Open Access Article
Open Access ArticleHighly effective photocatalytic performance of {001}-TiO2/MoS2/RGO hybrid heterostructures for the reduction of Rh B†
Ya Gaoa,
Yongjie Zheng *a,
Jixing Chaib,
Jingzhi Tiana,
Tao Jinga,
Deqing Zhangb,
Junye Cheng
*a,
Jixing Chaib,
Jingzhi Tiana,
Tao Jinga,
Deqing Zhangb,
Junye Cheng *c,
Huiqing Pengc,
Bin Liu
*c,
Huiqing Pengc,
Bin Liu *c and
Guangping Zhengd
*c and
Guangping Zhengd
aSchool of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, China. E-mail: yongjiezheng@163.com
bSchool of Materials Science and Engineering, Qiqihar University, Qiqihar 161006, China
cCenter of Super-Diamond and Advanced Films (COSDAF), Department of Materials Science and Engineering, City University of Hong Kong, Hong Kong 999077, China. E-mail: binliu@mail.ipc.ac.cn; jycheng4-c@my.cityu.edu.hk
dDepartment of Mechanical Engineering, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, China
First published on 17th May 2019
Abstract
Effective separation and rapid transfer of photogenerated electron–hole pairs are key features of photocatalytic materials with high catalytic activity, which could be achieved in co-catalysts. It is reported that the two-dimensional (2D) MoS2 is a promising co-catalyst due to its unique semi-conductive properties and graphene-like layered structure. However, the application of MoS2 as a co-catalyst is limited by its poor electrical conductivity. On the other hand, it is worth noting that TiO2 possesses reactive crystal facets, which is one of the dominant mechanisms for the separation of photogenerated electron–hole pairs. In this work, we prepared MoS2/RGO hybrids as co-catalysts which were doped to TiO2 with highly reactive {001} planes via the hydrothermal method. It was found that the {001}-TiO2/MoS2/RGO photocatalysts with 7 wt% MoS2/RGO co-catalyst show the highest photodegradation activity for the degradation of Rh B under visible light irradiation (λ > 400 nm), which could result from the synergy of the effective separation of electron–hole pairs by the {001} facets in TiO2 and the rapid transfer of electron–hole pairs in MoS2/RGO. The results show that the {001}-TiO2/MoS2/RGO hybrid is a low-cost and stable photocatalyst for the effective degradation of Rh B under visible light.
1. Introduction
Semiconducting photocatalysts exhibit great prospects in the removal of pollutants from water and air.1–4 Among the pursued semiconductors, titanium dioxide (TiO2) with a band gap of 3.2 eV between the conduction band (CB) and valence band (VB), has attracted attention as a photocatalytic material owing to its nontoxicity, chemical stability, low cost, high chemical inertness.5–10 The active photocatalytic characteristics of TiO2 are restricted due to the rapid recombination of photo-generated electron–hole pairs.11,12There are two strategies that have been carried out to enhance the photocatalytic performance of TiO2. One of the strategies is based on the design of unique morphology and structure of TiO2, such as mesoporous TiO2 hollow shells,13,14 TiO2 nanosheet arrays,15,16 and three-dimensional network TiO2 nanowire films.17 Another strategy is the loading of co-catalyst, including graphene (GN),18–20 metallic phases21,22 and nonmetallic phases23,24 of materials, as well as polymer-metallic catalysts.25,26 Zhang et al.20 reported a successful strategy to improve the photocatalytic performance of TiO2 via introducing the GN without defects to enhance the interfacial contact between GN and TiO2; Liu et al.22 prepared Ag/Ag(I)–TiO2 photocatalyst by a facile impregnated method in combination with a calcination process and the photocatalyst showed a higher visible-light photocatalytic activity; Qu et al.24 synthesized a S,N co-doping GQD/TiO2 composite though a facile hydrothermal and its photocatalytic performance (degradation of Rh B under visible light) was 10 times higher than that of P25; Jiang et al.26 reported a nanostructured TiO2 thin film accompanying with the effects of polymer conjugation and Mo-doping, as a result, the methylene blue dye degradation rate reached 90.6%. In the aforementioned methods, heterostructure photocatalysts consisting of two semi-conductive materials with different band gaps and electronic structures have been proved to be effective in photocatalysis.27–31
If developed into a co-catalyst material, MoS2 have some particular advantages due to its graphene-like nanostructures, relatively high activity and unique semiconducting properties.32–34 And there are numerous reports about MoS2-based heterostructure for photocatalytic production of H2. However the applications of MoS2 in the removal of pollutants are limited by its poor electrical conductivity.35–37 Generally, there are two synthesis routes in the fabrication of MoS2-based heterostructure with effective charge transfer processes. First, chemical lithium intercalation could induce a phase change in MoS2 from the 2H (showing semiconducting properties) to the 1T (showing metallic properties) phases, resulting in the heterostructure. Bai et al.38 firstly reported that compared with (2H) MoS2–TiO2, the (1T) MoS2–TiO2 exhibits excellent photocatalytic performance. Second, the introduction of conductive materials into MoS2 leads to a heterostructure. Peng et al.39 synthesized MoS2/RGO/CdS as an effective photocatalyst which can reduce and detoxify nitroaromatic compounds. The MoS2-based heterostructures prepared by these two methods have played an important catalytic role, while the latter is more favorable because of its simplicity and efficiency.
Moreover, it is reported that the reactive crystal facets of TiO2 are closely related with its photocatalytic efficiency, and the {001} facet-dominated single-crystal anatase TiO2 shows higher photocatalytic activity than P25.40–42 Enlightened by the abovementioned findings, it is suggested that the {001}-TiO2/MoS2/RGO should be an ideal photocatalyst. In this work, we prepared a {001}-TiO2/MoS2/RGO hybrid photocatalyst with low cost. The as-prepared {001}-TiO2/MoS2/RGO exhibited more outstanding photocatalytic performance for the degradation of Rhodamine B (Rh B) under visible light irradiation (λ > 400 nm), compared with the pure {001}-TiO2 and {001}-TiO2/MoS2. The enhanced photocatalytic activity could be ascribed to the effective charge separation and transfer due to the band gap of MoS2 and high conductivity of graphene.
2. Experimental
2.1 Materials
All chemicals were in analytical grade and used without further purification. Molybdic acid (H2MoO4 ≥ 85%) was purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. Thiourea (CH4N2S) was purchased from Tianjin Fuchen Chemical Reagent Factory. Graphene oxide dispersion were purchased from Nanjing Pioneer Nano Material Technology Co., Ltd. N-Butyl titanate (TBOT) was purchased from Tianjin Tianli Chemical Reagent Co. Ltd. Hydrofluoric acid (HF) was purchased from Tianjin Fuyu Chemical Reagent Co. Ltd.2.2 Synthesis of {001}-TiO2 nanosheets
The {001}-TiO2 nanosheets were prepared by a simple hydrothermal route. Firstly, 25 ml of n-butyl titanate and 3 ml of hydrofluoric acid were added into a 100 ml Teflon-lined autoclave, under magnetic stirring for 2 h. After heating and stirring for 24 h at 180 °C, the solution was naturally cooled to room temperature and washed with deionized water and ethanol for several times until its pH value reached 7 and dried at 60 °C in a vacuum oven to obtain the {001}-TiO2 nanosheets.2.3 Synthesis of {001}-TiO2/MoS2/RGO hybrid
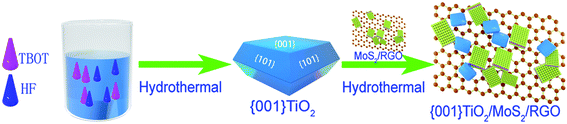
The nanohybrids were also prepared by a hydrothermal method. Firstly, the as-prepared MoS2/RGO (with 7 wt% RGO) which was prepared by a one-step hydrothermal method previously reported,43 was dispersed in 80 ml of DI. Secondly, 0.99 g of the as-prepared {001}-TiO2 nanosheets were added to the above-mentioned dispersions. After subjected to magnetic stirring for 1 h, the mixture was transfer to a 100 ml Teflon-lined autoclave. After reaction for 3 h at 180 °C, the solution was naturally cooled down to the room temperature. Then the {001}-TiO2/MoS2/RGO hybrid was washed with deionized water and ethanol for several times and dried at 60 °C in a vacuum oven. With the same method described above, the {001}-TiO2/MoS2/RGO samples with different MoS2/RGO loadings were prepared. The aforementioned facile synthesis route was summarized in Scheme 1.2.4 Characterization
The morphology of samples was characterized by the field emission scanning electron microscope (SEM, Hitachi S-3400) and transmission electron microscope (TEM, Hitachi H7650). The crystallization properties were measured by X-ray powder diffraction (XRD) patterns (Bruker D8) with a Cu-Kα radiation. The crystal structure was observed with the high-resolution electron microscopy (HRTEM, Tecnai F30). Element information was characterized by X-ray photoelectron spectrometer (ESCALAB250Xi, Thermofisher Co). Raman spectrum was measured by the equipment Lab RAM HA Evolution. Ultraviolet-visible (UV-vis) absorption spectra were performed using a spectrophotometer (Lambda 35, American). The photoluminescence spectra (PL) was examined with a fluorescence spectrophotometer (RF-5301PC, Shimadzu Corporation, Japan). The information on the Brunauer–Emmett–Teller (BET) surface areas of the sample powders was obtained on an automatic gas adsorption analyzer (Conta Instruments, USA).2.5 Evaluation of photocatalytic activity
The photocatalytic activities of the catalysts were evaluated through the degradation of Rh B under a light source of 300 W Xe arc lamp equipped with a cut-off filter (λ > 400 nm) for visible light. Firstly, 50 mg of photocatalyst was added into 50 ml Rh B solution (1 mg ml−1). Then, the suspensions were stirred in dark for 30 minutes to reach the adsorption–desorption equilibrium. Third, the suspensions were subjected to the illumination every 10 minutes; in the intervals of illumination 5 ml suspension was withdrawn and centrifuged to remove the photocatalyst particles and then was tested by a UV-vis spectrophotometer to determine the concentration of Rh B through measurements on the 554 nm absorption peak. The Rh B concentration after adsorption equilibrium is regarded as the initial concentration (C0).3. Results and discussion
3.1 Results of characterization
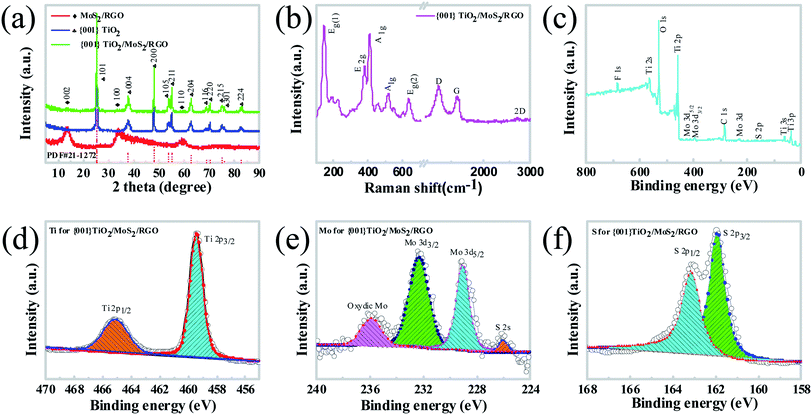
The crystal structures of the prepared samples are characterized determined by XRD, and the XRD patterns of pure {001}-TiO2, MoS2/RGO and {001}-TiO2/MoS2/RGO are shown in Fig. 1a. The diffraction peaks at 2θ = 25.28°, 37.8°, 48.04°, 53.89°, 55.06°, 62.68°, 68.76°, 70.30°, 75.02°, 76.01° and 82.65° can be observed in {001}-TiO2 and {001}-TiO2/MoS2/RGO samples, which are assigned to (101), (004), (200), (105), (204), (116), (220), (215), (301) and (224) planes of the anatase TiO2 (PDF 21-1272), respectively. It is also seen that there are only two extremely weak peaks of the co-catalyst in the pattern for {001}-TiO2/MoS2/RGO (the inset in Fig. 1a). In the patterns for the MoS2 and MoS2/RGO samples (Fig. S1a†), there are four peaks at 2θ = 14.6°, 32.8°, 39.8° and 58.5°, which are assigned to (002), (100), (103), and (110) planes of MoS2 (JCPDS37-1492), respectively. There are no obvious diffraction peaks for RGO mostly because of very small amount of RGOs in the samples.To confirm the presence of RGO in the {001}-TiO2/MoS2/RGO hybrid, Raman measurements were carried out. Fig. 1b shows the Raman spectrum of {001}-TiO2/MoS2/RGO hybrid, it is observed that the weak Eg(1), A1g and Eg(2) peaks for TiO2 are located at 142.97 cm−1, 515.22 cm−1 and 636.34 cm−1, respectively. It also can be clearly observed that the E2g and A1g peaks for MoS2 are located at 379.74 and 408.88 cm−1, respectively. The peaks of the D- and G-band for RGOs are located at 1348.53 and 1588.53 cm−1, respectively, and the I2D/IG ratio of RGO is 0.19 (0.07 < 0.19 < 0.3), suggesting that the hybrid have triple-layer graphene sheets.43 The results of Raman spectra thus prove the presence of RGO in the hybrid.
The interactions between the TiO2 and MoS2/RGO in the {001}-TiO2/MoS2/RGO hybrid are studied by XPS spectra. As shown in Fig. 1c, the Mo, S, Ti, O, C and F elements appear in the survey spectrum. The peak at the binding energy of 684.5 eV is assigned to F 1s, which is a typical value for the fluorinated TiO2 system such as the surficial Ti–F species.44 Fig. 1d and S2a† shows the high-resolution spectra of Ti 2p for TiO2 and {001}-TiO2/MoS2/RGO, respectively. The peaks located at the binding energies of 465.31 and 459.57 eV for TiO2 could correspond to Ti 2p1/2 and 4p3/2, respectively, which can be assigned to a Ti4+ oxidation state. Compared with pure TiO2, those peaks for {001}-TiO2/MoS2/RGO shift to 465.11 and 459.48 eV, indicating a strong interaction between MoS2/RGO and TiO2. As shown in the Mo spectrum of {001}-TiO2/MoS2/RGO (Fig. 1e), the bands located at the binding energies of 232.35 and 229.03 eV are assigned to Mo (3d3/2) and Mo (3d5/2) in the normal chemical state of Mo4+, respectively. The S spectra of {001}-TiO2/MoS2/RGO are observed to have two peaks located at 162.82 and 161.71 eV, as shown in Fig. 1f, which are consistent with those of MoS2. The C 1s spectrum can be deconvoluted into three peaks located at 288.75, 286.40, and 284.84 eV (Fig. S2b†), which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and C–C functionalities, respectively, and indicate the existence of the carboxy, epoxy, and hydroxyl groups in the graphene. The results indicate that most of the oxygen-containing functional groups are successfully removed. The reduction of GO to graphene will dramatically improve the electrical conductivity of the hybrids, and hence significantly enhancing their photocatalytic activity.45,46
O, C–O, and C–C functionalities, respectively, and indicate the existence of the carboxy, epoxy, and hydroxyl groups in the graphene. The results indicate that most of the oxygen-containing functional groups are successfully removed. The reduction of GO to graphene will dramatically improve the electrical conductivity of the hybrids, and hence significantly enhancing their photocatalytic activity.45,46
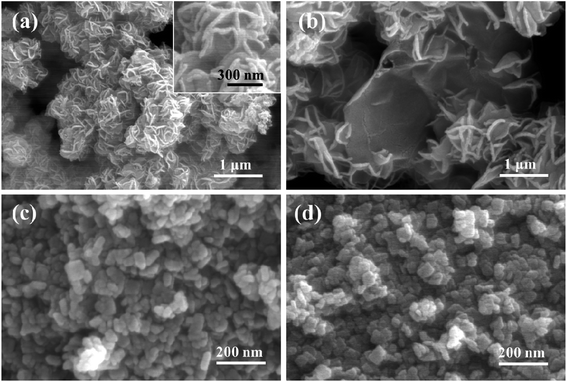
Typical morphology and microstructure of the as-prepared catalyst are characterized using scanning electron microscopy (SEM). Fig. 2a shows that a large number of stacked MoS2 nanosheets with significantly curled edges are observed. Fig. 2b shows the microstructure of MoS2/RGO, indicating that the small MoS2 nanosheets are well loaded onto the surfaces of RGOs. Fig. 2c shows the morphology of TiO2, which exhibits a rectangular shape with lengths of about 40–60 nm. Fig. 2d show the morphology of {001}-TiO2/MoS2/RGO. No obvious MoS2/RGO structures can be observed, which maybe caused by the low content of MoS2/RGO. To determine the composition of {001}-TiO2/MoS2/RGO, the sample is measured by the energy dispersive spectrometer (EDS) as shown in Fig. S3.† Fig. S3† shows that there are a large amount of Ti and O elements, a small amount of Mo and S elements and a few C elements. Moreover, the distribution of Ti and O elements is even and consistent, and the Mo and S elements also show similar features of distribution. Thus, the EDS results demonstrate that the sample shown in Fig. 3d could be {001}-TiO2/MoS2/RGO.
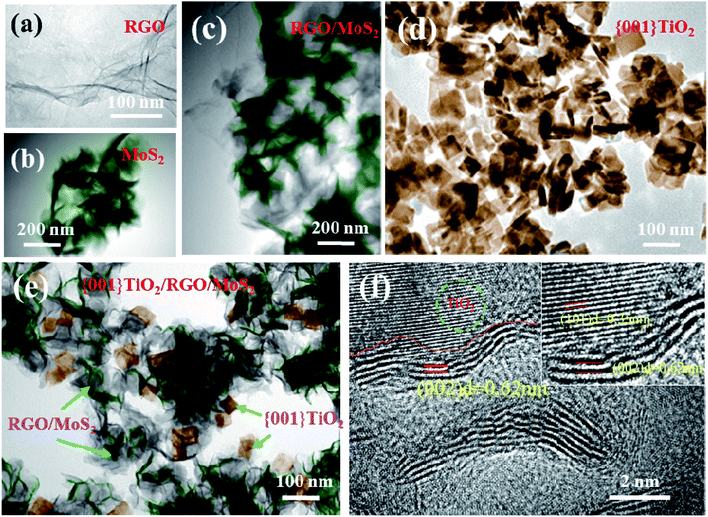 | ||
| Fig. 3 TEM images of RGO (a), MoS2 (b), MoS2/RGO (c), {001}-TiO2 (d), {001}-TiO2/MoS2/RGO (e); HRTEM image of {001}-TiO2/MoS2/RGO (f). | ||
Detailed microstructure of the samples can be clearly observed in the transmission electron microscopy (TEM) and high-resolution TEM (HRTEM). MoS2 shows an appearance of thin nanosheets that look like soft and light flowers (Fig. 3b), for MoS2/RGO hybrid, it can be clearly seen that the flower-like MoS2 in the dark region is evenly attached to the bright and light RGOs (Fig. 3a and c). The {001}-TiO2 has a thickness of 11 nm with average dimensions (length × width) of 64 × 38 nm and there is very few small sized {001}-TiO2 (Fig. 3d). Fig. 3e shows the TEM image of {001}-TiO2/MoS2/RGO. The {001}-TiO2 nanosheets and MoS2/RGO can be observed clearly. The HRTEM image of Fig. 3f shows that the lattice spacing of 0.35 nm and 0.62 nm can be assigned to (101) planes of MoS2 and (101) planes of anatase {001}-TiO2, respectively.
3.2 Photocatalytic study
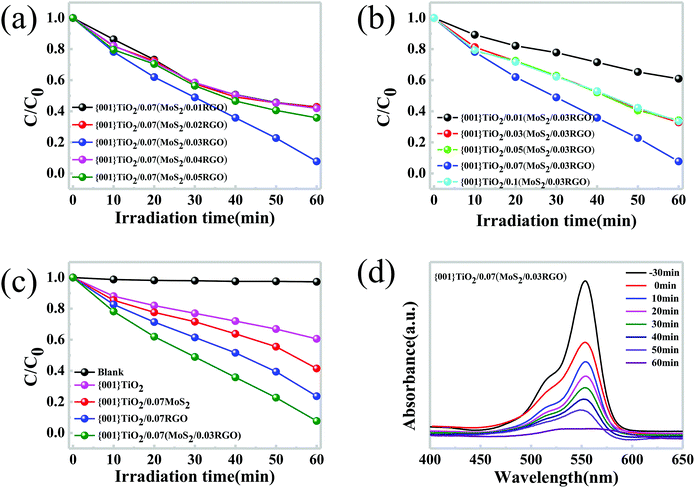
The photocatalytic activity of all the as-prepared samples was evaluated from measurements on the degradation of Rh B. Before illumination, the suspensions were prepared via stirring in dark for 30 minutes, such that they reached the adsorption–desorption equilibrium.In this study, firstly, we investigate the optimum ratio of MoS2 and RGO in a co-catalyst, as shown in Fig. 4a. The weight percentage of MoS2/RGO in the hybrid is kept at 7 wt%, and the {001}-TiO2/MoS2/RGO hybrid with a MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RGO ratio of 97
RGO ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 wt% shows the highest photodegradation rate. Then we studied the influence of the species and contents of co-catalyst on the photodegradation activity. As shown in Fig. 4c, the color dye without catalyst presents no photodegradation activity under visible light irradiation. It verifies that self-sensitisation couldn't cause the degradation. The pure {001}-TiO2 exhibits a low photocatalytic activity which could be caused by the rapid recombination of the CB electrons and the VB holes. While by the incorporation of MoS2, the photocatalytic activity of {001}-TiO2 is enhanced significantly, which is caused by the transfer of electrons from {001}-TiO2 to MoS2.43 In fact, the {001}-TiO2/MoS2 hybrid have exhibited excellent performance for the adsorption of Rh B under dark condition (as shown in Fig. S5c–f†). Through the incorporation of MoS2/RGO as the co-catalyst for {001}-TiO2, the photocatalytic activity of the hybrid could be further improved, as demonstrated in Fig. 4b, the {001}-TiO2/0.07(MoS2/0.03RGO) hybrids show the best photocatalytic performance. UV-visible investigation shows that after 60 min of visible light (λ > 400 nm) irradiation, 92.3% of Rh B has been degraded by {001}-TiO2/0.07(MoS2/0.03RGO) (as shown in Fig. 4d). It is suggested that the enhanced separation of photogenerated electrons and holes because of the high electron conductivity of RGO could result in the enhanced photocatalytic performance. In addition, the photocatalytic performance of {001}-TiO2/0.07(MoS2/0.03RGO) catalyst can also be evaluated by TOC removal. As shown in Fig. S5b,† the{001}-TiO2/0.07(MoS2/0.03RGO) hybrid catalyst shows the highest TOC removal rate of Rh B, which basically coincides with the result of the degradation rate, illustrating that the Rh B has been thoroughly mineralized. The color change of Rh B solution also reflects the efficiency of catalyst. We can clearly observe that the Rh B solution under the photocatalytic degradation of {001}-TiO2/MoS2/RGO hybrid is close to white (as shown in Fig. S4†).
3 wt% shows the highest photodegradation rate. Then we studied the influence of the species and contents of co-catalyst on the photodegradation activity. As shown in Fig. 4c, the color dye without catalyst presents no photodegradation activity under visible light irradiation. It verifies that self-sensitisation couldn't cause the degradation. The pure {001}-TiO2 exhibits a low photocatalytic activity which could be caused by the rapid recombination of the CB electrons and the VB holes. While by the incorporation of MoS2, the photocatalytic activity of {001}-TiO2 is enhanced significantly, which is caused by the transfer of electrons from {001}-TiO2 to MoS2.43 In fact, the {001}-TiO2/MoS2 hybrid have exhibited excellent performance for the adsorption of Rh B under dark condition (as shown in Fig. S5c–f†). Through the incorporation of MoS2/RGO as the co-catalyst for {001}-TiO2, the photocatalytic activity of the hybrid could be further improved, as demonstrated in Fig. 4b, the {001}-TiO2/0.07(MoS2/0.03RGO) hybrids show the best photocatalytic performance. UV-visible investigation shows that after 60 min of visible light (λ > 400 nm) irradiation, 92.3% of Rh B has been degraded by {001}-TiO2/0.07(MoS2/0.03RGO) (as shown in Fig. 4d). It is suggested that the enhanced separation of photogenerated electrons and holes because of the high electron conductivity of RGO could result in the enhanced photocatalytic performance. In addition, the photocatalytic performance of {001}-TiO2/0.07(MoS2/0.03RGO) catalyst can also be evaluated by TOC removal. As shown in Fig. S5b,† the{001}-TiO2/0.07(MoS2/0.03RGO) hybrid catalyst shows the highest TOC removal rate of Rh B, which basically coincides with the result of the degradation rate, illustrating that the Rh B has been thoroughly mineralized. The color change of Rh B solution also reflects the efficiency of catalyst. We can clearly observe that the Rh B solution under the photocatalytic degradation of {001}-TiO2/MoS2/RGO hybrid is close to white (as shown in Fig. S4†).
The reaction kinetics of Rh B reduction is determined by plotting the value of ln(C0/C) versus illumination time, and the reaction rate constants (K) are calculated by the pseudo-first order model. As shown in Fig. S5a,† the values of K for {001}-TiO2, {001}-TiO2/RGO, {001}-TiO2/MoS2 and {001}-TiO2/MoS2/RGO are 0.0084, 0.0201, 0.01285 and 0.03325, respectively. It can be seen that the {001}-TiO2/MoS2/RGO hybrid photocatalyst exhibits the highest rate (0.03325) of photodegradation.
The stability and reusability of {001}-TiO2/MoS2/RGO were also evaluated. Fig. S1b† shows the XRD patterns of {001}-TiO2/MoS2/RGO before and after photocatalysis, and it is observed that after photocatalysis the crystal structure has not changed. The inset of Fig. S5b† illustrates that the photocatalytic activity of {001}-TiO2/MoS2/RGO displays a slight decrease after running for five successive cycles, indicating that the {001}-TiO2/MoS2/RGO hybrid is stable and efficient photocatalyst.
3.3 Mechanisms of the enhanced photocatalytic activity
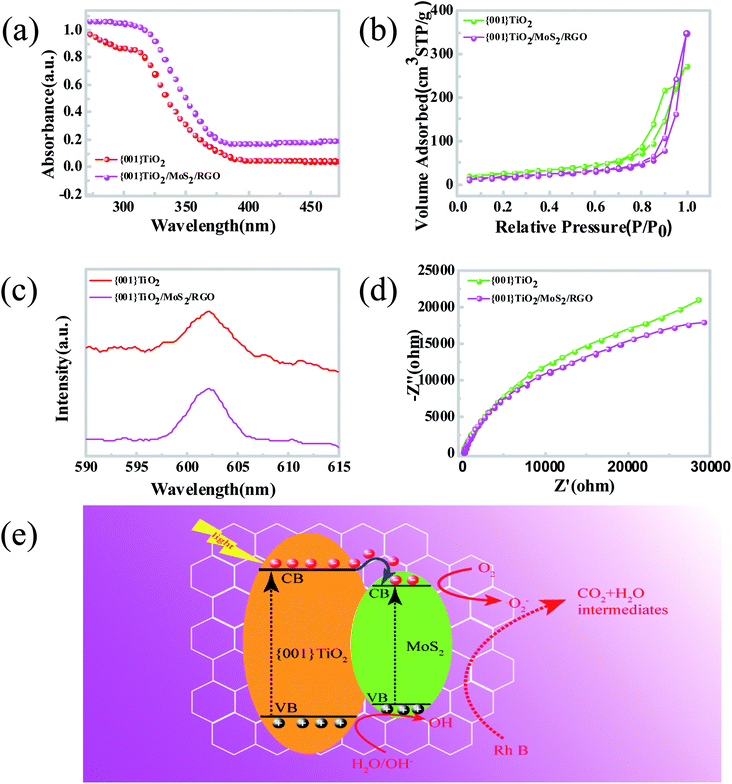
It is well known that the photocatalytic activity of semiconductive photocatalysts is intrinsically governed by three processes: (i) photoexcitation of semiconductor to generate electron–hole pairs, (ii) charge separation and diffusion to the surface of the catalysts, and (iii) surface activation for redox reactions.38 In the sections that follow, the mechanisms of the enhanced photocatalytic performance of {001}-TiO2/MoS2/RGO are elucidated separately based on the abovementioned processes.The UV-vis diffuse reflectance spectra of TiO2 and {001}-TiO2/MoS2/RGO are shown in Fig. 5a. Compared with pure {001}-TiO2, the {001}-TiO2/MoS2/RGO hybrid shows an enhanced visible light absorption. The band gaps of {001}-TiO2 and {001}-TiO2/MoS2/RGO are determined to be 3.2 and 3.04 eV, respectively, according to the onset of the absorption edge. The {001}-TiO2/MoS2/RGO hybrid exhibits a narrower band gap and higher absorption intensity, meaning that their redox potentials as well as the recombination of electrons and holes are reduced as compared with those of {001}-TiO2.47
Fig. 5b shows the nitrogen adsorption/desorption isotherms of {001}-TiO2 and {001}-TiO2/MoS2/RGO. The isotherms for pure {001}-TiO2 reveal a type-IV isotherm with an H3 hysteresis loop, which indicates that the holes are formed by the accumulation of flaky particles. The {001}-TiO2/MoS2/RGO hybrids display a type-IV isotherm with an H4 hysteresis loop, suggesting the slit-shaped pores are produced by the layered structure. Based on BET calculation, the surface area of {001}-TiO2 is 111.124 m2 g−1 and the surface area of {001}-TiO2/MoS2/RGO is 76.123 m2 g−1, revealing the information on their microstructures consistent with those observed in SEM images (Fig. 2c and d).
It is revealed that graphene could improve the photocatalytic property of {001}-TiO2/MoS2/RGO hybrid owning to the fact that graphene can rapidly transfer electrons to hinder the recombination of photogenerated electron–hole pairs. We can evaluate the probability of recombination of electron–hole pairs by PL spectroscopy, in that the higher fluorescence intensity represents higher probability of recombination. Fig. 5c shows the PL spectra under 400 nm excitation wavelengths for pure {001}-TiO2 and {001}-TiO2/MoS2/RGO samples, and a strong emission peak appears at about 602 nm.48 It is found that the PL intensity of {001}-TiO2/MoS2/RGO sample is lower than that of pure {001}-TiO2, which indicates that by the incorporation of MoS2/RGO co-catalyst, the hybrid can inhibit the recombination of excited electrons and holes in the {001}-TiO2/MoS2/RGO catalysts. The resistance of catalyst is determined by the electrochemical impedance spectroscopy (ESI). Fig. 5d shows the ESI spectrogram of {001}-TiO2 and {001}-TiO2/MoS2/RGO. Compared with pure {001}-TiO2, the {001}-TiO2/MoS2/RGO hybrid presents a smaller frequency semicircle, suggesting the resistance of {001}-TiO2/MoS2/RGO is lower than that of {001}-TiO2. Thus, it can be concluded that the introduction of MoS2/RGO co-catalysts into {001}-TiO2 can reduce the recombination of charge carriers, which is consistent with the results of PL spectrum.
The schematic of photocatalytic enhancement and electron transfer of {001}-TiO2/MoS2/RGO is shown in Fig. 5e. Under visible light irradiation, the VB electrons of MoS2 and {001}-TiO2 are excited to the CB, leaving holes (h+) in the VB.49,50 Meanwhile, the photoinduced electrons of {001}-TiO2 can transfer to MoS2 via the conductive network of RGO. It is worth noting that the separation of photogenerated electron–hole pairs of {001}-TiO2/MoS2/RGO is more rapid than that of conventional TiO2/MoS2/RGO owing to the especially reactive {001} facets of anatase TiO2. The photogenerated electrons on the CB of samples can react with O2 to produce superoxide radical anions ·O2−. Meanwhile those photoexcited holes on the VB of samples react with H2O and hydroxide ions to generate ·OH radicals. The powerful oxidizing agents ·O2− and ·OH could effectively decompose the Rh B into CO2, H2O or other intermediates. As listed in Table 1, we have compared {001}-TiO2/MoS2/RGO with other representative composites reported in literature. It can be found that the {001}-TiO2/MoS2/RGO exhibits desirable catalytic performance, which rapid and effective, indicating the promising perspective of {001}-TiO2/MoS2/RGO in the removal of pollutants applications.
| Catalyst | Method | Degradation (%) | Degradation target | Times (min) | Ref. |
|---|---|---|---|---|---|
| {001}-TiO2/MoS2/RGO | Hydrothermal | 92.3 | Rhodamine B | 60 | This work |
| BiPO4/MoS2/RGO | Hydrothermal | 92 | Rhodamine B | 90 | 51 |
| Bi2WO6/MoS2/RGO | Hydrothermal | 92 | Rhodamine B | 180 | 48 |
| TiO2/RGO | Hydrothermal | 94 | Rhodamine B | 120 | 52 |
| CeO2/SnO2/rGO | Hydrothermal | 95 | Methylene blue | 90 | 53 |
| rRGO–ZnO–MoS2 | Hydrothermal | 90 | Methylene blue | 75 | 54 |
| NiWO4–ZnO–NRGO | Microwave irradiation method | 89 | Methylene blue | 120 | 55 |
| {001}-TiO2/RGO | Hydrothermal | 82 | Methylene blue | 60 | 48 |
| TiO2/RGO/Ag | Sol–gel process photo-assisted reduction method | 93 | Methylene blue | 120 | 56 |
| rGO–ZnWO4–Fe3O4 | Microwave irradiation method | 97 | Methylene blue | 135 | 57 |
| TiO2/MoS2/RGO | Hydrothermal | 97 | Methylene blue | 100 | 49 |
4. Conclusion
In summary, we have successfully synthesized the {001}-TiO2/MoS2/RGO hybrids with effective catalytic performance using a facile hydrothermal method. The {001}-TiO2/MoS2/RGO photocatalysts with 7 wt% MoS2/RGO co-catalyst show the highest photodegradation activity for the degradation of Rh B under visible light irradiation (λ > 400 nm). The {001} facets of {001}-TiO2/MoS2/RGO accelerate the separation of photogenerated electron–hole pairs, and RGO rapidly transfer the photoinduced electrons from {001}-TiO2 to MoS2, which further enhances the charge carrier separation of the {001}-TiO2/MoS2/RGO hybrid. It is believed that such synergy effect promotes the photocatalytic performance of {001}-TiO2/MoS2/RGO hybrids. Taking into account their facile synthesis route and excellent photocatalytic performance, the MoS2/RGO hybrids are promising co-catalyst which can be widely used for co-photocatalysts and {001}-TiO2/MoS2/RGO hybrids are promising candidates for photocatalytic degradation of Rh B.Conflicts of interest
There is no conflict in the statement.Acknowledgements
This work was supported by Province Advantages and Distinctive Subjects Project of Heilongjiang (YSTSXK201810), Heilongjiang Provincial Education Department Project (LTSW201738), Qiqihar City Science and Technology Project (SFGG-201601), the Innovation Fund Project for Graduate Student of Qiqihar University (YJSCX2017-022X).References
- M. R. Hoffmann, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- N. Serpone and A. V. Emeline, J. Phys. Chem. Lett., 2012, 3, 673–677 CrossRef CAS PubMed.
- P. K. J. Robertson, J. M. C. Robertson and D. W. Bahnemann, J. Hazard. Mater., 2012, 211, 161–171 CrossRef PubMed.
- H. Kisch, Angew. Chem., Int. Ed., 2013, 52, 812–847 CrossRef CAS PubMed.
- S. P. Albu, A. Ghicov, J. M. Macak and P. Schmuki, Nano Lett., 2007, 7, 1286–1289 CrossRef CAS PubMed.
- T. Tachikawa, M. Fujitsuka and T. Majima, J. Phys. Chem. C, 2007, 111, 5259–5275 CrossRef CAS.
- J. Zhang, Q. Xu, Z. C. Feng, M. J. Li and C. Li, Angew. Chem., Int. Ed., 2010, 47, 1766–1769 CrossRef PubMed.
- M. M. Khan, S. A. Ansari, D. Pradhan and M. Omaish, J. Mater. Chem. A, 2013, 2, 637–644 RSC.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- R. Kaplan, B. Erjavec, G. Dražić, J. Grdadolnik and A. Pintar, Appl. Catal., B, 2015, 181, 465–474 CrossRef.
- H. Choi, A. Sofranko and D. Dionysiou, Adv. Funct. Mater., 2010, 16, 1067–1074 CrossRef.
- T. Fotiou, T. M. Triantis, T. Kaloudis, K. E. O'Shea, D. D. Dionysiou and A. Hiskia, Water Res., 2016, 90, 52–61 CrossRef CAS PubMed.
- J. B. Joo, Q. Zhang, M. Dahl and I. Lee, Energy Environ. Sci., 2012, 5, 6321–6327 RSC.
- J. B. Joo, I. Lee, M. Dahl and G. D. Moon, Adv. Funct. Mater., 2014, 23, 4246–4254 CrossRef.
- J. Wang, Z. Wang, H. Li, Y. T. Cui and Y. C. Du, J. Alloys Compd., 2010, 494, 372–377 CrossRef CAS.
- F. Li, J. Xu, L. Chen and B. B. Ni, J. Mater. Chem. A, 2012, 1, 225–228 RSC.
- X. Dong, J. Tao, Y. Y. Li, T. Wang and H. Zhu, Acta Phys.-Chim. Sin., 2009, 25, 1874–1882 CAS.
- J. C. Liu, L. Liu, H. G. Bai, Y. J. Wang and D. D. Sun, Appl. Catal., B, 2011, 106, 76–82 CrossRef CAS.
- S. D. Perera, R. G. Mariano, K. Vu and N. Nijem, ACS Catal., 2012, 2, 949–956 CrossRef CAS.
- Y. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Phys. Chem. Chem. Phys., 2012, 14, 9167–9175 RSC.
- N. Zhang, S. Liu, X. Fu and Y. J. Xu, J. Phys. Chem. C, 2011, 115, 9136–9145 CrossRef CAS.
- R. Liu, P. Wang, X. Wang, H. Yu and J. G. Yu, J. Phys. Chem. C, 2012, 116, 17721–17728 CrossRef CAS.
- R. Asahi, T. Morikawa, H. Irie and T. Ohwaki, Chem. Rev., 2014, 114, 9824–9852 CrossRef CAS PubMed.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. Zhang, D. Li, H. Q. Tan, Z. Zhao, Z. G. Xie and Z. C. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- A. Kubacka, M. Ferrer, M. Fernandezgarcia, C. Serrano, M. Cerrada and M. Fernández-García, Appl. Catal., B, 2011, 104, 346–352 CrossRef CAS.
- Y. Jiang, W. F. Chen, P. Koshy and C. C. Sorrell, J. Mater. Sci., 2019, 54, 5266–5279 CrossRef CAS.
- S. Liu, N. Zhang, Z. R. Tang and Y. J. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 6378–6385 CrossRef CAS PubMed.
- M. Wang, L. Sun, Z. Lin and J. H. Cai, Energy Environ. Sci., 2013, 6, 1211–1220 RSC.
- Z. A. Huang, Q. Sun, K. L. Lv, Z. H. Zhang, M. Li and B. Li, Appl. Catal., B, 2015, 164, 420–427 CrossRef CAS.
- X. Lin, D. Xu, Y. Xi, R. Zhao, L. N. Zhao, M. S. Song, H. J. Zhai, G. B. Che and L. M. Chang, Colloids Surf., A, 2017, 513, 117–124 CrossRef CAS.
- Y. Z. Hong, E. L. Liu, J. Y. Shi, X. Lin, L. Z. Sheng, M. Zhang, L. Y. Wang and J. H. Chen, Int. J. Hydrogen Energy, 2019, 44, 7194–7204 CrossRef CAS.
- H. Zeng, J. Dai, W. Yao, D. Xiao and X. Cui, Nat. Nanotechnol., 2012, 7, 490–493 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- Z. Yin, H. Li, H. Li, L. Jiang, Y. Shi, Y. Sun, G. Lu, Q. Zhang, X. Chen and H. Zhang, ACS Nano, 2012, 6, 74–80 CrossRef CAS PubMed.
- M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. S. Li and S. Jin, J. Am. Chem. Soc., 2013, 135, 10274–10277 CrossRef CAS PubMed.
- U. Gupta, B. S. Naidu, U. Maitra, A. Singh, S. N. Shirodkar, U. V. Waghmare and C. N. R. Rao, APL Mater., 2014, 2, 092802 CrossRef.
- X. Hu, H. Zhao, J. Tian, J. X. Gao, Y. J. Li and H. Z. Cui, Sol. Energy Mater. Sol. Cells, 2017, 172, 108–116 CrossRef CAS.
- S. Bai, L. Wang, X. Chen, J. t. Du and Y. j. Xiong, Nano Res., 2015, 8, 175–183 CrossRef CAS.
- W. C. Peng, Y. Chen and X. Y. Li, J. Hazard. Mater., 2016, 309, 173–179 CrossRef CAS PubMed.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914–11916 CrossRef CAS PubMed.
- J. Pan, G. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2011, 123, 2181–2185 CrossRef.
- N. Roy, Y. Sohn and D. Pradhan, ACS Nano, 2013, 7, 2532–2540 CrossRef CAS PubMed.
- D. Q. Zhang, J. X. Chai, J. Y. Cheng, X. Y. Yang, H. Wang, Z. L. Zhao, C. Han, G. C. Shan, W. J. Zhang, G. P. Zheng and M. S. Cao, Appl. Surf. Sci., 2018, 462, 872–882 CrossRef CAS.
- O. Akhavan, Carbon, 2015, 81, 158–166 CrossRef CAS.
- W. S. Wang, D. H. Wang, W. G. Qu, L. Q. Lu and A. W. Xu, J. Phys. Chem. C, 2012, 116, 19893–19901 CrossRef CAS.
- W. L. Song, X. T. Guan, L. Z. Fan, W. Q. Cao, C. Y. Wang and M. S. Cao, Carbon, 2015, 93, 151–160 CrossRef CAS.
- X. Liang, B. Quan, G. B. Ji, W. Liu, H. W. Zaho, S. S. Dai, J. Lv and Y. W. Du, ACS Sustainable Chem. Eng., 2017, 5, 10570–10579 CrossRef CAS.
- M. Liu, X. Xue, S. S. Yu, X. Y. Wang, Xi. Y. Hu, H. W. Tian, H. Chen and W. T. Zheng, Sci. Rep., 2017, 7, 3637 CrossRef PubMed.
- C. B. Liu, L. L. Wang, Y. H. Tang, S. L. Luo, Y. T. Liu, S. Q. Zhang, Y. X. Zeng and Y. Z. Xu, Appl. Catal., B, 2015, 164, 1–9 CrossRef CAS.
- D. B. Nimbalkar, H. H. Lo, P. V. R. K. Ramacharyulu and S. Y. C Ke, RSC Adv., 2016, 6, 31661–31667 RSC.
- H. Lv, Y. M. Liu, H. B. Tang, P. Zhang and J. J. Wang, Appl. Surf. Sci., 2017, 425, 100–106 CrossRef CAS.
- F. Wang and K. Zhang, J. Mol. Catal. A: Chem., 2011, 345, 101–107 CrossRef CAS.
- A. Priyadharsan, V. Vasanthakumar, S. Karthikeyan, V. Raj, S. Shanavas and P. M. Anbarasan, J. Photochem. Photobiol., A, 2017, 346, 32–45 CrossRef CAS.
- A. Priyadharsan, S. Shanavas and V. Vasanthakumar, Colloids Surf., A, 2015, 559, 43–53 Search PubMed.
- M. M. J. Sadiq, U. S. Shenoy and D. K. Bhat, J. Phys. Chem. Solids, 2017, 109, 124–133 CrossRef CAS.
- H. W. Tian, C. X. Wan, X. Xue and X. Xin, Catalysts, 2017, 7, 156 CrossRef.
- M. M. Ja, U. Sandhya and D. K. Bhat, RSC Adv., 2016, 6, 61821–61829 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02634g |
| This journal is © The Royal Society of Chemistry 2019 |