DOI:
10.1039/C9RA02763G
(Paper)
RSC Adv., 2019,
9, 20573-20581
Solvent-dependent regio- and stereo-selective reactions of 3-formylchromones with 2-aminobenzothiazoles and transacetalization efficiency of the product 3-((benzo[d]thiazol-2-ylimino)butyl)-4H-chromen-4-one†
Received
12th April 2019
, Accepted 16th May 2019
First published on 2nd July 2019
Abstract
Using 2-propanol as the solvent, 3-formylchromones and 2-aminobenzothiaoles formed corresponding imines, while 1° and 2°-alcohols formed the corresponding 2-alkoxy-3-enamines with selectivity for the Z-isomer. Changing the substrates with similar molecules such as 3-formylchromone with quinoline-, quinolone- and indole-3-carbaldehydes sometimes resulted in the formation of the corresponding imines, whereas replacing 2-aminobenzothiazole with amides resulted in the formation of acetals. Considering the effect of the solvent, replacing alcohols with the aprotic solvents THF and CH2Cl2 resulted in the formation of imines and enamines, which are the characteristic reactions of 2-propanol and other 1° and 2°-alcohols, respectively. 2-Alkoxy-3-enamines were found to undergo transacetalization with both short and long chain alcohols. The novelty of these reactions is that they did not require an external catalyst, all the reactions were performed at the same temperature, and purification was achieved by filtration. The transacetalization we performed herein is a new concept, which has not been reported to date. In contrast, other similar reactions, such as transalkoxylation, transalkylation, and transetherification, are performed on a commercial scale using expensive catalysts such as Otera's catalyst. The highly sensitive nature of 3-formylchromones towards variations in the substrates and solvents to form different products and the reason behind the selective formation of the Z-isomer of 2-alkoxy-3-enamines and its transacetalization efficiency need further studies to understand the reaction mechanism and possibly other factors such as solvent effects.
Introduction
Chromones are naturally occurring oxygen heterocyclic compounds, which are widely distributed in plants and form the basic nucleus of important compounds such as flavones, isoflavone, xanthones, and anthocyanins, and new natural products in this category are still being discovered regularly.1,2 Due to their wide range of pharmacological activities such as anti-inflammatory and antidiabetic activities,3–6 they are considered advantageous scaffolds in drug discovery. Similar to 2-naphthols, they are also bicyclic compounds. Towards our aim of developing single molecules as angiotensin converting enzyme (ACE) inhibitors and calcium channel blockers (CCB), which are denoted as ACE and CCB dual inhibitors, we designed, synthesized and screened several diarylalkylamines, such as 2-aminobenzothiazolobenzylnaphthols,7,8 xanthones9 and phenols,10 together with their corresponding arylalkylamine counterparts, i.e., 2-aminobenzothiazolomethylnaphthols, xanthones and phenols, which are under different stages of synthesis and screening. In search of bicyclic compounds with antihypertensive properties, we found that 3-(aminomethyl)chromone derivatives have potential antihypertensive properties, and a Scifinder search for commercially available 3-(aminomethyl)chromone derivatives with antihypertensive property listed hundreds of compounds. Narrowing down the results by eliminating compounds with substructures, such as flavone, isoflavone, and xanthone, resulted in 7 compounds. The structures of two of them are shown in Fig. 1.
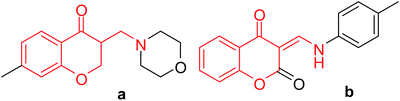 |
| | Fig. 1 Representative examples of commercially available 3-(aminomethyl)chromones with antihypertensive properties. | |
Based upon these reports, we expected 2-aminobenzothiazolobenzylchromones and 2-aminobenzothiazolomethylchromones, which are chromone analogues of our previous studies7 (Fig. 2), to have ACE and CCB dual inhibition properties.
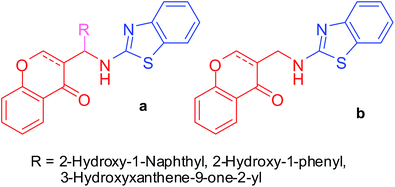 |
| | Fig. 2 Structures of the designed 2-aminobenzothiazolobenzylchromones and 2-aminobenzothiazolomethylchromones. | |
Several of our attempts to synthesize 2-aminobenzothiazolobenzylchromones 2a using our previous protocols in different solvents and temperatures were futile. Thus, temporarily putting their synthesis aside, we moved on to the synthesis of the corresponding methyl derivatives 2b. From a literature search, we found that the corresponding imines (Fig. 3a) can be synthesized in dry toluene with a catalytic amount of PTSA under Dean–Stark conditions with the possible formation of 1,4-adducts by the same amine or EtOH depending on the conditions with 60–65% step-wise yield.11 Moreover, 2-alkoxy-3-enamines (Fig. 3b) were found to be formed in EtOH using a catalytic amount of PTSA and heating at 50 °C for 30 min, followed by the addition of an ethanolic solution of the 2-aminobenzothiazole derivative and further stirring at RT, and filtration followed by recrystallization.12 Spectral data revealed that this method is applicable only to unsubstituted chromones. In the case of substituted chromones, imine formation in dry toluene under Dean–Stark conditions, followed by recrystallization was found to be suitable.11 Further, these publications described that the purification is difficult. The limited number of samples obtained were screened for their antimycobacterial12 and antifungal11,12 properties and found to do so.
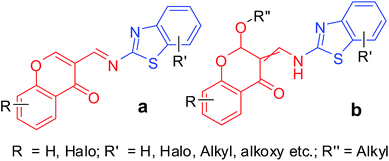 |
| | Fig. 3 Structurally similar chromone derivatives to our designed compounds. | |
These results indicate the possible wide range of biological applications of these compounds and the necessity to develop a method for their synthesis (Fig. 3). Due to the structural similarities of these compounds with our designed 2-aminobenzothiazolomethyl chromones (Fig. 2), we decided to develop a method for the synthesis of imine 3a and 2-alkoxy-3-enamine 3b, which is necessary for the screening of their ACE and CCB dual inhibition activities.
