoparticles with MoS2 nanosheets. Owing to the synergetic effect between RuxSe and MoS2, the hybrid catalyst demonstrates a superb electrocatalytic activity toward HER in basic media with an overpotential of only 45 mV to generate a current density of 10 mA cm−2, a small Tafel slope of 42.9 mV dec−1 and an extraordinary electrochemical stability. Furthermore, the hybrid catalyst also shows an enhanced catalytic activity for HER in acidic media in comparison with MoS2 and RuxSe.
2. Results and discussion
The crystal structure of the hybrid catalyst was characterized using an X-ray diffractometer (XRD). Fig. 1 shows the XRD pattern of RuxSe@MoS2 grown on carbon fiber paper. The diffraction peak around 26° is originated from the carbon fiber paper substrate. The peaks at 2θ = 14.5°, 32.7° and 58.3° could be well indexed to the (002), (100) and (110) planes of the standard XRD pattern of hexagonal 2H–Mo2S (JCPDS card no. 37-1492), respectively. It should be noted that RuxSe@MoS2 almost exhibits the same diffraction pattern as the pure MoS2 grown on the carbon fiber paper, suggesting the negligible effect of RuxSe nanoparticle deposition on the crystal structure of MoS2. However, due to the small loading as well as the low crystallinity, the diffraction peaks assigned to the deposited RuxSe nanoparticles could not be detected. To further determine the crystal characteristics of RuxSe nanoparticles, the powder product in the hydrothermal reaction for the synthesis of RuxSe@MoS2 hybrid was also collected for XRD measurement. As shown in Fig. 1, RuxSe powder exhibits two broad diffraction peaks around 2θ = 33.8° and 51.0° assigned to crystalline RuSe2 (JCPDS No. 65-3328), indicating the low crystallinity of RuxSe nanoparticles. It is worth to be pointed out that the crystallinity of RuxSe powder could be greatly enhanced by calcination at 400 °C (Fig. S1, ESI†).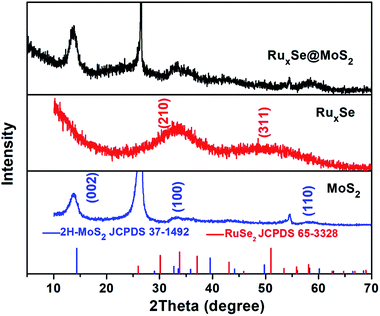 | ||
| Fig. 1 XRD patterns of MoS2, RuxSe and RuxSe@MoS2, and the standard patterns of 2H–MoS2 (JCPDS No. 37-1492) and RuSe2 (JCPDS No. 65-3328). | ||
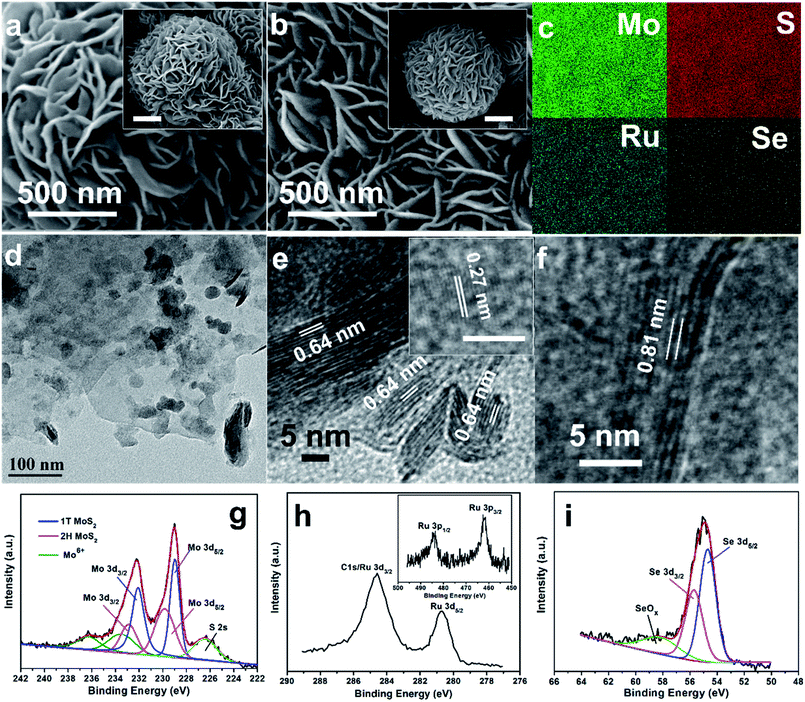
The microstructure of the pure MoS2 and RuxSe@MoS2 hybrid was investigated by scanning electron microscopy (SEM). As demonstrated in Fig. 2a and S2 (ESI†) for the morphology of MoS2 grown on the carbon fiber paper, the carbon fibers are covered with vertically aligned MoS2 sheets with a thickness of 20 nm, and hierarchical micro-flowers composed of interconnected MoS2 nanosheets with an average diameter of 2.5 μm are also formed on the top of MoS2 sheet layer. After integration with RuxSe via a further hydrothermal reaction with RuCl3 and Se powder as the Ru source and Se source, respectively, RuxSe nanoparticles were