Open Access Article
Open Access ArticleHighly stable binder free CNTs/rGO aerogel electrode for decolouration of methylene blue & palm oil mill effluent via electro-Fenton oxidation process
Muhammad Amirul Nazhif Mohd Nohana,
Chin Hua Chia *a,
Aina Shasha Hashimia,
Siew Xian Chinb,
Poi Sim Khiewc,
Sarani Zakariaa,
Azima Azmia,
Kam Sheng Laua and
Nur Fazlinda Razalia
*a,
Aina Shasha Hashimia,
Siew Xian Chinb,
Poi Sim Khiewc,
Sarani Zakariaa,
Azima Azmia,
Kam Sheng Laua and
Nur Fazlinda Razalia
aMaterials Science Program, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. E-mail: chia@ukm.edu.my
bASASIpintar Program, Pusat PERMATApintar, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
cCenter of Nanotechnology and Advanced Materials, Faculty of Engineering, University of Nottingham Malaysia Campus, Jalan Broga, 43500, Semenyih, Selangor, Malaysia
First published on 28th May 2019
Abstract
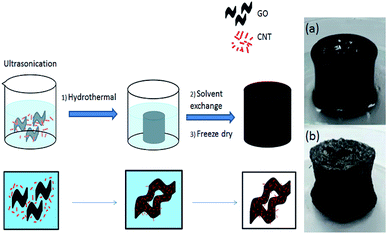
In this study, single wall carbon nanotubes (CNTs)/reduced graphene oxides (rGO) aerogels were prepared by a one-pot hydrothermal process without using a binder. The produced CNTs/rGO aerogel was used as cathode in electro-Fenton system for the decolouration of methylene blue (MB) and palm oil mill effluent (POME). The addition of CNTs increased the surface area, pore volume and conductivity of the rGO aerogel, which further enhanced their performance as cathode towards the decolouration of MB and POME via electro-Fenton reaction. Complete decolouration of MB using rGO aerogel without CNTs could not be achieved. The effect of electro-Fenton reaction parameters conducted using the aerogel samples including, current, electrolyte concentrations and pH, were investigated accordingly. The CNTs/rGO aerogel electrode also showed high stability and reusability for up to six consecutive treatment cycles for MB. Besides, the CNTs/rGO aerogel also showed good performance in treating POME with 69.8%, 47.6% and 58.1% of reduction in true colour, total organic carbon (TOC) and chemical oxygen demand (COD), respectively, via 60 minutes electro-Fenton reaction. This study showed that CNTs/rGO aerogels with high porosity and stability can be prepared using simple procedure without adding binder. This fully carbon-based aerogel can serve as effective cathode for decolouration of organic dye and effluent.
1 Introduction
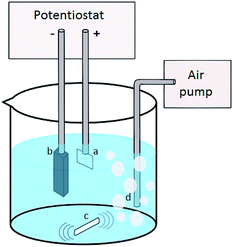
Advanced oxidation processes (AOP) have been popular methods for wastewater treatment, including ozonation, UV treatment, physical AOP (ultrasound, microwave, electro beam) and catalyst AOP (photocatalyst, Fenton reaction).1–6 Electro-Fenton process is known as electrochemical advanced oxidation process (EAOP) with high efficiency in decomposition of destructing persistent organic pollutants by hydroxyl radicals (˙OH).7,8 Hydrogen peroxide (H2O2) is produced from the cathode of the electro-Fenton system which is subsequently reacted with ferrous ions (Fe2+) to produce ˙OH as indicated in eqn (1).9–11| H2O2 + Fe2+ + H+ → ˙OH + H2O | (1) |
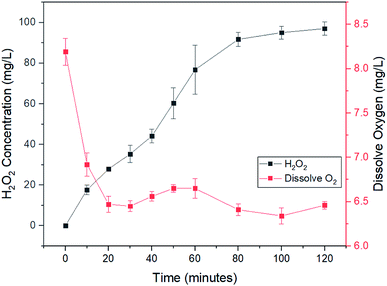
In situ generation of H2O2 is the core component for the production of ˙OH radicals in electro-Fenton process.11,12 H2O2 is reactive and unstable. In situ generations of H2O2 eliminates the risk of transportation and storage of H2O2.11,12 H2O2 is produced at cathode involving two-electrons oxygen reduction reaction as indicated in eqn (3).9,10 The efficiency of the H2O2 production is a key parameter of the electro-Fenton process.