Results and discussion
2-Aminobenzothiazoles and 3-formylchromes in alcohols
Synthesis of (Z)-3-((benzo[d]thiazol-2-ylamino)methylene)-2-methoxychroman-4-ones (3aa–bh) and 3-((benzo[d]thiazol-2-ylimino)methyl)-4H-chromen-4-one (4a–e). In general, imines can be synthesized by refluxing their corresponding aldehydes and amines in EtOH with PTSA. The existing literature on the designed compounds suggests that 2-alkoxy-3-enamine 3b can be formed under heating in EtOH. Thus, to start the reaction with the simplest possible protocol, we attempted to reflux a mixture of equimolar quantities of 2-aminobenzothiazole and 3-formylchrome in 20 mL MeOH (Table 1, Entry 1). The solution slowly turned into a yellow suspension. After 2 h, the product was formed in good yield. The as-formed solid was filtered and washed with MeOH and dried under vacuum to obtain the product in low yield (45%). 1H NMR analysis revealed the formation of the compound, but with slight impurities. However due to the possibility of three possible structures (Fig. 4) for the 1H NMR data obtained (notes and ESI-Page S5 and S18†), the structure assignment became difficult without single-crystal X-ray diffraction. Our further experiments to develop a method for the synthesis of pure product revealed that further heating the reaction mixture resulted in the decomposition of the product, which made the purification more difficult. Finally, we found that the purity of the product obtained was best when the reaction was performed at 30–40 °C, above which the products slowly decomposed, and below which, the reaction was a bit slow to consume all the substrates (Table 1). We now understood that both the substrate and the products formed are sensitive to changes in temperature.
Table 1 Stabilization of temperature for the reaction of 3-formylchromone and 2-aminobenzothiazole in MeOH
| Entry |
Temperature (°C) |
Time (h) |
Yield (%) |
| Presence of impurities. |
| 1 |
Reflux |
2 |
90a |
| 2 |
50 |
2 |
85a |
| 3 |
45 |
2.5 |
87a |
| 4 |
40 |
3 |
86 |
| 5 |
35 |
3.5 |
90 |
| 6 |
30 |
4 |
87 |
| 7 |
25 |
6 |
81 |
| 8 |
20 |
9 |
72a |
| 9 |
15 |
12 |
56a |
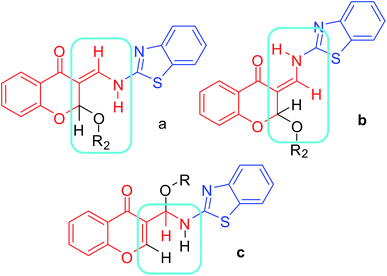 |
| | Fig. 4 Possible regio- and stereo-selective isomers of the compound obtained by heating 3-formylchromone and 2-aminobenzothiazole in MeOH under reflux. | |
To study the applicability of the method so developed, we performed reactions with a variety of 3-formylchromones, 2-aminobenzothiazoles and primary alcohols (Table 2, Entries 1–39), including branched alcohols and diols (Table 2, Entries 27–30). The products were obtained in good yields in about 10 h. However, with an increase in the carbon chain length, the rate of the reaction decreased slowly, and beyond propanol the yields slowly decreased due to purification losses (Table 2, Entries 22–30). The products from nitro-substituted 3-formylchromones were found to be difficult to purify and the nitro-substituted 2-aminobenzothiazoles did not react to form the product (Table 2, Entries 31 and 32). Finally, to identify the exact structure of the product formed, we successfully grew a crystal from one of the compounds, 3as (Table 2, Entry 19). Single crystal analysis revealed that the Z-isomer of 2-alkoxy-3-enamine was formed as a product with 100% regio- and stereo-selectivity, as shown in Fig. 4b, and its crystal structure is shown in Fig. 5. The reaction is schematically represented in Scheme 1.‡
Table 2 Reactions of chromone-3-carbaldehydes (1) with 2-aminobenzothiazoles (2) in alcohols (20 mL) at 30–40 °C
| Entry |
1 |
2 |
Alcohol |
Time (h) |
Product |
Yield (%) |
| Difficult to purify. No reaction. Reaction completed in 2 h under reflux. 4-Chloroaniline. |
| 1 |
H |
H |
MeOH |
10 |
3aa |
90 |
| 2 |
H |
6-Br |
MeOH |
10 |
3ab |
88 |
| 3 |
H |
6-OMe |
MeOH |
10 |
3ac |
91 |
| 4 |
H |
4,6-F2 |
MeOH |
10 |
3ad |
79 |
| 5 |
6-Cl |
H |
MeOH |
10 |
3ae |
86 |
| 6 |
6-Br |
H |
MeOH |
10 |
3af |
83 |
| 7 |
6-Cl |
6-OMe |
MeOH |
10 |
3ag |
89 |
| 8 |
6-Cl |
6-OCF3 |
MeOH |
10 |
3ah |
82 |
| 9 |
6-Cl |
4,6-F2 |
MeOH |
10 |
3ai |
83 |
| 10 |
6-Cl |
6-Br |
MeOH |
10 |
3aj |
88 |
| 11 |
6-Cl |
6-Me |
MeOH |
10 |
3ak |
91 |
| 12 |
6-Br |
6-Br |
MeOH |
10 |
3al |
92 |
| 13 |
6-Br |
4,6-F2 |
MeOH |
10 |
3am |
87 |
| 14 |
6-Br |
5-Cl-7-F |
MeOH |
10 |
3an |
88 |
| 15 |
6-OH |
H |
MeOH |
10 |
3ao |
82 |
| 16 |
6-Cl-8-Br |
4-Cl |
MeOH |
10 |
3ap |
84 |
| 17 |
H |
H |
EtOH |
12 |
3aq |
85 |
| 18 |
H |
6-OMe |
EtOH |
12 |
3ar |
83 |
| 19 |
H |
4-Cl |
EtOH |
12 |
3as |
84 |
| 20 |
6-Br |
6-Me |
EtOH |
12 |
3at |
86 |
| 21 |
6-Cl |
4-Cl |
1-Propanol |
24 |
3au |
79 |
| 22 |
6-Cl |
4-Cl |
1-Butanol |
48 |
3av |
75 |
| 23 |
6-Cl |
6-OMe |
1-Butanol |
48 |
3aw |
76 |
| 24 |
6-Cl |
4-Cl |
1-Hexanol |
96 |
3ax |
64 |
| 25 |
6-Cl |
4-Cl |
1-Heptanol |
96 |
3ay |
59 |
| 26 |
6-Cl |
4-Cl |
1-Octanol |
144 |
3az |
38 |
| 27 |
H |
4-Cl |
Isobutanol |
48 |
3ba |
74 |
| 28 |
6-Cl |
4-Cl |
Isobutanol |
48 |
3bb |
78 |
| 29 |
6-Cl |
H |
1,2-Ethanediol |
24 |
3bc |
76 |
| 30 |
6-Cl |
4-Cl |
1,5-Pentanediol |
72 |
3bd |
53 |
| 31 |
6-NO2 |
H |
MeOH |
24 |
—a |
— |
| 32 |
H |
6-NO2 |
MeOH |
24 |
—b |
— |
| 33 |
H |
H |
2-Propanol |
6 |
4a |
95 |
| 34 |
H |
6-Me |
2-Propanol |
6 |
4b |
95 |
| 35 |
6-Cl |
H |
2-Propanol |
6c |
4c |
96 |
| 36 |
6-Br |
H |
2-Propanol |
6c |
4d |
95 |
| 37 |
6-OH |
H |
2-Propanol |
6 |
4e |
96 |
| 38 |
6-Cl |
4-Cl |
2-Butanol |
48 |
3be |
74 |
| 39 |
H |
4-Cl |
2-Butanol |
48 |
3bf |
76 |
| 40 |
6-Cl |
4-Cl |
Cyclohexanol |
48 |
3bg |
45 |
| 41 |
6-Cl |
—d |
MeOH |
24 |
3bh |
29 |
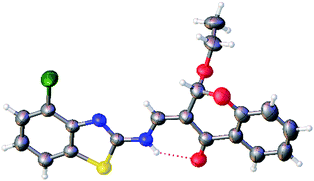 |
| | Fig. 5 Crystal structure of compound 3as. | |
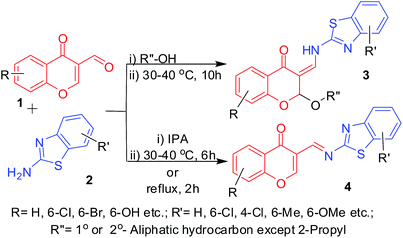 |
| | Scheme 1 Schematic representation of the reactions between 3-formylchromones (1) and 2-aminobenzothiazoles (2) using a variety of alcohols as the solvent at 30–40 °C. | |
In continuation of the experiments with other alcohols, we found that the reaction with the simplest 2°-alcohol, i.e., 2-propanol, gave imine 4, which was otherwise difficult to synthesize according to the reports11,12 in 6 h at about 95% yield (Scheme 1) (Table 2, Entries 33–37), while it was formed in just 2 h under reflux (Table 2, Entries 35 and 36) with a variety of substituents in both 3-formylchromone and 2-aminobenzothiazole. The solubility of imine 4 was found to be very low in any given solvent. However, in case of other 2°-alcohols, such as 2-butanol and cyclohexanol, product 3 was formed (Table 2, Entries 38–40). Using 3°-alcohols (tert-butanol), phenols and benzyl alcohols as the alcohol source, multiple products were formed, which could not be separated. It is noteworthy to mention that even though the reactions with phenols were not successful, 3-formylchromones with phenolic protons successfully formed the corresponding 2-alkoxy-3-enamines and imines in MeOH and 2-propanol, respectively (Table 2, Entries 15 and 37). To further study the applicability of this method to other amines, we successfully synthesized and purified the 2-alkoxy-3-enamine 3 using 4-chloroaniline as the amine source (Table 2, Entry 38), but at a low yield, which was due to purification losses. The product formed with benzylamines could not be purified, even through column due to its decomposition.
3-Formylchromones and amides in alcohols: synthesis of acetals
In continuation experiments for the synthesis of derivatives of compound 3 with different amines, we found that the reactions with amides (acetamide, formamide and benzamide) resulted in corresponding acetals, irrespective of the alcohol and amide used (Table 3, Entries 1–6), with good yields (71–82%) and even in the absence of amide (Table 3, Entry 7). These acetals are known to be formed by reactions in alcohols under both acidic13 and basic14,15 conditions mostly using Lewis acids as catalysts. However, their formation was unexpected when a primary amide is available as a substrate and this behaviour is contrary to its amine counter parts. Further, in the presence of amides, the formation of acetals was found to be fast. These results suggest that amides, such as formamide and acetamide, which are cheap and easy to purify from the reaction mixtures, can be used as catalysts in certain types of reactions, which in the present case replaced Lewis acids. The reaction is represented schematically in Scheme 2.
Table 3 Reactions of 3-Formylchromones with amides in alcohols (20 mL) at 30–40 °C over a period of 12 h
| Entry |
1 |
Amide (eq.) |
Alcohol |
Product |
Yield (%) |
| 1 |
6-Cl |
Acetamide (1) |
MeOH |
5a |
81 |
| 2 |
6-Cl |
Acetamide (5) |
MeOH |
5a |
80 |
| 3 |
6-Cl |
Acetamide (5) |
MeOH |
5a |
82 |
| 4 |
6-Cl |
Formamide |
MeOH |
5a |
86 |
| 5 |
6-Cl |
Benzamide |
MeOH |
5a |
71 |
| 6 |
H |
Acetamide |
1-Propanol |
5b |
72 |
| 7 |
H |
Formamide |
1-Propanol |
5b |
76 |
| 8 |
6-Cl |
— |
MeOH |
5a |
80 |
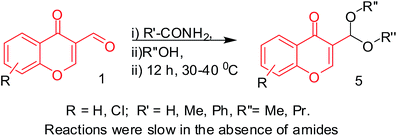 |
| | Scheme 2 Reactions of 3-formylchromones with alcohols in the presence and absence of amides. | |
Quinoline-, quinolone- and indole-3-carbaldehydes with 2-aminobenzothiazole in alcohol: synthesis of imines but not 2-alkoxy-3-enamines. To study the applicability of the method used for the synthesis of 2-alkoxy-3-enamine derivatives 3 to quinoline-, quinolone- and indole-3-carbaldehydes, which are the structural analogues of 3-formylchromone, we found that 4-chloroquinoline-3-carbaldehyde (Q1) and ethyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q4) both formed 4-oxo-1,4-dihydroquinoline-3-carbaldehyde (Q2), irrespective of the alcohol (20 mL) used. However, Q1 required reflux to form the product (Table 4, Entries 1–6 and 9). 4-Oxo-1,4-dihydroquinoline-3-carbaldehyde (Q2) formed the corresponding acetal 3-(dimethoxymethyl)quinolin-4(1H)-one (Q8) under reflux, while the reaction was sluggish under normal reaction conditions. The BoC-quinolone tert-butyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q3) and its indole analogues Q6 and Q7 formed the corresponding imines Q9, Q10 and Q11 under reflux and at 30–40 °C, respectively. However, the products formed from 4-chloroaniline decomposed in the column. The reactions are schematically represented in Scheme 3.