uniformly anchored on the vertically aligned nanosheets as well as the petals of the micro-flowers (Fig. 2b). It could be obviously noted that all the nanosheets preserve the original alignment, but the surface becomes rough with a thickness increasing to about 30 nm. Energy-dispersive X-ray spectroscopy (EDX) mapping images (Fig. 2c) suggest the uniform distribution of Mo, S, Ru and Se elements throughout the whole film, further confirming the homogeneous distribution of RuxSe nanoparticles on MoS2 nanosheets. The atomic ratio of Ru to Mo in the RuxSe@MoS2 hybrid is determined to be 2.95% according to the EDX spectrum (Fig. S3, ESI†), indicating a rather low usage of Ru for the fabrication of the hybrid catalysts.
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were recorded to further investigate the structural detail of the samples. As shown in Fig. 2d, RuxSe nanoparticles with a diameter range of 20–30 nm are highly dispersed on MoS2 nanosheets without any obvious agglomerates. However, the pure RuxSe synthesized under the same conditions exhibits severe particle aggregation revealed by TEM image shown in Fig. S4 (ESI†). Therefore, MoS2 nanosheets with a large specific area could facilitate the homogeneous distribution of RuxSe nanoparticles, enabling more exposed active sites for catalytic reactions. In the HRTEM images displayed in Fig. 2e and S5,† the lattice fringes with spaces of 0.64 nm and 0.27 nm could be attributed to the (002) and (100) crystallographic planes of 2H–MoS2, respectively. Additionally, the lattice fringe with a larger interplanar space of 0.81 nm in Fig. 2f corresponds to the (002) plane of 1T-MoS2,14 indicating a mixture phase of MoS2 in the RuxSe@MoS2 hybrid.
X-ray photoelectron spectroscopy (XPS) was carried out to further verify the valence states of different elements in RuxSe@MoS2. In the high-resolution Mo 3d spectrum (Fig. 2g), the peaks around 228.7 eV and 231.8 eV could be assigned to Mo 3d5/2 and Mo 3d3/2 of 1T-MoS2, respectively. Another two peaks located at binding energies of 229.6 eV and 232.6 eV are attributed to Mo 3d5/2 and Mo 3d3/2 of 2H–MoS2, respectively.13,14,69,70 The last two weak peaks around 233.3 eV and 236.1 eV are ascribed to Mo 3d5/2 (Mo6+) and Mo 3d3/2 (Mo6+), suggesting partial oxidation of the sample on the surface. Fig. 2h displays the high-resolution Ru 3d spectrum. Two peaks belonging to Ru 3d5/2 and Ru 3d3/2 are observed at the binding energies of 280.6 eV and 284.6 eV. Notably, the peak intensity of Ru 3d3/2 is much stronger than that of Ru 3d5/2 due to the coexist of C 1s peaks in the Ru 3d region.44 Accordingly, the characteristic peaks assigned to Ru 3p3/2 and Ru 3d1/2 are detected at 462.0 eV and 484.7 eV in the high resolution Ru 3p spectrum (Fig. 2h inset).44 For Se 3d spectrum (Fig. 2i), the doublet around 54.4 eV and 55.7 eV could be attributed to Se 3d3/2 and Se 3d5/2, respectively.65