| O2 + 2H+ + 2e− → H2O2 | (2) |
Hence, the improvement of cathode material to increase H2O2 production is of high importance for enhancing the efficiency of the electro-Fenton process. Carbonaceous materials, including graphite,13 carbon felt,14,15 carbon sponge,16 carbon aerogel,17 and activated carbon fibre18,19 are among the widely studied cathode materials for electro-Fenton process due to their advantages such as good stability, high conductivity, low cost, non toxic and low catalytic activity for H2O2 decomposition.11 Besides, uses of polymer binder in the production of carbonaceous electrode is avoided as it can limit the performance by reducing the conductivity of electrode.20 Therefore the design on binder-free electrodes are established with the presence of CNTs to improve mechanical and electrical properties of the electrodes.21–24
In this study, reduced-graphene oxide (rGO) aerogel is produced and used as cathode material for electro-Fenton process. To further enhance the conductivity of the electrode, CNTs are added into the rGO as conductive filler to further enhance the conductivity properties of the electrode. The CNTs are treated by nitric acid before used. This is because CNTs has high hydrophobic characteristic and low wettability which limit the access of ions and presence of active site for electron transfer. Chemical treatment of CNTs can increase oxygen-containing functional groups (carboxylic acid, carbonyl and hydroxyl), which can increase accessible sites for free electrolytes ions and help in enhancing the homogeneity of CNTs in GO solution for the preparation of well-dispersed CNTs/rGO aerogel electrode.23,25 Aggregation of CNTs can occur when electrical repulsive forces are lower than the van der Waals force which are important to induce repulsion force for better CNTs dispersion.26 Besides, dispersion of CNTs is important for high electrode stability and conductivity, while the presence of GO can act as surfactant which can enhance the dispersion of CNTs.27
The produced CNTs/rGO electrodes are further tested in the decolouration process of organic dye using an electro-Fenton system. Herein, few parameters are studied, such as CNTs loading, electric current intensity, pH, and electrolyte concentration, for the optimization of the electro-Fenton process. The stability and reusability of the electrodes are also determined. To further evaluate the viability of the CNTs/rGO aerogel for real application, the aerogel was used to treat palm oil mil effluent (POME) using the optimum electro-Fenton condition for MB. The electro-Fenton performance was evaluated in terms of decolouration, reduction of total organic carbon (TOC) and chemical oxygen demand (COD).
1.1 Materials and methods
2 Results and discussion
2.1 Characterization of aerogels
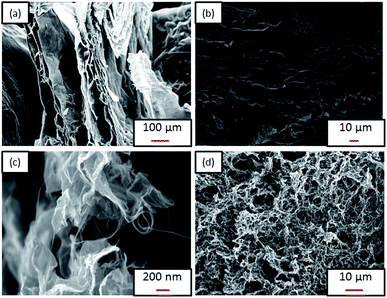 | ||
| Fig. 3 FESEM images of (a) rGO surface, (b) rGO cross section, (c) 60 wt%-CNTs/rGO surface, (d) 60 wt%-CNTs/rGO cross section. | ||
To further support findings obtained from the FESEM, surface area, pore volume and pore size of the aerogel samples were measured using BET analysis, as shown in Table 1. The presence of CNTs helps in increasing the surface area and active sites.24,32
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore (Å) |
|---|---|---|---|
| rGO | 38.83 | 0.3487 | 649.0 |
| 30%-CNTs/rGO | 156.7 | 0.678 | 381.1 |
| 60%-CNTs/rGO | 256.9 | 1.302 | 169.8 |
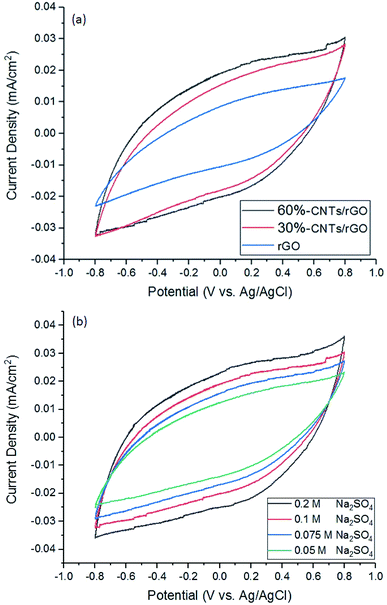
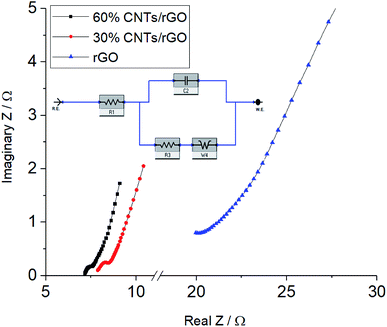
Due to the high roughness and porosity of the aerogel surfaces, bulk resistance was measured instead of sheet resistance. Bulk resistance of the rGO aerogel was decreased from approximately 20 kΩ to 40 and 18 Ω with the addition of 30% and 60% CNTs, respectively. The high resistance of the rGO aerogel could be due to it high porous structure in which the rGO sheets are not well interconnected. The CNTs added have served as conductive pathway to interconnect the rGO sheets. It is interesting to note that the addition of CNTs not only has increased the BET surface area and pore volume of the aerogels, but it has also significantly enhanced the bulk conductivity of the aerogel.
