Open Access Article
Open Access ArticleOne-step electrodeposition of cerium-doped nickel hydroxide nanosheets for effective oxygen generation†
Gang Fanga,
Jinhua Caib,
Zhipeng Huang *c and
Chi Zhang
*c and
Chi Zhang c
c
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, 212013, P. R. China
bCollege of Chemistry & Chemical Engineering, Jinggangshan University, Jian, Jiangxi Province 343009, P. R. China
cSchool of Chemical Science and Engineering, Tongji University, Shanghai, 200092, P. R. China. E-mail: zphuang@tongji.edu.cn
First published on 6th June 2019
Abstract
Efficient electrocatalysts catalyzing oxygen evolution reaction (OER) in alkaline media is highly desirable for large-scale hydrogen production from water splitting. Here we report the direct electrodeposition of cerium-doped nickel hydroxide nanosheets on carbon fiber paper and its prominent performance in catalyzing the OER. The composite generates a current density of 100 mA cm−2 at an overpotential of 320 mV, rivaling the performance of most reported OER catalysts and commercially available RuO2. X-ray photoelectron spectroscopy analysis shows strong electronic interaction between Ni(OH)2 and CeO2, making a great contribution to the OER enhancement.
Introduction
The fast-growing world population results in a significant increase in energy consumption. Due to the limited resource of fossil energy, the development of new technologies to provide clean, affordable, and renewable energy is urgently required.1,2 In the current hydrocarbon economic transformation, hydrogen is being actively promoted as a future energy carrier.3 In particular, electrochemical water splitting is being regarded as a green and sustainable route to convert water into hydrogen.3–5 However, because of the oxidative half-reaction, the oxygen evolution reaction (OER), is a 4-electron process that forms only one oxygen molecule, the kinetics of the OER is sluggish, and the OER has always been thought as the rate-limiting step in water splitting.6–9 Therefore, there is a need to develop an effective OER electrocatalyst, which possesses lower overpotential, faster catalytic kinetics, and therefore improves the energy conversion efficiency.10,11 Among many reported OER catalysts, RuO2 and IrO2 are the most active OER electrocatalysts, however, their scarcity and high cost greatly limit their large-scale applications.12,13 Substantial advances have been made in exploiting nonprecious oxygen-evolving catalysts, among which some transition metal hydr(oxy)oxides/oxides show superior intrinsic activity.14–19In general, doping catalysts with foreign elements can effectively modify electronic structure and reliably introduce defects to facilitate the adsorption and conversion of active intermediates with a lower energy barrier for OER.20 Transition metals, especially 3d transition metals such as Fe, Mn, Cr, and Co are widely used as dopants to regulate the chemical state of electrocatalysts.21–26 Recently, several groups reported the improvement of the performance of catalysts in hydrogen and oxygen generation by doping cerium into the host catalyst or constructing a heterogeneous structure between CeO2 and the active catalysts.27–34 For instance, CeO2-cluster-doped NiO shows better performance in the OER than CeO2 cluster surface-loaded NiO, due to promoted oxygen storage capacity and modified electronic structure of the active sites.35 The promotion of FeOOH catalysts for OER by the integration of CeO2 has been demonstrated due to the larger oxygen storage capacity of CeO2 and electron interaction between CeO2 and electrocatalysts.30 Another work has been represented by depositing a protective thin CeO2 layer on NiFeOx to improve stability by preventing ion leaching.36 We have also demonstrated that the introduction of Ce induced the amorphourization of CoOx and brought new oxygen defects.37 Although various approaches have been developed to introduce cerium into host catalysts, a convenient method for the one-step synthesis of metal hydr(oxy)oxides/oxides catalysts remains definitely unexploited.
In this study, we reported a convenient fabrication of OER electrocatalyst by one-step electrodeposition of cerium doped nickel hydroxide nanosheets (NSs) on carbon fiber paper (CFP) and its effective catalytic activity in the OER. The electrodeposited freestanding electrode possesses advantageous active site utilization and simple fabrication over conventional powder electrocatalysts, which is physically mixed with polymeric binder and conducting agent to make a slurry for coating on current collector.38 The optimization of the molar ratio of Ni to Ce in the deposition solution revealed that a 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ni
1 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce solution generated a film with the best performance. With the introduction of Ce, the optimized Ni(OH)2·0.75H2O–CeO2 NSs showed an tremendous increase in OER performance with the lowest overpotential of 320 mV at 100 mA cm−2 and Tafel slope of 126 mV dec−1 in alkaline electrolyte, as compared with that of Ni(OH)2·0.75H2O NSs(η100 = 460 mV, Tafel slope = 185 mV dec−1) deposited by the same method. Meanwhile, the Ni(OH)2·0.75H2O–CeO2 NS showed prominent stability at a high reaction rate for a long time and the faradaic efficiency of 92% during water oxidation. These results not only present a new 3d transition metal hydroxide doped with cerium as an effective OER catalysts, but also introduce a facile method for one step electrodeposition synthesis of electrocatalysts loaded onto conductive support.
Ce solution generated a film with the best performance. With the introduction of Ce, the optimized Ni(OH)2·0.75H2O–CeO2 NSs showed an tremendous increase in OER performance with the lowest overpotential of 320 mV at 100 mA cm−2 and Tafel slope of 126 mV dec−1 in alkaline electrolyte, as compared with that of Ni(OH)2·0.75H2O NSs(η100 = 460 mV, Tafel slope = 185 mV dec−1) deposited by the same method. Meanwhile, the Ni(OH)2·0.75H2O–CeO2 NS showed prominent stability at a high reaction rate for a long time and the faradaic efficiency of 92% during water oxidation. These results not only present a new 3d transition metal hydroxide doped with cerium as an effective OER catalysts, but also introduce a facile method for one step electrodeposition synthesis of electrocatalysts loaded onto conductive support.
Experimental
Reagents
Nickel nitrate hexahydrate (Ni(NO3)2·6H2O, AR), cerium nitrate hexahydrate (Ce(NO3)3·6H2O, REO), acetone (CH3COCH3, AR), and ethanol (C2H5OH, AR) were purchased from Tansoole Co., Ltd. CFP was commercially available from Hesen Co., Ltd. All chemicals were used as received without further purification.Synthesis
Prior to synthesis, a few pieces of CFP (1 cm × 2 cm) were washed ultrasonically with acetone, ethanol, and deionized water every 10 minutes in sequence. Ni(OH)2·0.75H2O–CeO2 NSs were directly electrodeposited on the CFP in a three-electrode cell, using the CFP as the working electrode, Pt as the counter electrode, and Hg/Hg2Cl2 as the reference electrode. The electrolyte solutions with different atomic ratio of Ni to Ce were prepared by mixing 10 mmol Ni(NO3)2·6H2O with different amounts of Ce(NO3)3·6H2O (i.e., 4, 1, 0.33, 0.25 mmol) into 100 ml deionized water and stirring them for 1 h. Electrodeposition was carried out in a three electrode system as described above using the potential static technique at −1.0 V for 20 min at room temperature; the corresponding electrodes are denoted as Ni(OH)2·0.75H2O–CeO2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (10
1) NSs, Ni(OH)2·0.75H2O–CeO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (30
1) NSs, Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, and Ni(OH)2·0.75H2O–CeO2 (40
1) NSs, and Ni(OH)2·0.75H2O–CeO2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, respectively. For the synthesis of Ni(OH)2·0.75H2O NSs, the method is similar to that of Ni(OH)2.0.75H2O–CeO2 NSs without adding the Ce(NO3)3·6H2O into the electrolyte. After deposition, the as-prepared products were washed with deionized water and absolute ethanol and then dried at 60 °C for 10 h. The mass loading of the products on CFP was approximately 6.8, 4.2, 7.5, 5.9, and 5.3 mg cm−2 for Ni(OH)2·0.75H2O–CeO2 (2.5
1) NSs, respectively. For the synthesis of Ni(OH)2·0.75H2O NSs, the method is similar to that of Ni(OH)2.0.75H2O–CeO2 NSs without adding the Ce(NO3)3·6H2O into the electrolyte. After deposition, the as-prepared products were washed with deionized water and absolute ethanol and then dried at 60 °C for 10 h. The mass loading of the products on CFP was approximately 6.8, 4.2, 7.5, 5.9, and 5.3 mg cm−2 for Ni(OH)2·0.75H2O–CeO2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (10
1) NSs, Ni(OH)2·0.75H2O–CeO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (30
1) NSs, Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (40
1) NSs, Ni(OH)2·0.75H2O–CeO2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs and Ni(OH)2·0.75H2O NSs, respectively. These results were obtained by weighing the electrodes carefully before and after deposition.
1) NSs and Ni(OH)2·0.75H2O NSs, respectively. These results were obtained by weighing the electrodes carefully before and after deposition.
Characterization
The morphology of the samples was characterized with field-emission scanning electron microscopy (FE-SEM, S-4800, Hitachi) and transmission electron microscopy (TEM, Tecnai G2 F30 S-TWIN, FEI). Powder X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance diffractometer with Cu Kα radiation (λ = 1.5406 Å). The surface characteristics of the samples were investigated using ESCALAB250Xi X-ray photoelectron spectrometer (XPS).Electrochemical measurement
Electrochemistry measurements were carried out on an electrochemistry workstation (CHI 760E, CH Instrument) in a three-port glass chamber. A mercury–mercury oxide electrode (MOE) was used as a reference electrode, and a graphite rod was used as a counter electrode. The counter electrode was separated from the chamber of working electrode by a porous glass frit. The electrolyte is an aqueous KOH solution (1 M). The RHE was determined by the open circuit potential of a clean Pt electrode in the solution of interest bubbled with H2 (99.999%). A scan rate of 5 mV S−1 was adopted for the measurement of polarization curves. The measured potentials were corrected with ohmic drop (iR), where i is the current corresponding to the experimental potential and R is the uncompensated cell resistance estimated by current interrupt method. The apparent Tafel slope was derived from the iR-corrected polarization curve by fitting experimental data to the equation η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j, where η is the iR-corrected potential, a is the Tafel constant, b is the Tafel slope, and j is the current density. Electrochemical impedance spectroscopy (EIS) were carried out at 1.55 V vs. RHE in the frequency range of 10−2 to 106 Hz with 10 mV sinusoidal perturbations and 12 steps per decade. Faradaic efficiency was measured by a method reported in our previous publications.39–41
j, where η is the iR-corrected potential, a is the Tafel constant, b is the Tafel slope, and j is the current density. Electrochemical impedance spectroscopy (EIS) were carried out at 1.55 V vs. RHE in the frequency range of 10−2 to 106 Hz with 10 mV sinusoidal perturbations and 12 steps per decade. Faradaic efficiency was measured by a method reported in our previous publications.39–41
Results and discussion
The XRD pattern was recorded to determine the component of products. Fig. 1 gives the XRD pattern of the two samples resulted from electro-deposition, including Ni(OH)2·0.75H2O and Ni(OH)2·0.75H2O–CeO2. In spite of the poor crystallinity of the deposited samples, both of the two XRD patterns have a number of peaks that can be indexed to Ni(OH)2·0.75H2O. The presence of Ni(OH)2·0.75H2O NSs were further examined using the HRTEM images. The detailed elemental composition and oxidation state of the hybrid arrays were further characterized using XPS analysis. The diffraction pattern for the as-prepared porous Ni(OH)2·0.75H2O–CeO2 NSs arrays on CFP, represented by the purple line, has seven obvious peaks at 28.5°, 33.0°, 47.5°, 56.3°, 59.0°, 69.4°, 76.7° and 79.0°, corresponding to (1 1 1), (2 0 0), (2 2 0), (3 1 1), (2 2 2), (4 0 0), (3 3 1), and (4 2 0) of cubic CeO2 (JCPDS PDF81-0792). It is therefore suggested that CeO2 was successfully doped into the Ni(OH)2·0.75H2O NSs. Owing to Ni(OH)2·0.75H2O NSs and Ni(OH)2·0.75H2O–CeO2 NSs growth on the CFP, peaks corresponding to the CFP were labeled by rhomboids.The morphologies of NSs of Ni(OH)2·0.75H2O and Ni(OH)2·0.75H2O–CeO2 supported by CFP were examined with the SEM. Fig. 2a and b show that Ni(OH)2·0.75H2O–CeO2 nanosheets were homogenously deposited on the surface of CFP with a three-dimensional (3D) open structure. Cracks can be found on the surface of CFP (shown in the Fig. S1†), with a width of 1.6 μm, which may be due to drying in the oven. The thickness of the sample on the surface of CFP was estimated to be 3 μm, according to the crack exposing the cross-section of the sample (shown in the Fig. S1†). Energy-dispersive X-ray spectroscopy (EDX) confirms the presences of Ni, Ce and O in the Ni(OH)2·0.75H2O–CeO2 NSs (Fig. S2 in the ESI†). With increasing Ce concentration, the atomic ratio of Ni to Ce changes from 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2.6
1 to 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2.27
1 to 2.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1.1
1 to 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (shown in the Table S1†), of which the values were determined by ICP-MS. The microstructures of Ni(OH)2·0.75H2O–CeO2 NSs were further assessed by TEM. Fig. 2c shows a high resolution TEM (HRTEM) image of Ni(OH)2·0.75H2O–CeO2 NSs, which shows well-resolved lattice fringes with an inter-plane distance of 0.27 nm, which corresponds to the (200) plane of CeO2, indicating the successfully introduction of CeO2 in the composite. And the part of the lattice that is not obvious corresponds to Ni(OH)2·0.75H2O, which is the same as HRTEM images of Ni(OH)2·0.75H2O NSs (Fig. S4a in the ESI†). The dark-field STEM image and the corresponding EDS mapping of the Ni(OH)2·0.75H2O–CeO2 NSs are shown in Fig. 2d–f. The element-mapping images of Ce, Ni, and O further confirm the Ni and Ce are homogeneously mixed with each other. For comparison, the pure Ni(OH)2·0.75H2O NSs grown on CFP was also synthesized by the same electro-deposition method without Ce source. The morphology of Ni(OH)2·0.75H2O NSs is analogous to that of Ni(OH)2·0.75H2O–CeO2 NSs, as shown by SEM images (Fig. S3 and S4 in the ESI†).
1 (shown in the Table S1†), of which the values were determined by ICP-MS. The microstructures of Ni(OH)2·0.75H2O–CeO2 NSs were further assessed by TEM. Fig. 2c shows a high resolution TEM (HRTEM) image of Ni(OH)2·0.75H2O–CeO2 NSs, which shows well-resolved lattice fringes with an inter-plane distance of 0.27 nm, which corresponds to the (200) plane of CeO2, indicating the successfully introduction of CeO2 in the composite. And the part of the lattice that is not obvious corresponds to Ni(OH)2·0.75H2O, which is the same as HRTEM images of Ni(OH)2·0.75H2O NSs (Fig. S4a in the ESI†). The dark-field STEM image and the corresponding EDS mapping of the Ni(OH)2·0.75H2O–CeO2 NSs are shown in Fig. 2d–f. The element-mapping images of Ce, Ni, and O further confirm the Ni and Ce are homogeneously mixed with each other. For comparison, the pure Ni(OH)2·0.75H2O NSs grown on CFP was also synthesized by the same electro-deposition method without Ce source. The morphology of Ni(OH)2·0.75H2O NSs is analogous to that of Ni(OH)2·0.75H2O–CeO2 NSs, as shown by SEM images (Fig. S3 and S4 in the ESI†).
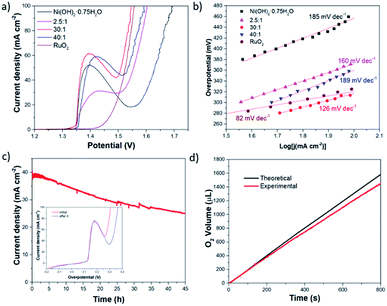
To demonstrate the activity of Ce for OER, the polarization curves were measured. The catalytic activity of Ni(OH)2·0.75H2O–CeO2 NSs and Ni(OH)2·0.75H2O NSs grown on CFP electrodes were studied initially by linear sweep voltammetry (LSV) in a standard three-electrode cell. A graphite rod was adopted as a counter electrode. All measured potentials were corrected with iR drop. Fig. 3a shows that the polarization curves of Ni(OH)2·0.75H2O and Ni(OH)2·0.75H2O–CeO2 with different atomic ratio of Ni to Ce. The overpotential required for a current density of 100 mA cm−2 (η100) is 460 mV for Ni(OH)2·0.75H2O NSs. Once Ce source was doped into the samples, the η100 of these Ni(OH)2·0.75H2O NSs changes with a huge decrease. With the increased atomic ratio of Ni to Ce, the OER activity appears as volcano-like trend, in which the Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs endows the best OER performance among the tested catalysts. The η100 is as small as 320 mV, making it among the best OER catalysts (shown in the Table S2†). To gain more insight on the OER activity, Tafel plots derived from polarization curves were constructed (Fig. 3b). The resulting Tafel slope of Ni(OH)2·0.75H2O–CeO2 (30
1) NSs endows the best OER performance among the tested catalysts. The η100 is as small as 320 mV, making it among the best OER catalysts (shown in the Table S2†). To gain more insight on the OER activity, Tafel plots derived from polarization curves were constructed (Fig. 3b). The resulting Tafel slope of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs was 126 mV dec−1, which is smaller than that of Ni(OH)2·0.75H2O NSs (185 mV dec−1), Ni(OH)2·0.75H2O–CeO2 (40
1) NSs was 126 mV dec−1, which is smaller than that of Ni(OH)2·0.75H2O NSs (185 mV dec−1), Ni(OH)2·0.75H2O–CeO2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (189 mV dec−1), and Ni(OH)2·0.75H2O–CeO2 (2.5
1) NSs (189 mV dec−1), and Ni(OH)2·0.75H2O–CeO2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (160 mV dec−1), indicating that the Ni(OH)2·0.75H2O–CeO2 (30
1) NSs (160 mV dec−1), indicating that the Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs exhibited better OER activity.
1) NSs exhibited better OER activity.
Long-term stability in oxygen generation is the prerequisite of a practically useful OER catalyst. The long-term stability of the Ni(OH)2·0.75H2O–CeO2 NSs was demonstrated by the chronoamperometry. The Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs can maintain 64% of its capability in catalyzing the OER in long-term water electrolysis after 45 hours, shown in Fig. 3c. And, the η100 is increased from 310 mV to 365 mV after the long-time electrolysis test (the inset plot in Fig. 3c). The decay of η100 is about 18%. The SEM images of Ni(OH)2·0.75H2O–CeO2 (30
1) NSs can maintain 64% of its capability in catalyzing the OER in long-term water electrolysis after 45 hours, shown in Fig. 3c. And, the η100 is increased from 310 mV to 365 mV after the long-time electrolysis test (the inset plot in Fig. 3c). The decay of η100 is about 18%. The SEM images of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs subjected to long-term potentiostatic electrolysis is shown in Fig. S5 in the ESI,† which shows that most Ni(OH)2·0.75H2O–CeO2 (30
1) NSs subjected to long-term potentiostatic electrolysis is shown in Fig. S5 in the ESI,† which shows that most Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs maintain their 3D open structure with some nanosheets peeling off on the surface of the catalyst. EDX shows that the atomic ratio of Ni to Ce changes from 6.29
1) NSs maintain their 3D open structure with some nanosheets peeling off on the surface of the catalyst. EDX shows that the atomic ratio of Ni to Ce changes from 6.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10.24
1 to 10.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with some loss of Ce (Fig. S6 in the ESI†), which may be one of the reasons for degradation of catalyst performance.
1 with some loss of Ce (Fig. S6 in the ESI†), which may be one of the reasons for degradation of catalyst performance.
The faradaic efficiency of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs during O2 evolution was evaluated by the comparison of the volume of generated gas and the theoretical volume in the potentiostatic electrolysis measurement, shown in Fig. 3d. The theoretical volumes were computed by assuming that all electrons passing through the circuit participate in the oxidation reaction of OH− (4OH− − 4e− → O2↑ + 2H2O), and the experiment volume was detected by the water displacement method. During electrolysis for 4000 s, the experiment and theoretical volumes are in good accordance, suggesting 92% faradaic efficiency.
1) NSs during O2 evolution was evaluated by the comparison of the volume of generated gas and the theoretical volume in the potentiostatic electrolysis measurement, shown in Fig. 3d. The theoretical volumes were computed by assuming that all electrons passing through the circuit participate in the oxidation reaction of OH− (4OH− − 4e− → O2↑ + 2H2O), and the experiment volume was detected by the water displacement method. During electrolysis for 4000 s, the experiment and theoretical volumes are in good accordance, suggesting 92% faradaic efficiency.
To understand the excellent OER activity, EIS was tested at the potential of 1.55 V vs. RHE. The results are shown in the Nyquist curves in the Fig. 4a, where the data were fit using an equivalent circuit shown in Fig. S7 and Table S3 in the ESI.† The semicircle in the low frequency range is related to the faradaic process (OER) on electrocatalyst surface. Corresponding resistance element is charge transfer resistance (Rct). Rct is usually used as an indicator of OER kinetics, a smaller Rct corresponds to a faster OER process. As shown in the Fig. 4a, the Rct is decreased with the introduction of Ce to Ni(OH)2·0.75H2O NSs, and the Rct of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (0.856 Ω) is smaller than those of Ni(OH)2·0.75H2O NSs (1.317 Ω), Ni(OH)2·0.75H2O–CeO2 (10
1) NSs (0.856 Ω) is smaller than those of Ni(OH)2·0.75H2O NSs (1.317 Ω), Ni(OH)2·0.75H2O–CeO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (0.928 Ω), Ni(OH)2·0.75H2O–CeO2 (40
1) NSs (0.928 Ω), Ni(OH)2·0.75H2O–CeO2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (0.948 Ω) and Ni(OH)2·0.75H2O–CeO2 (2.5
1) NSs (0.948 Ω) and Ni(OH)2·0.75H2O–CeO2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs (1.177 Ω) under the same condition, suggesting that Ni(OH)2·0.75H2O–CeO2 (30
1) NSs (1.177 Ω) under the same condition, suggesting that Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs possesses the fast electron transport ability for OER.
1) NSs possesses the fast electron transport ability for OER.
Electrochemically active surface area (ECSA) should play an important role in the high electrocatalytic performance, and it was derived from the specific capacitance measured by cyclic voltammetry (CV) scans (Fig. S8 in the ESI†). The potential range of the CV scans was selected at 1.1–1.2 V vs. RHE which did not include obvious electrochemical features corresponding to faradaic current. As a result, the dependence of the current on the scan rate in this region for both electrodes was linear. The ECSA of the Ni(OH)2·0.75H2O NSs, Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni (OH)2·0.75H2O–CeO2 (40
1) NSs, Ni (OH)2·0.75H2O–CeO2 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, Ni(OH)2·0.75H2O–CeO2 (10
1) NSs, Ni(OH)2·0.75H2O–CeO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs, and Ni(OH)2·0.75H2O–CeO2 (2.5
1) NSs, and Ni(OH)2·0.75H2O–CeO2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs were measured to be 3.4 cm2, 7.74 cm2, 6.33 cm2, 7.67 cm2, and 5.83 cm2 (shown in Fig. 4b). The ECSA of Ni(OH)2·0.75H2O–CeO2 (30
1) NSs were measured to be 3.4 cm2, 7.74 cm2, 6.33 cm2, 7.67 cm2, and 5.83 cm2 (shown in Fig. 4b). The ECSA of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs is 2.3 times that of Ni(OH)2·0.75H2O NSs, demonstrating that the introduction of Ce can remarkably enlarge the ECSA of Ni(OH)2·0.75H2O NSs.
1) NSs is 2.3 times that of Ni(OH)2·0.75H2O NSs, demonstrating that the introduction of Ce can remarkably enlarge the ECSA of Ni(OH)2·0.75H2O NSs.
To understand the better intrinsic OER performance of Ni(OH)2·0.75H2O–CeO2 NSs, the activation energy of the OER was determined by Arrhenius plot: log(j) = −0.434Ea/(RT) + const, where R is the Boltzmann constant (8.315 J g−1 mol−1 K−1), Ea is the activation energy expressed in J g−1 mol−1. Temperature related polarization curves of Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs and Ni(OH)2·0.75H2O NSs are shown in Fig. S9 in the ESI,† and corresponding Arrhenius plot at η = 150 mV can be found in the Fig. 5a. The Ea is 6.06 kJ mol−1 for Ni(OH)2·0.75H2O–CeO2 (30
1) NSs and Ni(OH)2·0.75H2O NSs are shown in Fig. S9 in the ESI,† and corresponding Arrhenius plot at η = 150 mV can be found in the Fig. 5a. The Ea is 6.06 kJ mol−1 for Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs and 9.77 kJ mol−1 for Ni(OH)2·0.75H2O NSs. That is the Ni(OH)2·0.75H2O–CeO2 (30
1) NSs and 9.77 kJ mol−1 for Ni(OH)2·0.75H2O NSs. That is the Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) NSs has a smaller apparent barrier height in the OER than Ni(OH)2·0.75H2O NSs.
1) NSs has a smaller apparent barrier height in the OER than Ni(OH)2·0.75H2O NSs.
 | ||
Fig. 5 (a) Arrhenius plots of Ni(OH)2·0.75H2O and Ni(OH)2·0.75H2O–CeO2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (b–d) XPS spectra of Ce 3d, O 1s, and Ni 2p. 1), (b–d) XPS spectra of Ce 3d, O 1s, and Ni 2p. | ||
In order to analyze the chemical composition of the prepared Ni(OH)2·0.75H2O NSs and Ni(OH)2·0.75H2O–CeO2 NSs electrocatalysts and to identify the chemical states of Ni, Ce, O elements in the samples, XPS measurements were carried out. As shown in Fig. 5b, XPS demonstrates that the element Ce mainly exists as CeO2, which is composed of 3d5/2 (vn) and 3d3/2 (un) spin–orbit components.30 For Ce 3d of CeO2, the spectra can be deconvoluted into 10 peaks, which consist of two pairs of doublets (v0/u0 and v′/u′) assigned to Ce3+ and three pairs of doublets (v/u, v′′/u′′, and v′′′/u′′′) assigned to Ce4+, indicating the coexistence of Ce3+ and Ce4+ in the Ni(OH)2·0.75H2O–CeO2 NSs.42,43 For the O 2p spectra (shown in Fig. 5c), peaks located at 530.87(O2), 832.60(O3) and 535.07(O4) eV are ascribed to the lattice oxygen, oxygen defects, and adsorbed oxygen species (water molecules), accordingly.44–47 Meanwhile, a peak near 528.94 eV is usually attributed to lattice Ce–O,30 reconfirming the existence of CeO2. As shown in the Ni 2p3/2 spectra (Fig. 5d), the XPS results demonstrate that the element Ni of Ni(OH)2·0.75H2O NSs and Ni(OH)2·0.75H2O–CeO2 NSs mainly exist as Ni(OH)2, containing Ni(II) species. However, there is a negative shift of 0.3 eV for Ni 2p3/2 in the Ni(OH)2·0.75H2O–CeO2 NSs, compared to those in the Ni(OH)2·0.75H2O NSs. Owing to the remarkable redox property of CeO2,48,49 This result suggests the electron transfer between Ni(OH)2 and CeO2, contributing to a strong electron interaction formed between Ni(OH)2 and CeO2, and accordingly enhancing the electrocatalytic performance.
Conclusions
In summary, cerium doped nickel hydroxide NSs were directly electrodeposited on CFP, and it exhibited superior OER activity compared to nickel hydroxide nanosheets. With the introduction of Ce, the optimized Ni(OH)2·0.75H2O–CeO2 NSs showed a tremendous increase in OER performance with the smallest η100 of 320 mV and Tafel slope of 126 mV dec−1 in alkaline electrolyte, as compared with that of Ni(OH)2·0.75H2O NSs deposited in the same electrodeposition method. Meanwhile, the catalyst is stable for 45 h at high current density and 92% faradaic efficiency. The introduction of Ce brings strong electronic interactions between Ni(OH)2 and CeO2 and more electrochemically active sites, which should play an essential role in enhancing the OER activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (51772214, 51432006), the Ministry of Science and Technology of China (2011DFG52970), the Ministry of Education of China (IRT14R23), 111 Project (B13025), Jiangsu Province (2011-XCL-019 and 2013-479).References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294 CrossRef CAS PubMed.
- R. Schloegl, Nat. Mater., 2008, 7, 772 CrossRef CAS PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332 CrossRef CAS PubMed.
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- C. C. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- I. Markoulaki, I. Papadas, I. Kornarakis and G. Armatas, Nanomaterials, 2015, 5, 1971–1984 CrossRef PubMed.
- X. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen and S. Yang, Angew. Chem., Int. Ed., 2014, 53(29), 7584–7588 CrossRef CAS PubMed.
- T. Sharifi, E. Gracia-Espino, X. Jia, R. Sandstrom and T. Wågberg, ACS Appl. Mater. Interfaces, 2015, 7(51), 28148–28155 CrossRef CAS PubMed.
- L. J. Foruzin, Z. Rezvani, Y. H. Shishavan and B. Habibi, Int. J. Hydrogen Energy, 2018, 43(1), 150–160 CrossRef CAS.
- J. Li, G. Liu, B. Liu, Z. Min, D. Qian, J. Jiang and J. Li, Int. J. Hydrogen Energy, 2018, 43(3), 1365–1374 CrossRef CAS.
- J. A. Koza, Z. He, A. S. Miller and J. A. Switzer, Chem. Mater., 2012, 24(18), 3567–3573 CrossRef CAS.
- R. Chen, H. Y. Wang, J. Miao, H. Yang and B. Liu, Nano Energy, 2015, 11, 333–340 CrossRef CAS.
- X. Zheng, B. Zhang, P. De Luna, Y. Liang, R. Comin, O. Voznyy and T. Regier, Nat. Chem., 2018, 10(2), 149 CrossRef CAS PubMed.
- R. D. Smith, M. S. Prévot, R. D. Fagan, S. Trudel and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135(31), 11580–11586 CrossRef CAS PubMed.
- B. Zhang, X. Zheng, O. Voznyy, R. Comin, M. Bajdich, M. García-Melchor and F. P. G. de Arquer, Science, 2016, 352(6283), 333–337 CrossRef CAS PubMed.
- R. Subbaraman, D. Tripkovic, K. C. Chang, D. Strmcnik, A. P. Paulikas, P. Hirunsit and N. M. Markovic, Nat. Mater., 2012, 11(6), 550 CrossRef CAS PubMed.
- J. W. D. Ng, M. García-Melchor, M. Bajdich, P. Chakthranont, C. Kirk, A. Vojvodic and T. F. Jaramillo, Nat. Energy, 2016, 1(5), 16053 CrossRef CAS.
- F. Dionigi and P. Strasser, Adv. Energy Mater., 2016, 6(23), 1600621 CrossRef.
- L. Han, S. J. Dong and E. K. Wang, Adv. Mater., 2016, 28, 9266 CrossRef CAS PubMed.
- C.-C. Lin and C. C. L. McCrory, ACS Catal., 2017, 7, 443 CrossRef CAS.
- T. Tang, W.-J. Jiang, S. Niu, N. Liu, H. Luo, Y.-Y. Chen, S.-F. Jin, F. Gao, L.-J. Wan and J.-S. Hu, J. Am. Chem. Soc., 2017, 139, 8320 CrossRef CAS PubMed.
- G. Wu, W. X. Chen, X. S. Zheng, D. P. He, Y. Q. Luo, X. Q. Wang, J. Yang, Y. E. Wu, W. S. Yan, Z. B. Zhuang, X. Hong and Y. D. Li, Nano Energy, 2017, 38, 167 CrossRef CAS.
- J. X. Feng, H. Xu, Y. T. Dong, S. H. Ye, Y. X. Tong and G. R. Li, Angew. Chem., Int. Ed., 2016, 55(11), 3694–3698 CrossRef CAS PubMed.
- X. F. Lu, L. F. Gu, J. W. Wang, J. X. Wu, P. Q. Liao and G. R. Li, Adv. Mater., 2017, 29(3), 1604437 CrossRef PubMed.