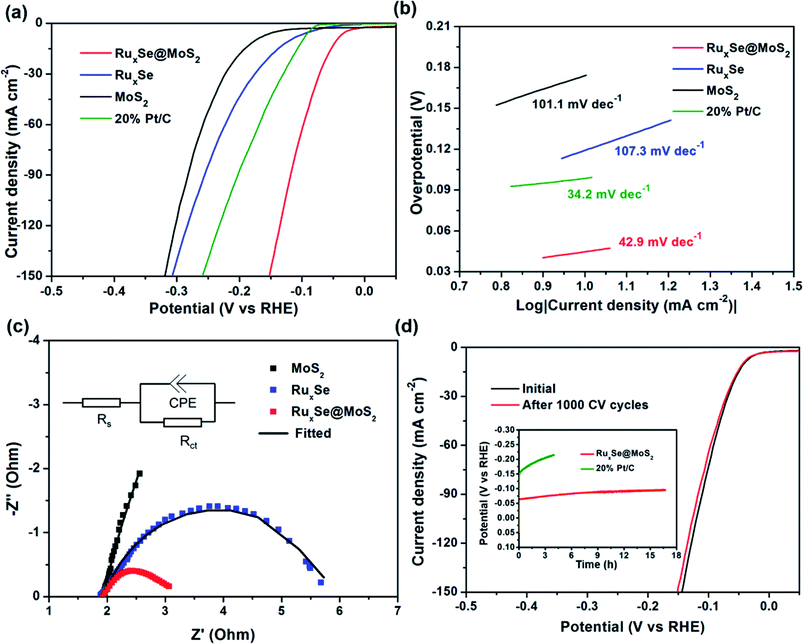
RuxSe@MoS2 was utilized as catalyst for hydrogen evolution reaction in 1.0 M KOH solution, and the HER activity was evaluated by measuring polarization curves using a standard three-electrode configuration. RuxSe@MoS2 grown on the carbon fiber paper with dimensions of 1 cm × 1 cm was directly used as the working electrode, the graphite rod and HgO/Hg electrode were employed as the counter electrode and reference electrode, respectively. For comparison, the HER activities of the pristine MoS2, RuxSe and commercial 20% Pt/C catalysts were also measured under the same conditions. The HER activities of different catalysts were characterized by IR-corrected polarization curves shown in Fig. 3a. It could be obviously seen that MoS2 exhibits a poor HER activity with a high overpotential of 177 mV to acquire a current density of 10 mA cm−2. RuxSe displays a higher HER activity with a smaller overpotential of 119 mV to deliver the same current density. Commercial 20% Pt/C catalyst demonstrates a better HER performance than both MoS2 and RuxSe, which could achieve a current density of 10 mA cm−2 at an overpotential of 98.0 mV. For RuxSe@MoS2 hybrid, the HER performance dramatically enhanced, and the overpotential to afford the same current density for Ru0.33Se@MoS2 reduces to 45 mV, just 132 mV and 74 mV lower than those of MoS2 and RuxSe, respectively. More importantly, the overpotential at 10 mA cm−2 for RuxSe@MoS2 hybrid catalyst is also among the best reported levels so far for MoS2-based HER catalysts in basic electrolyte (Table S1, ESI†).
Tafel slope could be used to provide further insight into the intrinsic HER kinetics of the electrocatalysts. A smaller Tafel slope implies a faster current increase with a specified overpotential change. Fig. 4b shows the corresponding Tafel plots transferred from the polarization curves. MoS2 exhibits a Tafel slope of 101.1 mV dec−1, almost the same as that of RuxSe (107.3 mV dec−1). After hybridization, RuxSe@MoS2 demonstrates an obviously decreased Tafel slope of 42.9 mV dec−1 comparable to the that of the commercial 20% Pt/C catalyst (34.2 mV dec−1), indicating a dramatically improved HER kinetics. The Tafel slope of RuxSe@MoS2 suggests a Volmer–Heyrovsky mechanism during the HER process and the hydrogen desorption is the rate-limiting step. Moreover, we also compared the Tafel slope of RuxSe@MoS2 with those of MoS2-based HER catalysts in basic media (Table S1, ESI†), further demonstrating the superb electrocatalytic HER kinetics of the hybrid catalyst.