| Parameter | rGO | 30% CNTs/rGO | 60% CNTs/rGO |
|---|---|---|---|
| R1 (ohm) | 20.37 | 7.872 | 7.154 |
| C2 (F) | 0.000128 | 0.000546 | 0.00111 |
| R3 (ohm) | 3.93 | 0.547 | 0.357 |
| W4 (S s(−1/2)) | 0.0254 | 0.311 | 0.376 |
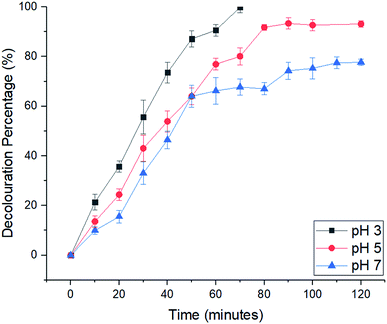
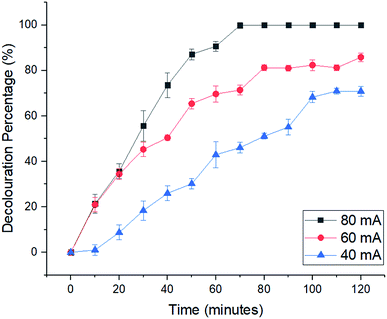
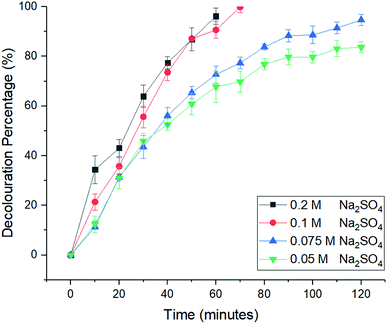
2.2 Electro-Fenton reaction for decolouration of MB
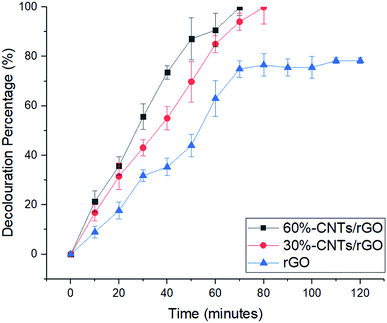 | ||
| Fig. 6 Decolouration of methylene blue in the electro Fenton system with different CNTs concentration as rGO electrode filler. Conditions: pH 3, current 80 mA and 0.1 M Na2SO4. | ||
As the reaction proceeds, MB colour faded indicate decrease of MB concentration. This can be described from collapse of MB molecule structure or chromoforic destruction. Decomposition of H2O2 plays a vital role in the generation of ˙OH radicals which are the main oxidizing agent of MB solution. The decomposition of MB which can be described by the breaking of the N–CH3, C–N, C–S and CH3 bonds to form intermediates that will be further oxidized into form CO2, H2O, Cl−, SO4− and NO3−.33
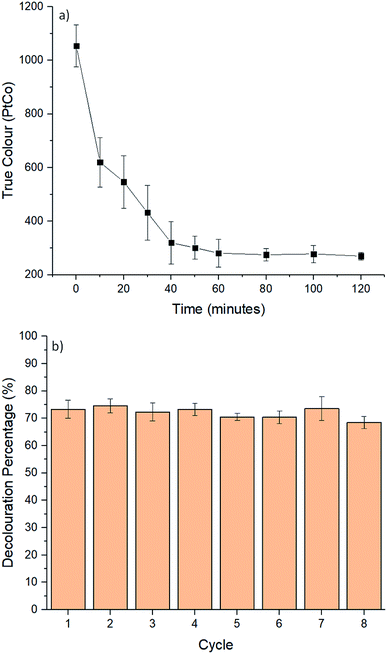
Table 3 presents the results of TOC and COD of POME before and after the electro-Fenton reaction. TOC and COD of the POME was reduced 47.63% and 58.1% respectively after 60 minutes of electro-Fenton reaction using the 60%-CNTs/rGO aerogel electrode. The decrease in TOC was caused by demineralization of dissolved organic compounds in the POME water and carbon dioxide by ˙OH radicals generated.