Table 4 Reactions of quinolone and indole-3-carbaldehyde derivatives Q1–Q7 with 2-aminobenzothiazole in MeOH (20 mL) at 30–40 °C
| Entry |
Q1–Q7 |
Time (h) |
Product |
Yield (%) |
| No reaction. Reflux. Reaction mixture decomposed. 2-propanol was used as the solvent. 4-Chloroaniline was used as the amine. Very slow reaction. Decomposed in column. |
| 1 |
Q1 |
144 |
—a |
— |
| 2 |
Q1 |
16b |
Q2 |
85 |
| 3 |
Q1 |
30b |
—c |
|
| 4 |
Q1 |
144d |
—a |
87 |
| 5 |
Q1 |
12b,d |
Q2 |
87 |
| 6 |
Q2 |
12 |
Q8 |
<10 |
| 7 |
Q2 |
8b |
Q8 |
83 |
| 8 |
Q3 |
24 |
Q9 |
55 |
| 9 |
Q4 |
24 |
Q2 |
77 |
| 10 |
Q5 |
12d |
—a |
— |
| 11 |
Q5 |
4b,d |
—c |
— |
| 12 |
Q5 |
12 |
—a |
— |
| 13 |
Q5 |
4b |
—c |
— |
| 14 |
Q6 |
12 |
Q10 |
81 |
| 15 |
Q6 |
12e |
—f |
— |
| 16 |
Q6 |
12b,e |
—g |
— |
| 17 |
Q7 |
48 |
Q11 |
51 |
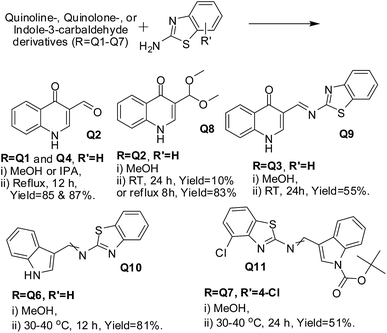 |
| | Scheme 3 Schematic representation of reactions of quinolone and indole derivatives Q1–Q7 with 2-aminobenzothiazole in MeOH (20 mL). | |
3-Formylchromone, 2-aminobenzothiazole and solvents except alcohols: synthesis of 2-alkoxy-3-enamines (3) in DCM and imines (4) in THF. To develop a method for the synthesis of 2-alkoxy-3-enamine derivatives 3 from viscous and solid alcohols, such as long chain unsaturated alcohols, benzylalcohols, and phenols, we made several attempts using 6-chloro-3-formylchromone and 2-amino-6-methoxybenzothiazole (Table 5) using different aprotic solvents (20 mL) (DMF, DMSO, CH3CN, THF, DCM, and xylene) and found that in THF, the imine product 4 is exclusively formed (Table 5, Entry 2). A detailed study on the effect of alcohols in the THF-mediated synthesis of imines 4 revealed that these imines are formed irrespective of the presence or absence of alcohol at temperatures ranging between RT to reflux (Table 6), even with chromones with phenolic protons (Table 6, Entry 8). Due to the very low solubility of derivatives of 4, we studied the effects of different alcohols without changing the substrates. The reaction is schematically represented in Scheme 4. On the other hand, the reactions in DCM with long chain unsaturated alcohols formed the expected 2-alkoxy-3-enamine in about 10% yield (Table 5, Entry 6). Further studies revealed that the products formed with phenols and benzyl alcohols were difficult to purify (Table 7, Entries 4 and 5) and long chain unsaturated alcohols formed the required 2-alkoxy-3-enamine in low yields (Table 7, Entries 6 and 7). The reaction is schematically represented in Scheme 5.
Table 5 Study of the influence of different solvents (20 mL) on the reactions between 6-chloro-3-formylchromone (1 mmol), 2-amino-6-methoxybenzothiazole (1 mmol) and alcohols (2 mmol)
| Entry |
Alcohol |
Solvent |
Time |
Product |
Yield (%) |
| Difficult to purify. Decomposed. Purified by column chromatography. |
| 1 |
n-Butanol |
DCM |
12 h |
—a |
— |
| 2 |
n-Butanol |
THF |
4 h |
4f |
94 |
| 3 |
n-Butanol |
DMF |
12 h |
—b |
— |
| 4 |
n-Butanol |
CH3CN |
12 h |
—b |
— |
| 6 |
1-Octanol |
DCMb |
10 h |
3bic |
10 |
| 7 |
1-Octanol |
DMF |
12 h |
—b |
— |
| 8 |
1-Octanol |
DMSO |
12 h |
—b |
— |
| 9 |
1-Octanol |
Xylene |
12 h |
—b |
— |
Table 6 Study of the influence of 1°,2°-alcohols on the formation of imines from 6-chloro-3-formylchromone (1) and 2-aminobenzothiazole (2) in THF (20 mL) at RT (10 h) and reflux (2 h)
| Entry |
Alcohol |
Eq. |
Product |
Yield (%) |
| No alcohol was used. 2-Aminobenzothiazole. 6-Hydroxy-3-formylchromone. |
| 1 |
1-BuOH |
1 |
4f |
96 |
| 2 |
1-BuOH |
10 |
4f |
95 |
| 3 |
2-BuOH |
1 |
4f |
96 |
| 4 |
Cyclohexanol |
1 |
4f |
95 |
| 5 |
—a |
— |
4f |
96 |
| 6 |
MeOHb |
1 |
4g |
95 |
| 7 |
—a,b |
— |
4g |
96 |
| 8 |
—a,b,c |
— |
4e |
95 |
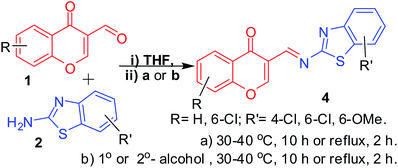 |
| | Scheme 4 Schematic representation of condensation of 3-formylchromones with 2-aminobenzothiazoles in THF (20 mL). | |
Table 7 Study of the synthesis of 2-alkoxy-3-enamine derivatives of long chain, unsaturated alcohols in DCM (20 mL) using 6-chloro-3-formylchromone (1) and 2-amino-6-methoxybenzothiazole (2) as substrates
| Entry |
Alcohol (eq.) |
Time |
Product |
Yield (%) |
| Difficult to purify. 2-Amino-4-chlorobenzothiazole. Purified by column chromatography. 3-Formylchromone. |
| 1 |
1-Octanol (1) |
10 h |
3bi |
11 |
| 2 |
1-Octanol (5) |
10 h |
3bi |
12 |
| 3 |
1-Octanol (10) |
10 h |
3bi |
12 |
| 4 |
Benzyl alcohol (1) |
7 h |
—a |
— |
| 5 |
4-ChloroPhenol (1) |
10 h |
—a |
— |
| 6 |
Geraniol (1) |
10 h |
3bjb,c |
7 |
| 7 |
5-Hexyne-1-ol (1) |
10 h |
3bkb,c,d |
6 |
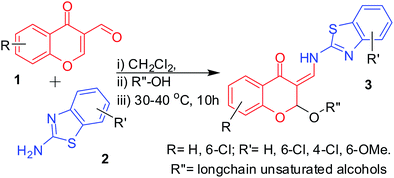 |
| | Scheme 5 Schematic representation of the synthesis of 2-alkoxy-3-enamine derivatives of long chain, unsaturated alcohols in CH2Cl2. | |
Trans-acetalization of 2-alkoxy-3-enamines: reactions of (Z)-2-butoxy-6-chloro-3-((6-methoxybenzo[d]thiazol-2-lamino) methylene)chroman-4-one (3aw) in MeOH (20 mL) and 1-octanol (20 mL) for the synthesis of corresponding derivatives 3ag and 3bi. During the purification of the derivatives of 2-alkoxy-3-enamines (3) formed from long chain alcohols and diols, which are highly viscous, we made several attempts to purify them by filtration under different conditions using different solvents. During this process, we observed that washing these highly viscous liquids with MeOH resulted in the displacement of that long chain alkoxy group with the methoxy group, which can be called transacetalization. We are well-versed with the commercial importance of transesterifications for the synthesis of polyesters16 using catalysts such as Otera's catalyst17 and transalkylations using zeolites,18 transamidifications19 and even transetherifications20,21 with limited substrate scope, i.e., either vinyl esters or vinyl ethers using expensive catalysts. The literature for the synthesis of unsymmetrical acetals22,23 are very limited and their transacetalization was not found to be reported. Thus, we made use of the 2-butoxy derivative 3aw as a substrate, dissolved it in either MeOH or 1-octanol (20 mL) with stirring at 30–40 °C over a period of 5 days. The corresponding products 3ag and 3bi were formed in good yields, but as usual, the purification losses were found to be more in octanol reactions and resulted in a final yield of12%. Using 1–10 mmol of alcohol (MeOH or 1-octanol) and using xylene (20 mL) as the solvent did not result in the formation of any product, but the substrate started decomposing with a gradual increase in temperature beyond 50 °C (Table 8). The reaction is schematically represented in Scheme 6, and the structural features favouring this transacetalization is expected to be the (Z)-3-(aminomethylene)-2-methoxy-2H-pyran-4(3H)-one skeleton, which is shown in Fig. 6 (brown square). Thus, further studies are necessary to understand how to exploit this property.
Table 8 Studies on the transacetalization efficiency of 2-alkoxy-3-enamines using (Z)-2-butoxy-6-chloro-3-((6-methoxybenzo[d]thiazol-2-ylamino)methylene)chroman-4-one (3aw) as a substrate
| Entry |
Alcohol (20 mL) |
Solvent |
Time (h) |
Product |
Yield (%) |
| 1 eq. of 1-Octanol. Decomposed. No alcohol. No reaction. 50–100 °C. |
| 1 |
MeOH |
MeOH |
24 |
3ag |
91 |
| 2 |
1-Octanol |
1-Octanol |
120 |
3bi |
12 |
| 3 |
1-Octanola |
Xylene |
24 |
—b |
— |
| 4 |
—c |
Xylene |
6 |
—d |
— |
| 5 |
—c |
Xylene |
6e |
—b |
— |
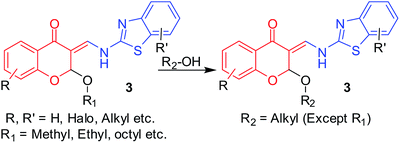 |
| | Scheme 6 Transacetalization reactions of 2-alkoxy-3-enamine (3). | |
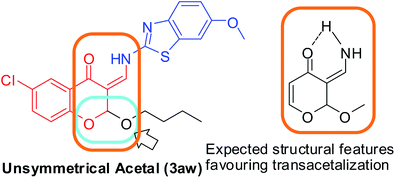 |
| | Fig. 6 Position of unsymmetrical acetal in a representative compound 3aw (blue square) and possible structural features driving the transacetalization reaction (brown square). | |
Materials and methods
Synthesis of quinoline- and quinolone-3-carbaldehydes (Q1, Q2, Q4 and Q5) and BoC-protected derivatives Q3 and Q7
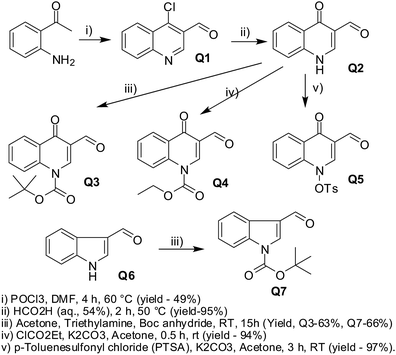
The experiments were performed following the existing literature.24 BoC protections were performed using the general protocols. The reactions are schematically represented in Scheme 7.
 |
| | Scheme 7 Schematic representation of the synthesis of derivatives of quinoline-, quinolone- and indole-3-carbaldehyde derivatives Q1–Q5 and Q7. | |
Synthesis of 4-chloroquinoline-3-carbaldehyde (Q1) and 1,4-dihydro-4-oxoquinoline-3-carbaldehyde (Q2). To 50 mL of dry DMF, POCl3 (23 mL, 246.8 mmol, 6 eq.) was added dropwise at 0 °C, and stirred for 15 min under nitrogen at RT. To this mixture, 2-aminoacetophenone (5 mL, 41.1 mmol, 1 eq.) was added dropwise and heated at 60 °C for 4 h. The reaction mixture was then quenched with the slow addition of ice, neutralized with saturated NaHCO3 solution, and the solid formed was filtered. The solid was dissolved in 400 mL CHCl3 and washed with water. The organic layer was concentrated, and the resulting solid was purified by column chromatography using CH2Cl2 as the eluent, and crystallized from a mixture of ethyl acetate and hexane to afford 4-chloroquinoline-3-carbaldehyde (Q1) as a white solid (3.8 g; 49%). A suspension of 3.3 g of Q1 (17.3 mmol) in 40 mL of 54% aqueous formic acid was heated at 50 °C for 2 h. The resulting suspension was frozen, and the solid formed was filtered and washed with water. Pure 1,4-dihydro-4-oxoquinoline-3-carbaldehyde (Q2) was collected as a white solid without further purification (2.7 g, 95%).
tert-Butyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q3) and tert-butyl 3-formyl-1H-indole-1-carboxylate (Q7). To a solution of 1,4-dihydro-4-oxoquinoline-3-carbaldehyde (Q2, 386 mg, 2.23 mmol) or indole-3-carbaldehyde (Q6, 324 mg, 2.23 mmol) and TEA (1.24 mL, 8.93 mmol) in CH2Cl2 (20 mL), (BoC)2O (1.46 g, 6.7 mmol) was added under an N2 atmosphere at 5–10 °C. The reaction mixture was stirred overnight at RT, diluted with water and extracted with CH2Cl2. The organic layer was washed with water and brine, and dried over Na2SO4 and concentrated to obtain the corresponding tert-butyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q3, 384 mg, 63%) or tert-butyl 3-formyl-1H-indole-1-carboxylate (Q7, 361 mg, 66%) in good yields.
Ethyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q4). To a suspension of 1,4-dihydro-4-oxoquinoline-3-carbaldehyde (Q2) (701 mg, 4.05 mmol, 1 eq.) in 50 mL acetone, anhydrous K2CO3 (1.12 g, 8.10 mmol, 2 eq.) was added and the mixture was stirred at RT for 30 min. To this mixture, 3 ethyl chloroformate (0.61 mL, 6.08 mmol, 1.5 eq.) was added, and the mixture was stirred at RT for 30 min. The reaction mixture was filtered, washed with acetone, and the filtrate was concentrated and extracted with water and chloroform. The organic layer was concentrated under vacuum to obtain pure ethyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q4) as a yellowish solid (920 mg, 94%).
1,4-Dihydro-4-oxo-1-(4-toluolsulfonyl)quinoline-3-carbaldehyde (Q5). To a suspension of 1,4-dihydro-4-oxoquinoline-3-carbaldehyde (200 mg, 1.16 mmol, 1 eq.) in 20 mL acetone, anhydrous K2CO3 (320 mg, 2.32 mmol, 2 eq.) was added, and stirred at RT for 30 min. To this mixture, p-toluenesulfonyl chloride (332 mg, 1.74 mmol, 1.5 eq.) was added, and the resulting mixture was stirred at RT for 3 h. The reaction mixture was filtered, the solid was washed with acetone and the filtrate was concentrated. The residue was purified by column chromatography (silica gel, 60–120 mesh), using CH2Cl2 as the eluent followed by a mixture of CH2Cl2–acetone (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solid obtained was recrystallized from a mixture of CH2Cl2-light petroleum to give 1,4-dihydro-4-oxo-1-(4-toluolsulfonyl)quinoline-3-carbaldehyde (Q5) as a white solid (369 mg, 97%).
1). The solid obtained was recrystallized from a mixture of CH2Cl2-light petroleum to give 1,4-dihydro-4-oxo-1-(4-toluolsulfonyl)quinoline-3-carbaldehyde (Q5) as a white solid (369 mg, 97%).
(Z)-3-((Benzo[d]thiazol-2-ylamino)methylene)-2-methoxychroman-4-one and its aliphatic alcohol derivatives (3aa–3bh)
Equimolar quantities of chromone-3-carbaldehyde (174.2 mg, 1 mmol) and 2-aminobenzothiazole (150.2 mg, 1 mmol) were dissolved in MeOH (20 mL) and stirred at 30–40 °C for about 12 h. The yellow as-formed solid was filtered, washed with a little MeOH and dried under vacuum to obtain pure 3aa as a yellow solid (304 mg, 90% yield). The same conditions were applicable to a variety of 3-formyl chromones, 2-aminobenzothiazoles and 1°,2°-alcohols, except 2-propanol where formation of imine 4 was observed. The reaction time may very between 10 h to 3 days depending on the substituents and the alcohol used (Table 2). An increase in the alcohol chain length was found to be associated with an increase in reaction time. It is noteworthy that even though a few substrates were able to withstand a higher temperature (∼60 °C), a few of them decomposed at this temperature and purification of the product was difficult and sometimes not possible. This stability was found to be completely dependent on the substituents on both the chromone-3-carbaldehyde (1 mmol) and 2-aminobenzothiazole.
(Z)-6-Chloro-3-((6-methoxybenzo[d]thiazol-2-ylamino)methylene)-2-(octyloxy)chroman-4-one and its long chain unsaturated alcohol derivatives (3bi–3bk)
Equimolar quantities of 6-chloro-4-oxo-4H-chromene-3-carbaldehyde (209 mg, 1 mmol) and 2-amino-6-methoxybenzothiazole (180 mg, 1 mmol) were dissolved in 20 mL of CH2Cl2. To this mixture, 2 eq. of 1-octanol (260 mg, 320 μL) was added and stirred at 30–40 °C for about 12 h, the as-formed yellow solid was refrigerated overnight, filtered, washed with small quantities of ice cold MeOH (20 mL × 3) and dried under vacuum, then purified by flash column to obtain pure 3bi as a lemon-yellow solid in 11% (55 mg) yield. It is noteworthy to mention that these compounds readily decomposed in the column, and hence rapid column chromatography is suggested. We were not able to purify the products formed from phenols and benzylalcohols due to their decomposition.
Synthesis of 3-((benzo[d]thiazol-2-ylimino)methyl)-4H-chromen-4-one (4a) and its derivatives (4a–g)
Method A: equimolar quantities of chromone-3-carbaldehyde (174 mg, 1 mmol) and 2-aminobenzothiazole (150 mg, 1 mmol) were dissolved in IPA (20 mL) and stirred at 30–40 °C for about 6 h. The as-formed pale-yellow solid was filtered, washed with a little MeOH (20 mL × 2) and dried under vacuum to obtain 291 mg of pure 4a (95% yield). The reaction was completed in just 2 h under reflux conditions and resulted in good yield (Table 2). It is important to note that the solubility of these imines was very low in any given solvent, including CHCl3, acetone, DMSO and DMF.
Method B: equimolar quantities of chromone-3-carbaldehyde (174 mg, 1 mmol) and 2-aminobenzothiazole (150 mg, 1 mmol) were dissolved in THF (20 mL), and to this mixture, 2 eq. of alcohol was added and stirred at 30–40 °C for about 6 h. The as-formed pale-yellow solid was filtered, washed with a little MeOH and dried under vacuum to obtain 291 mg of pure 4a (95% yield). Even though the reaction did not need an alcohol to proceed, adding a little alcohol, such as MeOH, EtOH, and IPA, allowed the reaction to be completed in 6 h, which otherwise took about 10 h. The reaction was completed in less than 2 h under reflux conditions, resulting in good yields (Table 2).
6-Chloro-3-(dimethoxymethyl)-4H-chromen-4-one (5)
To a solution of 6-chloro-3-formylchromone (209 mg, 1 mmol) in MeOH (20 mL), acetamide (59 mg, 50 μL, 1 mmol) was added and stirred at for 30–40 °C 12 h. The wheat-brown solid obtained was filtered, washed with MeOH (20 mL × 3) and dried under vacuum to obtain 5a in 81% yield (Table 3). This method was applicable to other amides, such as formamide and benzamide, and other alcohols such as 1-propanol.
4-Oxo-1,4-dihydroquinoline-3-carbaldehyde (Q2) from 4-chloroquinoline-3-carbaldehyde (Q1) and ethyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q4)
Equimolar quantities of Q1 (192 mg, 1 mmol) or Q4 (245 mg, 1 mmol) and 2-aminonenzothiazole (150 mg, 1 mmol) were dissolved in MeOH (20 mL) and stirred under reflux for 12 h, and the as-formed solid was filtered and washed with 15 mL of MeOH to yield the product Q2 as a wheat-brown solid in 85% (147 mg) and 77% (133 mg) yield, respectively (Table 4).
3-(Dimethoxymethyl)quinolin-4(1H)-one (Q8)
To a solution of Q2 (173 mg, 1 mmol) in MeOH (20 mL), 2-aminobenzothiazole (150 mg, 1 mmol) was added and stirred under reflux for 8 h. The solid obtained was filtered and washed with a minimum amount of MeOH to obtain the product Q8 as a yellow solid in 83% (182 mg) yield.
3-((Benzo[d]thiazol-2-ylimino)methyl)quinolin-4(1H)-one (Q9)
A solution of equimolar quantities of tert-butyl 3-formyl-4-oxoquinoline-1(4H)-carboxylate (Q3) and 2-aminobenzothiazole in MeOH was stirred at RT for 24 h. Even though multiple impurity spots were visible from TLC, filtration of the reaction mixture followed by washing with a minimum amount of MeOH yielded pure Q9 as a light-yellow solid in 55% yield. This method was applicable to indole derivatives Q6 and Q7 for the synthesis of their imines N-((1H-indol-3-yl)methylene)benzo[d]thiazol-2-amine (Q10) and tert-butyl 3-((4-chlorobenzo[d]thiazol-2-ylimino)methyl)-1H-indole-1-carboxylate (Q11) with 81% and 51% yields over a period of 12 h and 48 h, respectively.
Conclusions
In conclusion, using 2-propanol as the solvent, 3-formylchromone and 2-aminobenzothiazole were found to form imines; whereas, with other 1° and 2°-alcohols they formed the Z-isomer of 2-alkoxy-3-enamine. Replacing 3-formylchromone with quinoline and indole derivatives did not yield the corresponding 2-alkoxy-3-enamines as expected, whereas replacing 2-aminobenzothiazole with an amide formed the corresponding acetal. Replacing alcohols with aprotic solvents THF and CH2Cl2 and an alcohol as the substrate formed corresponding imines and 2-alkoxy-3-enamines. This highlights the reactivity differences and highly selective nature of 3-formylchromones. Further the 2-alkoxy-3-enamines formed above were found to have the unique ability to undergo transacetalization, which is first of its kind. In addition, these reactions do not require any external catalyst and all the reactions were performed at or just above room temperature, and purification was achieved by filtration. Transacetalization, which was successfully performed here, is a new concept with no previous reference in the literature. In contrast, other similar reactions, namely transalkoxylation, transalkylation, transamidification, transetherification are performed on a commercial scale using expensive catalysts such as Otera's catalyst. Further studies may reveal the potential applications of the properties of 3-formylchromones and the applicability of the present method to other compounds.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
OA is thankful to China Scholarship Council (CSC) for awarding the fellowship. CV is thankful to management, CJEX Biochem. Pvt. Ltd., India, for their support in performing this research.
Notes and references
- W. Li, Y. Yang, W. Dong, H. Wang, F. Kong, C. Cai, W. Mei and H. Dai, Fitoterapia, 2019, 133, 12–16 CrossRef CAS PubMed.
- H.-X. Huo, Y.-F. Gu, Z.-X. Zhu, Y.-F. Zhang, X.-N. Chen, P.-W. Guan, S.-P. Shi, Y.-L. Song, Y.-F. Zhao, P.-F. Tu and J. Li, Phytochemistry, 2019, 158, 46–55 CrossRef CAS PubMed.
- J. Reis, A. Gaspar, N. Milhazes and F. Borges, J. Med. Chem., 2017, 60, 7941–7957 CrossRef CAS PubMed.
- C. F. M. Silva, D. C. G. A. Pinto and A. M. S. Silva, ChemMedChem, 2016, 11, 2252–2260 CrossRef CAS PubMed.
- S. K. Sharma, S. Kumar, K. Chand, A. Kathuria, A. Gupta and R. Jain, Curr. Med. Chem., 2011, 18, 3825–3852 CrossRef CAS PubMed.
- R. S. Keri, S. Budagumpi, R. K. Pai and R. G. Balakrishna, Eur. J. Med. Chem., 2014, 78, 340–374 CrossRef CAS PubMed.
- P. K. Kalavagunta, P. K. Bagul, A. Jallapally, S. Kantevari, S. K. Banerjee and N. Ravirala, Eur. J. Med. Chem., 2014, 83, 344–354 CrossRef CAS PubMed.
- P. K. Kalavagunta, R. Pala, U. R. Pathipati and N. Ravirala, J. Agric. Food Chem., 2014, 62, 6571–6576 CrossRef CAS PubMed.
- V. Cherkadu, P. K. Kalavagunta, M. Ningegowda, N. S. Shivananju, M. Madegowda and B. S. Priya, Synlett, 2016, 27, 1116–1120 CrossRef CAS.
- V. Cherkadu, P. K. Kalavagunta, N. Ravirala, N. S. Shivananju and B. S. Priya, Synlett, 2016, 27, 2795–2798 CrossRef CAS.
- V. P. Sharma, P. Kumar and M. Sharma, Asian J. Chem., 2011, 23, 4616–4620 CAS.
- H. M. El-Shaaer, P. Foltínová, M. Lácová, J. Chovancová and H. Stankovičová, Il Farmaco, 1998, 53, 224–232 CrossRef CAS PubMed.
- S. Madabhushi, K. K. R. Mallu, N. Chinthala, C. R. Beeram and V. S. Vangipuram, Tetrahedron Lett., 2012, 53, 697–701 CrossRef CAS.
- A. Clerici, N. Pastori and O. Porta, Tetrahedron, 1998, 54, 15679–15690 CrossRef CAS.
- C. K. Ghosh, C. Bandyopadhyay and N. Tewari, J. Org. Chem., 1984, 49, 2812–2815 CrossRef CAS.
- W. Riemenschneider and H. M. Bolt, Ullmann's Encyclopedia of Industrial Chemistry, 2005, DOI: DOI:10.1002/14356007.a09_565.pub2.
- J. Otera, N. Danoh and H. Nozaki, J. Org. Chem., 1991, 56, 5307–5311 CrossRef CAS.
- J. L. G. de Almeida and J. L. B. Tejero, US Pat., US20100022814A1, Petroquimica Espanola, S. A. Petresa, 2010.
- G. Hillion, I. Durand, A. Sinquin and M. Velly, US Pat., US006476081B1, Institut Francais Du Petrole, 2002.
- M. Bosch and M. Schlaf, J. Org. Chem., 2003, 68, 5225–5227 CrossRef CAS PubMed.
- W. H. Watanabe and L. E. Conlon, J. Am. Chem. Soc., 1957, 79, 2828–2833 CrossRef CAS.
- Y. Zong and Y. Rao, Org. Lett., 2014, 16, 5278–5281 CrossRef CAS PubMed.
- M.-L. Wang and S.-W. Chang, Ind. Eng. Chem. Res., 1995, 34, 3696–3702 CrossRef CAS.
- R. S. G. R. Seixas, A. M. S. Silva, I. Alkorta and J. Elguero, Monatsh. Chem., 2011, 142, 731–742 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1908522. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra02763g |
| ‡ Single crystal data for the compound 3as can be found in CCDC, depository number: 1908522. Spectral data for the compound (Z)-3-((benzo[d]thiazol-2-ylamino)methylene)-2-methoxychroman-4-one (3aa): Color & state: lemon yellow solid. Yield 90%, m.p.: 144–146 °C. 1H NMR (CDCl3, 500 MHz): δ 12.29 (d, 1H, J = 10.9 Hz), 8.05–7.95 (m, 2H), 7.79–7.71 (m, 2H), 7.52 (t, 1H, J = 8.4 Hz), 7.43 (t, 1H, J = 7.9 Hz), 7.28 (t, 1H, J = 7.6 Hz), 7.13 (t, 1H, J = 7.5 Hz), 7.06 (d, 1H, J = 8.1 Hz), 5.72 (s, 1H), 3.54 (s, 3H); 13C NMR (DMSO-d6, 75 MHz): δ 188.3, 174.9, 166.4, 163.4, 155.6, 152.7, 135.2, 130.9, 126.7, 125.4, 125.3, 124.6, 120.8, 120.0, 118.8, 117.7, 48.6; IR: νmax, 2929, 1649, 1588, 1529, 1465, 1267, 1207, 1146, 1066, 1015, 967, 938, 752, 717 cm−1. EI-MS: M–H, 337. EI-HRMS: calcd for. C18H14N2O3S = 338.0725. Found: 338.0724. |
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *b and
Jing Shang*b
*b and
Jing Shang*b






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solid obtained was recrystallized from a mixture of CH2Cl2-light petroleum to give 1,4-dihydro-4-oxo-1-(4-toluolsulfonyl)quinoline-3-carbaldehyde (Q5) as a white solid (369 mg, 97%).
1). The solid obtained was recrystallized from a mixture of CH2Cl2-light petroleum to give 1,4-dihydro-4-oxo-1-(4-toluolsulfonyl)quinoline-3-carbaldehyde (Q5) as a white solid (369 mg, 97%).