In order to further evaluate the inherent HER activity, the exchange current density (J0) at the thermodynamic redox potential (η = 0) was obtained by extrapolating the horizontal intercept of the linear region of the Tafel plot. Accordingly, our hybrid catalyst gives a J0 of 0.91 mA cm−2, far larger than the values for MoS2 (0.19 mA cm−2) and RuxSe (0.78 mA cm−2). The highest J0 for RuxSe@MoS2 implies the most favorable electron transfer kinetics under zero overpotential, leading to the superior electrocatalytic performance toward HER in alkaline media. In addition, the exchange current density for RuxSe@MoS2 is also comparable or larger than the values for those reported MoS2-based alkaline HER catalysts.29,33
To validate the superior charge-transfer kinetics of RuxSe@MoS2 under HER-operating conditions, electrochemical impedance spectroscopy (EIS) measurements were carried out under an overpotential of −200 mV. As shown in Fig. 3c, the semicircle in the low-frequency region of the Nyquist plot represents the charge- transfer resistance between the catalysts and the substrates. The smaller the charge- transfer resistance (Rct) is, the more favorable charge transfer kinetics will be. Compared with RuxSe (3.93 Ω) and MoS2 (27.8 Ω) electrodes, RuxSe@MoS2 electrode exhibits a substantially lower Rct of 1.16 Ω, indicating a superior inherent conductivity which is probably induced by the synergy between RuxSe and MoS2. Therefore, the hybrid affords a more rapid electron transport process and a faster HER kinetics.
The exceptional HER activity of RuxSe@MoS2 may stem from the synergetic coupling effect of MoS2 nanosheets and RuxSe nanoparticles: (i) Ru promotes the dissociation of H2O into Hads and OH−. While Se sites and/or the edge sites of MoS2 with a moderate hydrogen absorption energy are favorable for speeding up the hydrogen generation kinetics. (ii) The homogeneous dispersion of RuxSe nanoparticles on MoS2 nanosheets greatly suppresses particle aggregation, thus ensuring more exposed active sites and enhancing the utilization efficiency of RuxSe. (iii) The hybridization greatly improves catalyst conductivity and enhances the charge transfer efficiency, thus accelerating the electrocatalytic process.
Electrochemical durability of the hybrid catalyst is assessed by a long-term cycling test. Fig. 3d compares the polarization curves recorded before and after 1000 cyclic voltammetry (CV) cycles. The almost overlapped curves indicate a highly electrochemical stability of the hybrid catalyst during the long-term continuous HER test. Moreover, we also conducted the long-term HER test under a constant current density of –20 mA cm−2, and the corresponding time-dependent potential change was recorded in Fig. 3d (inset). Obviously, the potential shows an insignificant variation during a 16 h test, further evidencing the superb stability of our hybrid catalyst. Moreover, the morphology RuxSe@MoS2 hybrid after the long-term stability test was also investigated by SEM and TEM. As shown in Fig. S6 and S7 (ESI†), there is no significant change in the micro-structure of RuxSe@MoS2, indicating a high structural stability of RuxSe@MoS2 during the continuous HER test. Unfortunately, commercial 20% Pt/C catalyst exhibits a rather poor long-term stability for HER test in basic media. As shown in the Fig. 4d (inset), the potential at −20 mA cm−2 reduced from −0.15 V to −0.22 V during only a 4 h continuous HER operation.
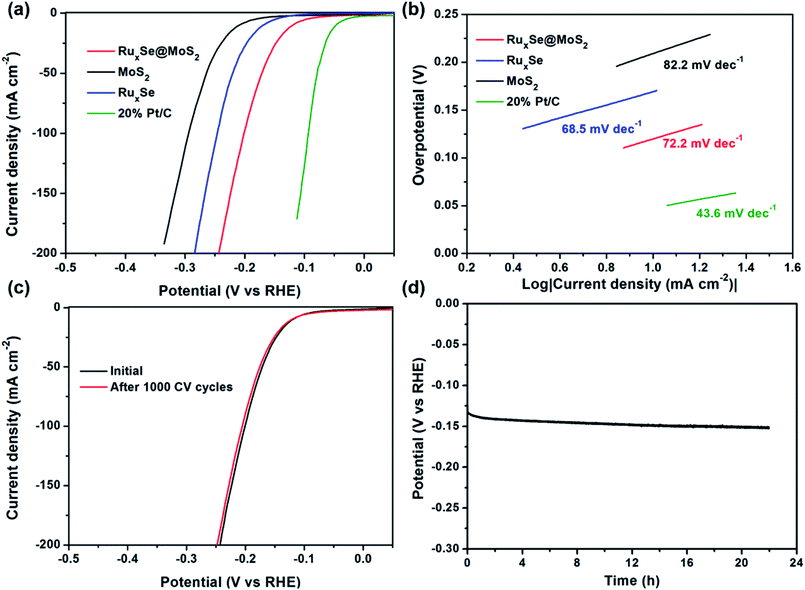