| Initial (mg L−1) | Final (mg L−1) | Percentage removal (%) | |
|---|---|---|---|
| TOC | 105 | 44 | 47.6 |
| COD | 401 | 210 | 58.1 |
3 Conclusions
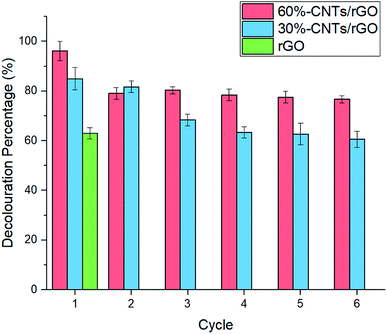
This study described the preparation and utilization of CNTs/rGO aerogel as a electrode for electro-Fenton reaction. The 60%-CNTs/rGO aerogel showed good decolouration performance towards MB and POME as compared to the neat rGO due to its high surface area, porosity and bulk conductivity. It is also found that the 60%-CNTs/rGO aerogel can be reused for more than six cycles of electro-Fenton reaction to decolorize MB and POME without significant reduction in performance.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Centre of Research and Instrumentation (CRIM), UKM for the research grant GUP-2018-159 provided.Notes and references
- D. Kanakaraju, B. D. Glass and M. Oelgem, J. Environ. Manag., 2018, 219, 189–207 CrossRef CAS PubMed.
- D. B. Miklos, C. Remy, M. Jekel, K. G. Linden and J. E. Drewes, Water Res., 2018, 139, 118–131 CrossRef CAS PubMed.
- Y. Lin, S. Wu, C. Yang, M. Chen and X. Li, Appl. Catal., B, 2019, 245, 71–86 CrossRef CAS.
- Y. Lin, S. Wu, X. Li, X. Wu, C. Yang, G. Zeng, Y. Peng, Q. Zhou and L. Lu, Appl. Catal., B, 2018, 227, 557–570 CrossRef CAS.
- S. Wu, H. Li, X. Li, H. He and C. Yang, Chem. Eng. J., 2018, 353, 533–541 CrossRef CAS.
- S. Wu, H. He, X. Li, C. Yang, G. Zeng, B. Wu, S. He and L. Lu, Chem. Eng. J., 2018, 341, 126–136 CrossRef CAS.
- Q. Wang, S. Zhou, S. Xiao, F. Wei, X. Zhao, J. Qu and H. Wang, RSC Adv., 2018, 8, 14775–14786 RSC.
- X. Wang, K. Zhu, X. Ma, Z. Sun and X. Hu, RSC Adv., 2018, 8, 19971–19978 RSC.
- Q. Peng, Z. Zhang, Z. Huang, W. Ren and J. Sun, RSC Adv., 2014, 4, 60168–60175 RSC.
- L. Bounab, O. Iglesias, E. González-Romero, M. Pazos and M. Ángeles Sanromán, RSC Adv., 2015, 5, 31049–31056 RSC.
- S. Qiu, L. Yu, D. Tang, W. Ren, K. Chen and J. Sun, Ind. Eng. Chem. Res., 2018, 57, 4907–4915 CrossRef CAS.
- B. Hou, B. Ren, R. Deng, G. Zhu, Z. Wang and Z. Li, RSC Adv., 2017, 7, 15455–15462 RSC.
- T. X. H. Le, T. Van Nguyen, Z. Amadou Yacouba, L. Zoungrana, F. Avril, D. L. Nguyen, E. Petit, J. Mendret, V. Bonniol, M. Bechelany, S. Lacour, G. Lesage and M. Cretin, Chemosphere, 2017, 172, 1–9 CrossRef CAS PubMed.