- S. H. Ye, Z. X. Shi, J. X. Feng, Y. X. Tong and G. R. Li, Angew. Chem., Int. Ed., 2018, 57(10), 2672–2676 CrossRef CAS PubMed.
- J. A. Haber, Y. Cai, S. Jung, C. X. Xiang, S. Mitrovic, J. Jin, A. T. Bell and J. M. Gregoire, Energy Environ. Sci., 2014, 7, 682 RSC.
- Y.-R. Zheng, M.-R. Gao, Q. Gao, H.-H. Li, J. Xu, Z.-Y. Wu and S.-H. Yu, Small, 2015, 11, 182 CrossRef CAS PubMed.
- J. W. D. Ng, M. García-Melchor, M. Bajdich, P. Chakthranont, C. Kirk, A. Vojvodic and T. F. Jaramillo, Nat. Energy, 2016, 1, 16053 CrossRef CAS.
- J.-X. Feng, S.-H. Ye, H. Xu, Y.-X. Tong and G.-R. Li, Adv. Mater., 2016, 28, 4698 CrossRef CAS PubMed.
- Z. Q. Liu, N. Li, H. Y. Zhao, Y. Zhang, Y. H. Huang, Z. Y. Yin and Y. P. Du, Chem. Sci., 2017, 8, 3211 RSC.
- W. Gao, M. Yan, H.-Y. Cheung, Z. M. Xia, X. M. Zhou, Y. B. Qin, C.-Y. Wong, J. C. Ho, C.-R. Chang and Y. Q. Qu, Nano Energy, 2017, 38, 290 CrossRef CAS.
- X. Wang, Y. Yang, L. Diao, Y. Tang, F. He, E. Liu and S. Ji, ACS Appl. Mater. Interfaces, 2018, 10(41), 35145–35153 CrossRef CAS PubMed.
- M. Favaro, W. S. Drisdell, M. A. Marcus, J. M. Gregoire, E. J. Crumlin, J. A. Haber and J. Yano, ACS Catal., 2017, 7(2), 1248–1258 CrossRef CAS.
- W. Gao, Z. Xia, F. Cao, J. C. Ho, Z. Jiang and Y. Qu, Adv. Funct. Mater., 2018, 28(11), 1706056 CrossRef.
- K. Obata and K. Takanabe, Angew. Chem., Int. Ed., 2018, 57(6), 1616–1620 CrossRef CAS PubMed.
- S. Xu, C. Lv, T. He, Z. Huang and C. Zhang, J. Mater. Chem. A, 2019, 7, 7526–7532 RSC.
- H. Zhang, H. Ning, J. Busbee, Z. Shen, C. Kiggins, Y. Hua and J. M. Zuo, Sci. Adv., 2017, 3(5), e1602427 CrossRef PubMed.
- C. Lv, Z. Huang, Q. Yang, G. Wei, Z. Chen, M. G. Humphrey and C. Zhang, J. Mater. Chem. A, 2017, 5, 22805–22812 RSC.
- C. Lv, Q. Yang, Q. Huang, Z. Huang, H. Xia and C. Zhang, J. Mater. Chem. A, 2016, 4, 13336–13343 RSC.
- L. Jin, H. Xia, Z. Huang, C. Lv, J. Wang, M. G. Humphrey and C. Zhang, J. Mater. Chem. A, 2016, 4, 10925–10932 RSC.
- C. Hardacre, G. M. Roe and R. M. Lambert, Surf. Sci., 1995, 326(1–2), 1–10 CrossRef CAS.
- B. Lin, Y. Liu, L. Heng, J. Ni, J. Lin and L. Jiang, Catal. Commun., 2017, 101, 15–19 CrossRef CAS.
- W. X. Guo, W. W. Sun and Y. Wang, ACS Nano, 2015, 9, 11462 CrossRef CAS PubMed.
- T. V. Thi, A. K. Rai, J. Gim and J. Kim, J. Power Sources, 2015, 292, 23 CrossRef CAS.
- B. P. Payne, M. C. Biesigner and N. S. McIntyre, J. Electron Spectrosc. Relat. Phenom., 2012, 185, 159 CrossRef CAS.
- J. Bao, X. D. Zhang, B. Fan, J. J. Zhang, M. Zhou, W. L. Yang, X. Hu, H. Wang, B. C. Pan and Y. Xie, Angew. Chem., 2015, 127, 7507 CrossRef.
- C. T. Campbell and C. H. F. Peden, Science, 2005, 309, 713 CrossRef CAS PubMed.
- J. Paier, C. Penschke and J. Sauer, Chem. Rev., 2013, 113, 3949 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02682g |
| This journal is © The Royal Society of Chemistry 2019 |