Next, we further examined the HER performance of RuxSe@MoS2 in a 0.5 M H2SO4 electrolyte. Linear sweep voltammetry (LSV) curves of different catalysts were recorded using a three-electrode setup with an Ag/AgCl (saturated KCl solution) electrode as the reference electrode. As expected, commercial 20% Pt/C catalyst shows a superb HER performance with a small overpotential of 47.1 mV to give a 10 mA cm−2 current density and a Tafel slope of 43.6 mV dec−1. RuxSe@MoS2 hybrid catalyst requires an overpotential of 120 mV to achieve a current density of 10 mA cm−2, much smaller than those for pure MoS2 (209 mV) and RuxSe (169 mV), respectively. The Tafel slopes of different catalysts were also calculated according to the LSV curves in order to probe the HER kinetics in acidic media. Compared with the Tafel slopes obtained in alkaline media, both MoS2 and RuxSe demonstrate smaller Tafel slopes under acidic conditions, suggesting faster HER kinetics in acidic media. However, RuxSe@MoS2 hybrid catalyst showed a larger Tafel slope of 72.2 mV dec−1, indicating a decreased HER kinetics under acidic conditions in comparison with that in alkaline situation, as acidic HER process is predominated by the intermediate hydrogen adsorption kinetics without the water dissociation process which would be accelerated by Ru in alkaline media. The exchange current densities for different catalysts in 0.5 M H2SO4 were also calculated. RuxSe@MoS2 shows a larger J0 value of 0.22 mA cm−2 than RuxSe (0.034 mA cm−2) and MoS2 (0.029 mA cm−2), further corroborating the faster electron transfer kinetics in the hybrid catalyst.
The stability of the hybrid catalyst in acidic electrolyte was also assessed by 1000 cycles of CV scanning at a scan rate of 100 mV s−1. As displayed in Fig. 4c, the polarization curve recorded after continuous CV scanning is almost identical to the initial one, indicating an ultrahigh durability under the operation conditions. The long-term hydrogen evolution reaction catalyzed by RuxSe@MoS2 in acidic media was also carried out at a constant current density of −20 mA cm−2. Fig. 4d shows the corresponding time-dependent curve. The potential shows an insignificant variation even after conducting the HER for more than 20 h, further corroborating the supreme stability of RuxSe@MoS2 under acidic conditions. For comparison, we also conducted a long-time HER test for commercial 20% Pt/C catalyst at a same current density. It could be obviously seen from the time-dependent potential curve (Fig. S8, ESI†) that the potential to maintain the same current density various between −60 mV to −130 mV during the long-term HER test, indicating an inferior electrochemical stability of commercial 20% Pt/C catalyst.
3. Conclusions
In summary, we demonstrate a facile two-step hydrothermal method for the decoration of MoS2 nanosheets with RuxSe nanoparticles. Compared with pure MoS2 and RuxSe, the obtained RuxSe@MoS2 hybrid exhibits a superior electrocatalytic activity toward HER in both acidic and alkaline media, which could be ascribed to the synergy effect between MoS2 and RuxSe. The synergy effect endows the hybrid catalyst with an enhanced HER kinetics induced by the proliferated active sites and the accelerated charge-transfer process. The outstanding HER performance in basic media could be also related to the bifunctionality of RuxSe@MoS2 hybrid, in which Ru promotes the water adsorption and dissociation to provide ample intermediate hydrogen, and the nearby Se atoms, unsaturated Mo and/or S atoms act as active sites for the intermediate hydrogen adsorption followed by an electrochemical desorption step to evolve H2. The facile fabrication and impressive catalytic properties for RuxSe@MoS2 make this hybrid a great potential candidate for practical application in electrocatalytic hydrogen evolution reaction. More importantly, our strategy to fabricate highly efficient electrocatalysts based on the low-cost and abundant transitional-metal with the aid of a low usage of noble metal paves a new way for the design of other hybrid systems for electrocatalytic energy conversion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 11504091, U1804138), the Key Research Programs in Universities of Henan Province (18A430023).Notes and references
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Adv. Mater., 2019, 31, 1807134 CrossRef PubMed.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 29, 1605838 CrossRef PubMed.
- W. Zhong, Z. Lin, S. Feng, D. Wang, S. Shen, Q. Zhang, L. Gu, Z. Wang and B. Fang, Nanoscale, 2019, 11, 4407 RSC.
- Q. Ding, B. Song, P. Xu and S. Jin, Chem, 2016, 1, 699 CAS.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529 RSC.
- Z. Wu, B. Fang, A. Bonakdarpour, A. Sun, D. P. Wilkinson and D. Wang, Appl. Catal., B, 2012, 125, 59 CrossRef CAS.
- D. Wang, D. Zhang, C. Tang, P. Zhou, Z. Wu and B. Fang, Catal. Sci. Technol., 2016, 6, 1952 RSC.
- X. Geng, W. Sun, W. Wu, B. Chen, A. Al-Hilo, M. Benamara, H. Zhu, F. Watanabe, J. Cui and T. P. Chen, Nat. Commun., 2016, 7, 10672 CrossRef CAS PubMed.
- J. Wang, N. Wang, Y. Guo, J. Yang, J. Wang, F. Wang, J. Sun, H. Xu, Z.-H. Liu and R. Jiang, ACS Sustainable Chem. Eng., 2018, 6, 13435 CrossRef CAS.
- Y. Li, M. B. Majewski, S. M. Islam, S. Hao, A. A. Murthy, J. G. DiStefano, E. D. Hanson, Y. Xu, C. Wolverton, M. G. Kanatzidis, M. R. Wasielewski, X. Chen and V. P. Dravid, Nano Lett., 2018, 18, 7104 CrossRef CAS PubMed.
- Q. Zhang, H. Bai, Q. Zhang, Q. Ma, Y. Li, C. Wan and G. Xi, Nano Res., 2016, 9, 3038 CrossRef CAS.
- K. Chang, X. Hai, H. Pang, H. Zhang, L. Shi, G. Liu, H. Liu, G. Zhao, M. Li and J. Ye, Adv. Mater., 2016, 28, 10033 CrossRef CAS PubMed.
- M. A. R. Anjum, H. Y. Jeong, M. H. Lee, H. S. Shin and J. S. Lee, Adv. Mater., 2018, 30, 1707105 CrossRef PubMed.
- Z. Wu, C. Tang, P. Zhou, Z. Liu, Y. Xu, D. Wang and B. Fang, J. Mater. Chem. A, 2015, 3, 13050 RSC.
- Z. Wu, B. Fang, Z. Wang, C. Wang, Z. Liu, F. Liu, W. Wang, A. Alfantazi, D. Wang and D. P. Wilkinson, ACS Catal., 2013, 3, 2101 CrossRef CAS.
- L. Yang, W. Zhou, J. Lu, D. Hou, Y. Ke, G. Li, Z. Tang, X. Kang and S. Chen, Nano Energy, 2016, 22, 490 CrossRef CAS.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807 CrossRef CAS PubMed.
- C. Tsai, H. Li, S. Park, J. Park, H. S. Han, J. K. Norskov, X. Zheng and F. Abild-Pedersen, Nat. Commun., 2017, 8, 15113 CrossRef PubMed.
- T. Sun, J. Wang, X. Chi, Y. Lin, Z. Chen, X. Ling, C. Qiu, Y. Xu, L. Song, W. Chen and C. Su, ACS Catal., 2018, 8, 7585 CrossRef CAS.
- S. Wei, X. Cui, Y. Xu, B. Shang, Q. Zhang, L. Gu, X. Fan, L. Zheng, C. Hou, H. Huang, S. Wen and W. Zheng, ACS Energy Lett., 2019, 4, 368 CrossRef CAS.
- Y. Cheng, S. Lu, F. Liao, L. Liu, Y. Li and M. Shao, Adv. Funct. Mater., 2017, 27, 1700359 CrossRef.
- Y. Shi-Ze, G. Yongji, M. Priyanka, Z. Yu-Yang, Y. Gonglan, C. Shuangming, S. Li, T. P. Sokrates, M. A. Pulickel, F. C. Matthew and Z. Wu, Adv. Mater., 2018, 30, 1803477 CrossRef PubMed.
- Y. Shi, Y. Zhou, D. R. Yang, W. X. Xu, C. Wang, F. B. Wang, J. J. Xu, X. H. Xia and H. Y. Chen, J. Am. Chem. Soc., 2017, 139, 15479 CrossRef CAS PubMed.
- Z. Luo, Y. Ouyang, H. Zhang, M. Xiao, J. Ge, Z. Jiang, J. Wang, D. Tang, X. Cao, C. Liu and W. Xing, Nat. Commun., 2018, 9, 2120 CrossRef PubMed.
- L. X. Chen, Z. W. Chen, Y. Wang, C. C. Yang and Q. Jiang, ACS Catal., 2018, 8, 8107 CrossRef CAS.
- Z. Zhao, F. Qin, S. Kasiraju, L. Xie, M. K. Alam, S. Chen, D. Wang, Z. Ren, Z. Wang, L. C. Grabow and J. Bao, ACS Catal., 2017, 7, 7312 CrossRef CAS.
- S. Park, J. Park, H. Abroshan, L. Zhang, J. K. Kim, J. Zhang, J. Guo, S. Siahrostami and X. Zheng, ACS Energy Lett., 2018, 3, 2685 CrossRef CAS.
- Y. Luo, X. Li, X. Cai, X. Zou, F. Kang, H. M. Cheng and B. Liu, ACS Nano, 2018, 12, 4565 CrossRef CAS PubMed.
- X. Zhang and Y. Liang, Adv. Sci., 2018, 5, 1700644 CrossRef PubMed.
- H. Zhang, L. Yu, T. Chen, W. Zhou and X. W. Lou, Adv. Funct. Mater., 2018, 28, 1807086 CrossRef.
- M. Kim, M. A. R. Anjum, M. Lee, B. J. Lee and J. S. Lee, Adv. Funct. Mater., 2019, 29, 1809151 CrossRef.
- J. Zhang, T. Wang, P. Liu, S. Liu, R. Dong, X. Zhuang, M. Chen and X. Feng, Energy Environ. Sci., 2016, 9, 2789 RSC.
- J. Liu, J. Wang, B. Zhang, Y. Ruan, H. Wan, X. Ji, K. Xu, D. Zha, L. Miao and J. Jiang, J. Mater. Chem. A, 2018, 6, 2067 RSC.
- B. Zhang, J. Liu, J. Wang, Y. Ruan, X. Ji, K. Xu, C. Chen, H. Wan, L. Miao and J. Jiang, Nano Energy, 2017, 37, 74 CrossRef CAS.
- M. Watanabe and S. Motoo, J. Electroanal. Chem. Interfacial Electrochem., 1975, 60, 267 CrossRef CAS.
- D. J. Chen and Y. J. Tong, Angew. Chem., Int. Ed. Engl., 2015, 54, 9394 CrossRef CAS PubMed.
- D. Strmcnik, P. P. Lopes, B. Genorio, V. R. Stamenkovic and N. M. Markovic, Nano Energy, 2016, 29, 29 CrossRef CAS.
- R. Ge, S. Wang, J. Su, Y. Dong, Y. Lin, Q. Zhang and L. Chen, Nanoscale, 2018, 10, 13930 RSC.
- Y. Wang, Z. Liu, H. Liu, N. T. Suen, X. Yu and L. Feng, ChemSusChem, 2018, 11, 1 CrossRef.
- T. Liu, S. Wang, Q. Zhang, L. Chen, W. Hu and C. M. Li, Chem. Commun., 2018, 54, 3343 RSC.
- J. Q. Chi, W. K. Gao, J. H. Lin, B. Dong, K. L. Yan, J. F. Qin, B. Liu, Y. M. Chai and C. G. Liu, ChemSusChem, 2018, 11, 743 CrossRef CAS PubMed.
- Z. Pu, I. S. Amiinu, Z. Kou, W. Li and S. Mu, Angew. Chem., Int. Ed., 2017, 56, 11559 CrossRef CAS PubMed.
- J. Yu, Y. Guo, S. She, S. Miao, M. Ni, W. Zhou, M. Liu and Z. Shao, Adv. Mater., 2018, 30, 1800047 CrossRef PubMed.
- T. Liu, B. Feng, X. Wu, Y. Niu, W. Hu and C. M. Li, ACS Appl. Energy Mater., 2018, 1, 3143 CrossRef CAS.
- W. Li, Y. Liu, M. Wu, X. Feng, S. A. T. Redfern, Y. Shang, X. Yong, T. Feng, K. Wu, Z. Liu, B. Li, Z. Chen, J. S. Tse, S. Lu and B. Yang, Adv. Mater., 2018, 30, 1800676 CrossRef PubMed.
- J. Zhang, P. Liu, G. Wang, P. P. Zhang, X. D. Zhuang, M. W. Chen, I. M. Weidinger and X. L. Feng, J. Mater. Chem. A, 2017, 5, 25314 RSC.
- B. K. Barman, D. Das and K. K. Nanda, Sustainable Energy Fuels, 2017, 1, 1028 RSC.
- J. Ohyama, D. Kumada and A. Satsuma, J. Mater. Chem. A, 2016, 4, 15980 RSC.
- Q. Wang, M. Ming, S. Niu, Y. Zhang, G. Fan and J.-S. Hu, Adv. Energy Mater., 2018, 9, 1801698 CrossRef.
- R. Ye, Y. Liu, Z. Peng, T. Wang, A. S. Jalilov, B. I. Yakobson, S. H. Wei and J. M. Tour, ACS Appl. Mater. Interfaces, 2017, 9, 3785 CrossRef CAS.
- F. Li, G. F. Han, H. J. Noh, I. Ahmad, I. Y. Jeon and J. B. Baek, Adv. Mater., 2018, 30, 1803676 CrossRef.
- X. Cheng, H. Wang, M. Ming, W. Luo, Y. Wang, Y. Yang, Y. Zhang, D. Gao, J. Bi and G. Fan, ACS Sustainable Chem. Eng., 2018, 6, 11487 CrossRef CAS.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2016, 138, 16174 CrossRef CAS PubMed.
- J. Mahmood, F. Li, S. M. Jung, M. S. Okyay, I. Ahmad, S. J. Kim, N. Park, H. Y. Jeong and J. B. Baek, Nat. Nanotechnol., 2017, 12, 441 CrossRef CAS PubMed.
- J. Yu, Y. Guo, S. Miao, M. Ni, W. Zhou and Z. Shao, ACS Appl. Mater. Interfaces, 2018, 10, 34098 CrossRef CAS.
- S. Liu, Q. Liu, Y. Lv, B. Chen, Q. Zhou, L. Wang, Q. Zheng, C. Che and C. Chen, Chem. Commun., 2017, 53, 13153 RSC.
- J. Liu, Y. Zheng, D. Zhu, A. Vasileff, T. Ling and S. Z. Qiao, Nanoscale, 2017, 9, 16616 RSC.
- K. Yang, P. Xu, Z. Lin, Y. Yang, P. Jiang, C. Wang, S. Liu, S. Gong, L. Hu and Q. Chen, Small, 2018, 14, 1803009 CrossRef PubMed.
- J. Xu, T. Liu, J. Li, B. Li, Y. Liu, B. Zhang, D. Xiong, I. Amorim, W. Li and L. Liu, Energy Environ. Sci., 2018, 11, 1819 RSC.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069 CrossRef CAS.
- Y. Liu, S. Shen, J. Zhang, W. Zhong and X. Huang, Appl. Surf. Sci., 2019, 478, 762 CrossRef CAS.
- W. Zhong, S. Shen, S. Feng, Z. Lin, Z. Wang and B. Fang, CrystEngComm, 2018, 20, 7851 RSC.
- W. Zhong, W. Tu, S. Feng and A. Xu, J. Alloys Compd., 2019, 772, 669 CrossRef CAS.
- F. Wang, Y. Li, T. A. Shifa, K. Liu, F. Wang, Z. Wang, P. Xu, Q. Wang and J. He, Angew. Chem., Int. Ed., 2016, 55, 6919 CrossRef CAS PubMed.
- H. Zhou, F. Yu, Y. Liu, J. Sun, Z. Zhu, R. He, J. Bao, W. A. Goddard, S. Chen and Z. Ren, Energy Environ. Sci., 2017, 10, 1487 RSC.
- K. Wang, Q. Chen, Y. Hu, W. Wei, S. Wang, Q. Shen and P. Qu, Small, 2018, 14, 1802132 CrossRef PubMed.
- J. Deng, H. Li, J. Xiao, Y. Tu, D. Deng, H. Yang, H. Tian, J. Li, P. Ren and X. Bao, Energy Environ. Sci., 2015, 8, 1594 RSC.
- Q. Liu, X. Li, Q. He, A. Khalil, D. Liu, T. Xiang, X. Wu and L. Song, Small, 2015, 11, 5556 CrossRef CAS PubMed.
- H. Wang, Z. Lu, D. Kong, J. Sun, T. M. Hymel and Y. Cui, ACS Nano, 2014, 8, 4940 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02873k |
| This journal is © The Royal Society of Chemistry 2019 |