- C. Zhang, M. Zhou, G. Ren, X. Yu, L. Ma, J. Yang and F. Yu, Water Res., 2015, 70, 414–424 CrossRef CAS PubMed.
- Y. Wang, Y. Liu, K. Wang, S. Song, P. Tsiakaras and H. Liu, Appl. Catal., B, 2015, 165, 360–368 CrossRef CAS.
- S. O. Ganiyu, T. X. Huong Le, M. Bechelany, G. Esposito, E. D. Van Hullebusch, M. A. Oturan and M. Cretin, J. Mater. Chem. A, 2017, 5, 3655–3666 RSC.
- W. Ren, Q. Peng, Z. Huang, Z. Zhang, W. Zhan, K. Lv and J. Sun, Ind. Eng. Chem. Res., 2015, 54, 8492–8499 CrossRef CAS.
- H. Zhao, L. Qian, X. Guan, D. Wu and G. Zhao, Environ. Sci. Technol., 2016, 50, 5225–5233 CrossRef CAS PubMed.
- J. Hu, J. Sun, J. Yan, K. Lv, C. Zhong, K. Deng and J. Li, Electrochem. Commun., 2013, 28, 67–70 CrossRef CAS.
- X. Wang, W. Guo, Y. Zhu, X. Liang, F. Wang and P. Peng, Appl. Sci., 2018, 8, 2101 CrossRef.
- J. H. Kim, J. Y. Hwang, H. R. Hwang, H. S. Kim, J. H. Lee, J. W. Seo, U. S. Shin and S. H. Lee, Sci. Rep., 2018, 8, 1–11 CrossRef PubMed.
- F. Schütt, S. Signetti, H. Krüger, S. Röder, D. Smazna, S. Kaps, S. N. Gorb, Y. K. Mishra, N. M. Pugno and R. Adelung, Nat. Commun., 2017, 8, 1–10 CrossRef PubMed.
- S. A. Shamsuddin, M. N. Derman, U. Hashim, M. Kashif, T. Adam, N. H. A. Halim and M. F. M. Tahir, AIP Conf. Proc., 2016, 1756, 090002 CrossRef.
- Q. Yang, S. K. Pang and K. C. Yung, J. Power Sources, 2016, 311, 144–152 CrossRef CAS.
- J. Shen, A. Liu, Y. Tu, G. Foo, C. Yeo, M. B. Chan-Park, R. Jiang and Y. Chen, Energy Environ. Sci., 2011, 4, 4220–4229 RSC.
- H. He, Y. Cheng, C. Yang, G. Zeng, C. Zhu and Z. Yan, J. Environ. Sci., 2017, 54, 135–141 CrossRef PubMed.
- M. N. Mohd Zubir, A. Badarudin, S. N. Kazi, H. Nay Ming, R. Sadri and A. Amiri, J. Dispersion Sci. Technol., 2016, 37, 1395–1407 CrossRef CAS.
- N. M. Huang, H. N. Lim, C. H. Chia, M. A. Yarmo and M. T. Muhammad, Int. J. Nanomed., 2011, 6, 3443–3448 CrossRef CAS PubMed.
- S. Pei and H. M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- M. Bowman, J. Chem. Educ., 1949, 1, 103–104 CrossRef.
- W. Yang, Y. Chen, J. Wang, T. Peng, J. Xu, B. Yang and K. Tang, Nanoscale Res. Lett., 2018, 13, 181 CrossRef PubMed.
- B. Hashwan, S. Saeed, M. F. Fatin, A. R. Ruslinda, M. K. Md Arshad, U. Hashim and R. M. Ayub, Appl. Mech. Mater., 2015, 754–755, 1156–1160 Search PubMed.
- Q. Wang, S. Tian, J. Cun and P. Ning, Desalin. Water Treat., 2013, 51, 5821–5830 CrossRef CAS.
- A. Babuponnusami and K. Muthukumar, International Journal of Advance Innovations, Thoughts & Ideas, 2017, 3, 439–451 Search PubMed.
- E. Alfaya, O. Iglesias, M. Pazos and M. A. Sanromán, RSC Adv., 2015, 5, 14416–14424 RSC.
- M. Radwan, M. Gar Alalm and H. Eletriby, Journal of Water Process Engineering, 2018, 22, 155–162 CrossRef.
- H. He and Z. Zhou, Crit. Rev. Environ. Sci. Technol., 2017, 1–32 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |