Open Access Article
Open Access ArticlePlasmonic nanoprobes based on the shape transition of Au/Ag core–shell nanorods to dumbbells for sensitive Hg-ion detection
Ling Chen*,
Rui Li and
Ping Yang *
*
School of Materials Science and Engineering, University of Jinan, Jinan 250022, China. E-mail: mse_chenl@ujn.edu.cn; mse_yangp@ujn.edu.cn
First published on 5th June 2019
Abstract
We report a sensitive and selective localized surface plasmon resonance (LSPR) nanoprobe for the detection of mercuric ions (Hg2+) using gold/silver core–shell nanorods as an optical nanosubstrate. Sulfide can quickly react with silver atoms to generate Ag2S at room temperature in the presence of oxygen. The transformation from Ag shell to Ag2S on the nanorod surface results in its LSPR absorption band shifting to a longer wavelength, which is attributed to their different refractive indices. Interestingly, the morphology also changed from a rod-like to dumbbell shape. However, in the presence of Hg2+, this morphology transformation is inhibited because the sulfide reacts with free Hg2+ prior to the Ag atoms. The amount of Ag2S reduced with the increasing concentration of Hg2+, and the absorption band shift was also decreased. According to this “rod-like to dumbbell or not” shape change, a sensitive and selective LSPR nanoprobe was established, assisted by UV-Vis absorption spectroscopy. The detection limit of this probe for Hg2+ was as low as 13 nM. The efficiency of this probe in complex samples was evaluated by the detection of Hg2+ in spiked water samples.
Introduction
Water pollution arising from toxic heavy metal ions has become a serious threat to human health and the ecological environment.1,2 Heavy metal ions mainly exist in polluted water, with some also occurring in the atmosphere and solid waste. Heavy metals cannot be biodegraded, but they are bioaccumulative and can thus directly threaten higher organisms, including humans. Among these heavy metal ions, toxic mercury(II) ions in the form of organic or inorganic species are considered as a particularly harmful pollutant to human health, and such species are mainly distributed in water due to their water solubility3,4 and can be enriched in the environment and in organisms causing damage to the brain, heart, kidneys, lungs, and immune system.5 Therefore, the recognition and detection of mercury ions (Hg2+) cannot be ignored. Current traditional analysis techniques for Hg2+ detection include atomic absorption spectroscopy, atomic emission spectroscopy, atomic fluorescence spectroscopy and inductively coupled plasma mass spectroscopy,6–9 and these element-specific detection techniques are generally coupled with other accurate but sophisticated techniques, such as gas chromatography, high performance liquid chromatography and capillary electrophoresis.10 Although these methods can provide good detection sensitivity, most of them require complex and sophisticated instruments and complicated operation procedures, which are time-consuming and laborious. Therefore, it is necessary to develop a simple, fast and low-cost method for the analysis of mercury ions.Noble metallic nanoparticles with excellent localized surface plasmon resonance (LSPR) properties play a central role in optical nanoprobes for chemical and biological analysis.11–14 Typically, plasmonic nanoparticle-based optical nanoprobes, such as colorimetric surface-enhanced Raman resonance probes, have been well developed for the detection and imaging of ions, small molecules, proteins, and cells.15,16 In particular, colorimetric nanoprobes based on nanomaterials have attracted much attention due to their simplicity, rapidity and naked-eye detection.17–19 In recent years, a series of optical nanoprobes for Hg2+ sensing have been developed based on plasmonic nanomaterials.20–25 Most of these methods are based on noble metallic (gold or silver) nanomaterials with excellent optical properties. Specific recognition ligands, such as DNA aptamers, fluorescent molecules and small organic molecules, were employed for the specific recognition and detection of Hg2+ by using a colorimetric method assisted by absorption spectroscopy. For example, Yang's team developed a nano-sensing method that could be used to visualize and fluorescently respond to Hg2+ by using the principle that Hg2+ can induce configurational changes in thymine base-rich DNA and different electrostatic binding forces between single-stranded DNA/double-stranded DNA and gold nanoparticles.26 Based on the strong affinity between gold and mercury, Campiglia et al. developed a colorimetric method based on the morphological changes of gold nanorods that could directly detect Hg2+ with quite low detection limit.27 From the perspective of methodological research, these novel plasmonic nanoprobes show the application of gold or silver nanoparticles incisively and vividly, and also provide a new strategy for the detection of Hg2+.
As a typical anisotropic nanoparticle, nanorods have two dimensions of width and length; their dipole plasmon resonance is split between transverse and longitudinal dipole resonances,28 showing two absorption peaks in the spectrum. The position of the transverse and longitudinal absorption peaks depend on both the aspect ratio of the nanorods and the absolute dimensions of the particles. Different components of nanoparticles can also cause changes in the LSPR frequency as well as changes in the colloid's colour.29,30 As for metal–metal core–shell nanoparticles, the plasmon resonance of the outer shell dominates; for instance, a blue-shift would strongly occur at the resonance of the Au/Ag core–shell particles.31–33 As in a previous investigation, silver nanorods are more difficult to make than gold nanorods.34 In this case, easily prepared-gold nanorods could be employed as the core section and their surface could be covered with a silver layer to fabricate pseudo-silver nanorods, which would also show the characteristic absorption peak of silver. This strategy offers a good candidate for diverse plasmonic nanoprobes.
In view of this characteristic, we initially prepared gold/silver core–shell composite nanorods and then established a suspension-based nanoprobe for the sensitive detection of mercury ions. Sulfide can quickly react with silver atoms to generate Ag2S at room temperature in the presence of oxygen. The transformation from Ag shell to Ag2S on the nanorod surface results in its LSPR absorption band shifting to a longer wavelength, attributed to their different refractive indices. However, in the presence of mercury ions, this morphology transformation would be inhibited because the sulfides react with free Hg2+ prior to Ag atoms. Thus, the sensing system for the detection of mercury ions was based on the different reaction abilities among sulfide anions, the silver shell and mercury ions.
Experimental section
Materials and methods
Hydrogen tetrachloroaurate(III) tetrahydrate (HAuCl4·4H2O), sodium borohydride (NaBH4), cetyltrimethylammonium bromide (CTAB), AgNO3, ascorbic acid (AA), hydrochloric acid and sodium hydroxide were received from Sinopharm Chemical Reagent Co., Ltd, China. Sodium sulfide, CoCl2·6H2O, Cd(NO3)2, Pb(NO3)2, CuSO4, Zn(NO3)2, MgSO4 and so on were purchased from Aladdin. The standard of Hg(NO3)2 (1000 ng mL−1) was obtained from the CRM/RM Information Center of China. Because sodium sulfide is deliquescent, desiccative sodium sulfide was employed to prepare the sulfide solution. 0.12 g Na2S·9H2O was weighed and dissolved in water in a 50 mL volumetric flask. The final concentration of sulfide was 0.01 mol L−1. All the standards and stock solutions were stored at 4 °C in a refrigerator until further use. All of the chemicals and reagents were of analytical grade and used without further purification, and deionized ultrapure water was used for the preparation of the aqueous solutions in all of the experiments.Deionized water (18.2 MΩ cm specific resistances) was purified by a Cascada TM LS Ultrapure water system (Pall Corp., USA). UV-Vis-NIR absorption spectra were measured on a HITACHI U-4100 spectrophotometer (Japan). The morphology of the synthesized samples was analyzed by transmission electron microscopy (TEM, JEM-2100, JEOL, Ltd, Japan). For sample preparation, the sediments were dropped on a copper mesh and dried in an oven at room temperature for observation by the transmission electron microscope. All the glasswares used in the following procedures was cleaned in a bath of freshly prepared aqua regia (HCl/HNO3, 3v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1v), rinsed thoroughly in water and dried in air.
1v), rinsed thoroughly in water and dried in air.
Preparation of Au/Ag core–shell nanoparticles
Gold nanorods (Au NRs) were initially prepared via the most common method, namely “seed-mediated” growth as per a previous report35 with some necessary modifications. Typically, for the seed solution, a freshly prepared, ice-cold aqueous NaBH4 solution (0.01 M, 0.6 mL) was injected into a mixture solution of HAuCl4 (0.05 M, 50 μL) and CTAB (0.1 M, 10 mL) under stirring at 28 °C. The resultant solution was stirred for 1 min and then kept at room temperature for 2 h. For the growth solution, HAuCl4 (0.05 M, 0.4 mL), AgNO3 (0.01 M, 0.4 mL) and CTAB (0.1 M, 40 mL) were mixed together, and then, a freshly prepared ascorbic acid solution (0.1 M, 0.32 mL) and an aqueous HCl solution (1.0 M, 0.8 mL) were added. After the resultant solution was mixed by stirring, the seed solution (100 μL) was rapidly injected with continuous stirring for 30 s. The reaction mixture was then left undisturbed overnight, with the temperature maintained at 26 °C.For the growth of the silver shell, 4.0 mL of the as-synthesized Au NRs were purified by centrifugation for 20 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and re-dispersed with deionized water in the same volume. Then, 2.0 mL CTAB (0.1 M) was added to the Au NR solution with vigorous stirring. Afterwards, 300 μL 0.1 M AA and 400 μL 10 mM AgNO3 were added to the mixture, followed by 600 μL 0.1 M NaOH to start the coating reaction within a few minutes. This reaction continued for 6 h. The resultant nanoparticles were then stored in glass media bottles and sealed at 4 °C in a refrigerator for the following experiments. The core–shell nanoparticles were washed by centrifugation before use in the sensing system.
000 rpm, and re-dispersed with deionized water in the same volume. Then, 2.0 mL CTAB (0.1 M) was added to the Au NR solution with vigorous stirring. Afterwards, 300 μL 0.1 M AA and 400 μL 10 mM AgNO3 were added to the mixture, followed by 600 μL 0.1 M NaOH to start the coating reaction within a few minutes. This reaction continued for 6 h. The resultant nanoparticles were then stored in glass media bottles and sealed at 4 °C in a refrigerator for the following experiments. The core–shell nanoparticles were washed by centrifugation before use in the sensing system.
Procedure for the sensing of mercury ions (Hg2+) in aqueous solution
For Hg2+ sensing, the different concentrations of Hg2+ were first mixed with sulfide ions (2 μL, 0.01 M) in 0.5 mL buffer solution, and then the Au/Ag core–shell NRs (100 μL) were added. The total volume of the mixture was 1.5 mL. After incubation for several minutes, the absorption spectra were measured by using a UV-Vis spectrophotometer.In the selectivity experiments, other metal ions (Co2+, Cd2+, Pb2+, Cu2+, Zn2+, Ni2+, Mg2+) were tested in a similar way.
Analysis of mercury ions in real water samples
Drinking water and tap water samples obtained from our institute were filtered through a 0.2 μm membrane. The water samples were spiked with standard Hg2+ ions at certain concentrations and then mixed with the stock solutions containing the Au/Ag core–shell NRs probe and an acetic acid–sodium acetate (HAc–NaAc) buffer solution (pH 5.0).Results and discussion
Proposed mechanism of the Au/Ag nanorod-based plasmonic sensor for Hg2+ detection
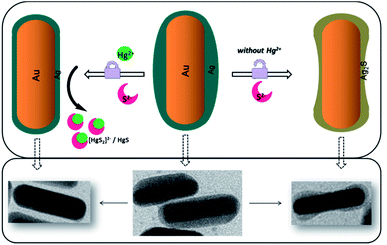
Scheme 1 represents the proposed mechanism of the Au/Ag core–shell nanoparticle-based colorimetric assay for the detection of Hg2+ in an aqueous solution. The fabrication procedure of silver nanorods was much more difficult than that of gold nanorods. Therefore, the Au/Ag core–shell nanorods were designed by embedding a silver shell on the gold nanorods core section, whereby a blue-shift was expected and the resultant hybrid nanorods also possessed excellent LSPR properties. As shown in Fig. 1, the dominant absorption peak of the synthesized Au/Ag nanoparticles was around 630 nm, indicating the resonance of the outer silver shell, which also resulted in a deep green colour of the resultant colloid. Fig. 1B shows the rod-like shape of Au/Ag NRs having a 60 × 20 nm Au NR core and 2.5 nm Ag shell approximately. Interestingly, the resultant hybrid nanoparticle revealed that an anisotropic coating of silver occurred, resulting in partly orange slice-like nanocrystals. Different growth rates for the {110} side facets accounted for this shape.36 Among the four {110} side facets, two of the neighbouring {110} facets grew quickly and another two grew comparatively slowly, thus inducing the anisotropic deposition of the silver shell.In the presence of oxygen, sulfide ions can selectively and quickly react with silver atoms to generate Ag2S at room temperature,37,38 under basic conditions:
| 4Ag + 2S2− + O2 + 2H2O → 2Ag2S + 4OH− |
| 4Ag + 2S2− + O2 + 4H+ → 2Ag2S + 2H2O |
In this case, the formation of Ag2S on an Au/Ag core–shell nanoparticle surface would result in its LSPR maximum shifting to a longer wavelength because the refractive indices of Ag (∼0.17) and Ag2S (∼2.2) are substantially different. As shown in Fig. 1A, curves (a) and (b), the absorption peak shifted from 630 nm to 700 nm after the introduction of sulfide anions. The colloid colour also changed from blue-green to blue-grey.
According to Fig. 1B(b), the TEM image of Au/Ag NRs with a certain amount of S2− indicated that the resultant shell was irregular. Meanwhile, interestingly, after the reaction with sufficient sulfide ions, the morphology of the nanoparticles was transformed from rod-like to dumbbell shaped, according to the SEM images in Fig. 2. We assume that this transformation was mainly attributed to the following reasons. The volume of one Ag2S unit is larger than that of two Ag atoms following the incorporation of S2−.39 During the sulfidation reaction, the new Ag2S shell expands outward and forms irregular stacks at specific points. It is worth noting that Ag2S gives priority to stacking at the endcap site with a small curvature radius,38 and finally forms a dumbbell shape with sufficient sulfide ions. The high-resolution TEM image (Fig. 2c) confirmed the formation of the Ag2S shell near the endcap sites with an interplane distance of the lattice fringes of ∼0.28 nm, corresponding to that of the (−112) facets of Ag2S.40
 | ||
| Fig. 2 SEM images of Au/Ag core–shell nanorods (A) and Au/Ag2S nanoparticles (B). High-resolution TEM image of Au/Ag2S nanoparticles (C). | ||
Then, wonderfully, mercury ions could “inhibit” this change due to the strong affinity between Hg and S. Herein, mercury ions could preferentially consume sulfides, resulting in decreased Ag2S formation. It is also noted that the pKsp of Ag2S was 48.7, HgS was 52.4,41 and according to the stoichiometric ratio, log[Ag–S] was −16.23 and log[Hg–S] was −26.2, indicating the stronger affinity between Hg and S. As in a previous representative report, Hg2+ could bind with S2− by forming HgS with the equivalent S2− or [HgS2]2− species with excess S2−.42 The obvious sensing of Hg2+ in this well-designed system was confidently expected.
As suggested by Fig. 1A, curve (c), while adding a moderate concentration of Hg2+, the absorption peak was retained at 650 nm. The solution colour changes were in accordance with the absorption spectra change. According to the TEM images, after adding the sulfide, the Au/Ag core–shell nanorods were invaded and the shell morphology transformed to be an irregular partly dumbbell shape (Fig. 1B(b)), while in the presence of Hg2+, the nanoparticles stayed in a rod-like shape (Fig. 1B(c)); in this case, sulfide prior reacted first with Hg2+ due to the strong interaction between S and Hg species. On the basis of this “rod-like to dumbbell or not” phenomenon, a sensing method for Hg2+ detection was established based on Au/Ag nanorods with the assistance of sulfide.
It is well known that the frequency of LSPR can be tuned by changing the material composition, size, shape and dielectric environment.43 Other reports of mercury sensors based on nanorods or nanoparticles involved aggregation,44 amalgamation,45 etching46,47 or other effects that can be modulated by mercury; hence, discussing these effects is necessary. The aggregation of nanoparticles indeed can lead to red-shifts and assembly via side by side or end to end. In the recent work by Zhu et al., cysteine was modified on the surface of nanorods to mediate the assembly.44 In this work, no ligands were introduced to induce the aggregating behaviour of nanoparticles. As indicated by the TEM images in Fig. 1B, the nanoparticles exhibited a well-monodispersed state. Besides, the colloid colour was transparent and no precipitate emerged after staying for a few days. Therefore, we can firmly state that the red-shift of the absorbance spectra could be attributed to the substantially different refractive indices of Ag (∼0.17) and Ag2S (∼2.2).
Mercury is known to form amalgams with gold and silver. Jin and Han describe how the formation of such amalgams can alter the SPR of gold nanorods.45 They use ascorbic acid as a reducing agent. Here, the Au/Ag core–shell nanorods were washed by centrifugation before using in the sensing system. The effect of the ascorbic acid involved in the synthesis procedure was negligible. Ying Qi et al. developed a typical determination strategy for Hg2+ based on the surface etching of Au–Ag core–shell triangular nanoplates.47 It is reported that the formation of a chemical complex will reduce the activity of ions and therefore reduce the redox potential.48,49 In this work, it is worth noting that Hg2+ was mixed with sulfide first, and then the Au/Ag core–shell nanorods were added. In this case, HgS or HgS22− should be considered instead of bare Hg2+. According to the Nernst equation,  , due to the formation of HgS or HgS22−, the potential will be substantially decreased. Thus the potential energy barrier between Hg2+ and Ag is increased. According to the TEM images, the etching by Hg2+ was difficult to observe.
, due to the formation of HgS or HgS22−, the potential will be substantially decreased. Thus the potential energy barrier between Hg2+ and Ag is increased. According to the TEM images, the etching by Hg2+ was difficult to observe.
Optimal sensing conditions of the proposed plasmonic nanoprobe
To obtain the best performance for the sensing system, the sensing conditions were examined in detail, such as pH condition, concentration of sulfide, the effects of other anions and the reaction time. The LSPR maximum shift, Δλmax value, was employed to evaluate the absorption peak shift.According to the respective reaction of Ag and Hg with sulfide ions, acidic condition is beneficial to the reaction system.50 Thus, to avoid the H2S gas escaping in acidic condition with low pH, and promote the reaction between Ag or Hg with S, acid buffer solutions with a relatively higher pH were introduced into the sensing system. As can be seen in Fig. 3 A, the proposed sensing system was not quite sensitive to pH 4.2–6.0. Accordingly, HAc–NaAc buffer solution (pH ∼5.0) was chosen for further experiments.
Under acidic conditions, H2S will be the major species. This also could be confirmed by calculating the distribution fractions of the existing sulfide species. H2S is a typical diacid; the two dissociation constants are Ka1 = 1.3 × 10−7, Ka2 = 7.1 × 10−15, and41 δ2, δ1, δ0 represent the distribution fraction of H2S, HS−, S2−, respectively. In theory,
According to the above formulas, the values of δ2, δ1, δ0 can be calculated, where, δ2 = 0.9872, which means H2S will be the major species.
In the practical sensing system, four reactions are involved as below.
| H2S ⇌ H+ + HS− | (1) |
| HS− ⇌ H+ + S2− | (2) |
| 4Ag + 2S2− + O2 + 4H+→ 2Ag2S + 2H2O | (3) |
| Hg2+ + S2− → HgS | (4) |
The latter two reactions (eqn (3) and (4)) are irreversible; the formation constant of Ag2S and HgS are sufficiently high, and thus the two reversible reactions are driven to dissociation, promoting the sensing behaviour.
Sulfides can quickly react with silver atoms in the presence of oxygen by generating Ag2S at room temperature. Because of the different refractive indices of Ag and Ag2S, the formation of Ag2S on the Au/Ag core–shell nanoparticle surface would result in its LSPR maximum shifting to a longer wavelength.51 Increasing amounts of sulfide were added to the nanoparticles, whereby a red-shift of the LSPR absorption band occurred gradually. The Δλmax value was recorded to evaluate the absorption peak shift. Finally, 13.3 μM was chosen, at which the increasing trend of Δλmax value reached maximum, as shown in Fig. 3B. In this case, while adding Hg2+, a remarkable absorption band change along this trend can be expected. The effects of inorganic sulfur ions (S2O32−, SO32−, SCN−) and other anions (NO3−, CH3COO−, PO43−, CO32−) on the sensing system were also examined. As indicated in Fig. 3C, no comparable LSPR responses from other species were detected because of the much lower solubility of Ag2S (pKsp = 50.83) than other Ag compounds.
As the reaction proceeds, the absorbance spectra of the nanoparticles changed gradually. Thus, the spectra were recorded continuously to indicate the reaction extent. The reaction time was also studied under the optimized pH in the absence and presence of Hg2+. The results showed that the maximum band shift was obtained in 5 min after the addition of Hg2+ and remained unchanged with further increases in reaction time. Additionally, the time gap between the additions of nanorods after sulfide interaction was also tested. It was found that the effects of different time gaps could be negligible (inset of Fig. 3D).
Feasibility for the detection of Hg2+
To quantify the concentration of Hg2+, R (%) = (λS − λHg)/(λS − λblank), this ratio value was employed to indicate the extent of inhibition by Hg2+ against the sulfide, where λS, λHg, and λblank represent the LSPR absorbance band of Au/Ag nanorod colloid solution in three different conditions: in the presence of a certain amount of sulfide alone, sulfide and Hg2+, absence of both, respectively. Fig. 4B shows a corresponding calibration plot of the ratio value R with the concentration of Hg2+ ranging from 0 to 3.0 μM. As shown by the inset of Fig. 4B, a good linear relationship was exhibited from 0.04 to 1.5 μM (R2 = 0.99), and the limit of detection was estimated to be about 13 nM at a signal-to-noise ratio of 3. This value is particularly attractive because it is lower than the maximum level (30 nM) permitted by the World Health Organization (WHO).52 Skillfully, the sensing range can be tuned by the amount of nanoparticles involved in the sensing behaviour, that is to say, more nanoparticles in the sensing system will expand the sensing range, and vice versa. Compared with other works, this proposed method does not need a ligand to functionalize the nanoparticle. According to the parameters given in Table 1, this work also exhibits competitive performance for mercury ion detection, including the detection limit and sensing range.| Optical techniques | Fabrication of sensors | Limit of detection (nM) | Linear range | References |
|---|---|---|---|---|
| Colorimetry/UV-Vis | 3-Mercaptopropionate acid-Au NPs | 100 | 0.2–0.5 μM | 53 |
| Cysteine-AuNPs | 25 | 0.05–1, 1–10 μM | 54 | |
| Peptite-AuNPs | 20 | 10–45 μM | 55 | |
| Mercaptopropionate acid/AMP-Au NPs | 500 | 0.5–3.5 μM | 56 | |
| Dithioerythritol-AuNPs | 24 | 0.1–0.5, 0.5–5 μM | 22 | |
| Chitosan-MoSe2 nanosheets with TMB | 3.5 | 0.025–2.5 μM | 57 | |
| Fluorescence | BAS/R6G/MPA-AuNPs | 20 | 0.1–2.5 μM | 58 |
| UV-Vis | Au/Ag core–shell nanorods | 13 | 0.04–1.5 μM | This work |
A specific response towards a target is of great significance for a nanoprobe. The excellent selectivity of this method for mercury ions over competing species can be expected by comparing the stability constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf) of metallic ions with sulfide ions. We next examined the selectivity of the proposed sensing method by obtaining the absorbance spectra in the presence of other metal ions, such as Co2+, Cd2+, Pb2+, Cu2+, Zn2+, Ni2+, and Mg2+. As shown in Fig. 5, among all the metal ions examined, only Hg2+ caused a significant response. Other metal ions could not bind with the S anions effectively, and their effects on the sensing behaviour were negligible. It is known that those metal ions (e.g. Cu2+, Pb2+, Zn2+) can interact with sulfide ions to form respective metal sulfides. The difference in their formation constants was employed here. We note that the log
Kf) of metallic ions with sulfide ions. We next examined the selectivity of the proposed sensing method by obtaining the absorbance spectra in the presence of other metal ions, such as Co2+, Cd2+, Pb2+, Cu2+, Zn2+, Ni2+, and Mg2+. As shown in Fig. 5, among all the metal ions examined, only Hg2+ caused a significant response. Other metal ions could not bind with the S anions effectively, and their effects on the sensing behaviour were negligible. It is known that those metal ions (e.g. Cu2+, Pb2+, Zn2+) can interact with sulfide ions to form respective metal sulfides. The difference in their formation constants was employed here. We note that the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf of Hg(ITC) n was ca. 21.8, whereas those of Cu2+, Pb2+, Zn2+ were 10.4, 1.48, 2.0,59 respectively, indicating that Hg2+ has the higher affinity toward sulfide species. Under the optimal conditions, the absorption spectra were recorded and no obvious response was obtained with other metal ions. Therefore, the selective detection of Hg2+ stems indeed from the strong and selective interaction between Hg2+ and sulfide.
Kf of Hg(ITC) n was ca. 21.8, whereas those of Cu2+, Pb2+, Zn2+ were 10.4, 1.48, 2.0,59 respectively, indicating that Hg2+ has the higher affinity toward sulfide species. Under the optimal conditions, the absorption spectra were recorded and no obvious response was obtained with other metal ions. Therefore, the selective detection of Hg2+ stems indeed from the strong and selective interaction between Hg2+ and sulfide.
Practical analysis of Hg2+ in water samples
The efficiency of the sensing performance of the established plasmonic nanoprobe was further investigated for its practical applicability towards Hg2+ analysis. Drinking water and tap water samples were collected and employed as a complicated matrix. No mercury ions were detected in the initial water samples. Different concentrations of mercury ions were introduced into the samples to simulate polluted water samples, and then the added Hg2+ was detected using our proposed nanoprobe. As shown in Table 2, satisfactory recoveries were obtained in the range of 96.7–102.7% for the two practical water samples, drinking water and tap water, with spiked concentration levels of Hg2+. The results clearly confirmed that this proposed nanoprobe is potentially applicable for the accurate determination of mercury ions in real samples. In this Au/Ag core–shell nanorod-based sensing system, Hg2+ ions were detected indirectly assisted by sulfide ions. From the angle of methodology, we designed a method for Hg2+ sensing based on the shell morphology change of plasmonic nanoparticles. As for the application in more complicated samples, which may themselves contain sulfide/sulfate ions, limitations exist objectively. Integrating the methodology and application, an additional job-plot could be established in real samples to avoid the presence of sulfide ions.| Samples | Spiked (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| a nd: not detected. | ||||
| Drinking water | 0 | nd | — | — |
| 300 | 290.1 | 96.7 | 0.72 | |
| 600 | 612.5 | 102.1 | 1.13 | |
| 1000 | 968.5 | 96.85 | 1.05 | |
| Tap water | 0 | nd | — | — |
| 300 | 308.2 | 102.7 | 1.03 | |
| 600 | 615.5 | 102.6 | 1.24 | |
| 1000 | 998.7 | 99.87 | 1.45 | |
Conclusions
In conclusion, a sensitive and selective localized surface plasmon resonance (LSPR) nanoprobe for the detection of mercuric ions was fabricated by using gold/silver core–shell nanorods. Sulfides play an important role by quickly reacting with silver atoms to generate Ag2S at room temperature in the presence of oxygen. The transformation from the silver shell to Ag2S on the nanorod surface resulted in its LSPR absorption band shifting to a longer wavelength because of their different refractive indices. Interestingly, the morphology also changed from rod-like to dumbbell shaped. But, in the presence of mercury ions, this morphology transformation was inhibited because the sulfide could preferentially react with free Hg2+ instead of Ag atoms. The amount of Ag2S was reduced with the increasing concentration of Hg2+, and the absorption band shift also decreased. According to this “rod-like to dumbbell or not” shape change, a sensitive and selective LSPR nanoprobe was developed that was assisted by UV-Vis absorption spectroscopy. A good linear relationship was obtained between the absorption band shift and Hg2+ concentrations. The detection limit of this sensor towards Hg2+ was as low as 13 nM, which is lower than the limitation concentration (30 nM) proposed by the World Health Organization. The efficiency of this probe in complex samples was evaluated by allowing the detection of Hg2+ in spiked water samples. A satisfactory recovery was obtained, which indicates its promising potential for application in complicated environmental areas.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (51501071, 51572109 and 51772130), the Outstanding Young Scientists Foundation Grant of Shandong Province (BS2014CL037), General Grant Financial from China Postdoctoral Science Foundation (Grant No: 2016M592124).References
- A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling and W. R. Dichtel, Nature, 2016, 529, 190–194 CrossRef CAS PubMed.
- D. Banerjee, D. Kim, M. J. Schweiger, A. A. Kruger and P. K. Thallapally, Chem. Soc. Rev., 2016, 45, 2724–2739 RSC.
- R. Das, C. D. Vecitis, A. Schulze, B. Cao, A. F. Ismail, X. Lu, J. Chen and S. Ramakrishna, Chem. Soc. Rev., 2017, 46, 6946–7020 RSC.
- J. R. Miller, J. Rowland, P. J. Lechler, M. Desilets and L.-C. Hsu, Water, Air, Soil Pollut., 1996, 86, 373–388 CrossRef CAS.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- K. Leopold, M. Foulkes and P. Worsfold, Anal. Chim. Acta, 2010, 663, 127–138 CrossRef CAS PubMed.
- S. R. Segadea and Ju. F. Tyson, Talanta, 2007, 71, 1696–1702 CrossRef PubMed.
- M. Leermakers, W. Baeyens, P. Quevauviller and M. Horvat, Trends Anal. Chem., 2005, 24(5), 383–393 CrossRef CAS.
- K. Leopold, M. Foulkes and P. J. Worsfold, Trends Anal. Chem., 2005, 28(4), 426–435 CrossRef.
- F. F. Yang, J. H. Li, W. H. Lu, Y. Y. Wen, X. Q. Cai, J. M. You, J. P. Ma, Y. J. Ding and L. X. Chen, Electrophoresis, 2014, 35, 474–481 CrossRef CAS PubMed.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 RSC.
- A. Kumar, S. Kim and J.-M. Nam, J. Am. Chem. Soc., 2016, 138, 14509–14525 CrossRef CAS PubMed.
- P. K Jain, X. H. Huang, I. H. EL-Sayed and M. A. EL-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef PubMed.
- L. X. Chen, Y. Q. Wang, X. L. Fu, L. Chen, Novel Optical Nanoprobes for Chemical and Biological Analysis, 2014, DOI:10.1007/978-3-662-43624-0.
- S. K. Kailasa, J. R. Koduru, M. L. Desai, T. J. Park, R. K. Singhal and H. Basu, TrAC, Trends Anal. Chem., 2018, 105, 106–120 CrossRef CAS.
- D. Liu, Z. Wang and X. Jiang, Nanoscale, 2011, 3, 1421–1433 RSC.
- V. N. Mehta and S. K. Kailasa, RSC Adv., 2015, 5, 4245–4255 RSC.
- S. K. Kailasa, M. Chandel, V. N. Mehta and T. J. Park, Spectrochim. Acta, Part A, 2018, 195, 120–127 CrossRef CAS PubMed.
- J. Du, L. Jiang, Q. Shao, X. Liu, R. S. Marks, J. Ma and X. D. Chen, Small, 2013, 9, 1467–1481 CrossRef CAS PubMed.
- Y. Ding, S. Wang, J. Li and L. Chen, Trends Anal. Chem., 2016, 82, 175–190 CrossRef CAS.
- H. Zhang and Y. S. Xia, ACS Sens., 2016, 1, 384–391 CrossRef CAS.
- L. L. He, L. Cheng, Y. Lin, H. F. Cui, N. Hong, H. Peng, D.-R. Kong, C. –D. Chen, J. Zhang, G. B. Wei and H. Fan, J. Electroanal. Chem., 2018, 814, 161–167 CrossRef CAS.
- C. Zhang, C. Kong, Q. Liu and Z. Chen, Analyst, 2019, 144, 1205–1209 RSC.
- D. Huang, X. Liu, C. Lai, L. Qin, C. Zhang, H. Yi, G. Zeng, B. Li, R. Deng, S. Liu and Y. Zhang, Microchim. Acta, 2019, 186, 31 CrossRef PubMed.
- H. Wang, Y. Wang, J. Jin and R. Yang, Anal. Chem., 2008, 80, 9021–9028 CrossRef CAS PubMed.
- M. Rex, F. E. Hernandez and A. D. Campiglia, Anal. Chem., 2006, 78, 445–451 CrossRef CAS PubMed.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410–8426 CrossRef CAS.
- J. Becker, I. Zins, A. Jakab, Y. Khalavka, O. Schubert and C. Sönnichsen, Nano Lett., 2008, 8, 1719–1723 CrossRef CAS PubMed.
- Y. Xiang, X. Wu, D. Liu, Z. Li, W. Chu, L. Feng, K. Zhang, W. Zhou and S. Xie, Langmuir, 2008, 24, 3465–3470 CrossRef CAS PubMed.
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713–3735 CrossRef CAS PubMed.
- Y. Xiang, X. Wu, D. Liu, Z. Li, W. Chu, L. Feng, K. Zhang, W. Zhou and S. Xie, Langmuir, 2008, 24, 3465–3470 CrossRef CAS PubMed.
- T. Kim, Q. Zhang, J. Li, L. Zhang and J. V. Jokerst, ACS Nano, 2018, 12, 5615–5625 CrossRef CAS PubMed.
- D. Seo and H. Song, J. Am. Chem. Soc., 2009, 131, 18210–18211 CrossRef CAS PubMed.
- Y. D. Zheng, M. D. Xiao, S. X. Jiang, F. Ding and J. F. Wang, Nanoscale, 2013, 5, 788–795 RSC.
- Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153–1175 CrossRef CAS.
- S. Lilienfeld and C. E. White, J. Am. Chem. Soc., 1930, 52, 885–892 CrossRef CAS.
- J. Zeng, J. Tao, D. Su, Y. Zhu, D. Qin and Y. Xia, Nano Lett., 2011, 11, 3010–3015 CrossRef CAS PubMed.
- G. Park, C. Lee, D. Seo and H. Song, Langmuir, 2012, 28, 9003–9009 CrossRef CAS PubMed.
- Y. Du, B. Xu, T. Fu, M. Cai, F. Li, T. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470–1471 CrossRef CAS PubMed.
- Wuhan University, Analytical Chemistry, addenda, Solubility product of slightly soluble compounds, 2008, pp. 402–403 Search PubMed.
- S. Y. Ding, M. Dong, Y. W. Wang, Y. T. Chen, H. Z. Wang, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed.
- P. K. Jain, K. S. Lee, I. H. EI-Sayed and M. A. EI-Sayed, J. Phys. Chem. B, 2006, 110, 7238–7248 CrossRef CAS PubMed.
- J. Zhu, B. Z. Zhao, Y. Qi, J. J. Li, X. Li and J. W. Zhao, Sens. Actuators, B, 2018, 255, 2927–2935 CrossRef CAS.
- L. H. Jin and C. S. Han, Sens. Actuators, B, 2014, 195, 239–245 CrossRef CAS.
- J. Zhu, T. T. Jia, J. J. Li and J. W. Zhao, Spectrochim. Acta, Part A, 2019, 207, 337–347 CrossRef CAS PubMed.
- Y. Qi, J. Zhao, G. J. Weng, J. J. Li, J. Zhu and J. W. Zhao, Sens. Actuators, B, 2018, 267, 181–190 CrossRef CAS.
- J. Rodríguez-Fernández, J. Pérez-Juste, P. Mulvaney and L. M. Liz-Marzán, J. Phys. Chem. B, 2005, 109, 14257–14261 CrossRef PubMed.
- L. Shang, L. H. Jin and S. J. Dong, Chem. Commun., 2009, 3077–3079 RSC.
- D. Liu, S. Wang, M. Swierczewska, X. Huang, A. A. Bhirde, J. Sun, Z. Wang, M. Yang, X. Jiang and X. Chen, ACS Nano, 2012, 6(12), 10999–11008 CrossRef CAS PubMed.
- B. Xiong, R. Zhou, J. Hao, Y. Jia, Y. He and E. S. Yeung, Nat. Commun., 2013, 4, 1708, DOI:10.1038/ncomms2722.
- World Health Organization, Guidelines for drinking-water quality: incorporating 1st and 2nd addenda, volume 1–Recommendations, World Health Organization, Geneva, 3rd edn, 2008, http://www.who.int/water_sanitation_health/dwq/fulltext.pdf Search PubMed.
- C. C. Huang and H. T. Chang, Chem. Commun., 2007, 12, 1215–1217 RSC.
- N. Ding, H. Zhao, W. Peng, Y. He, Y. Zhou, L. Yuan and Y. Zhang, Colloids Surf., A, 2012, 395, 161–167 CrossRef CAS.
- S. Si, A. Kotal and T. K. Mandal, J. Phys. Chem. C, 2007, 111(3), 1248–1255 CrossRef CAS.
- C. J. Yu and W. L. Tseng, Langmuir, 2008, 24(21), 12717–12722 CrossRef CAS PubMed.
- L. Huang, Q. Zhu, J. Zhu, L. Luo, S. Pu, W. Zhang, W. Zhu, J. Sun and J. Wang, Inorg. Chem., 2019, 58, 1638–1646 CrossRef CAS PubMed.
- H. Y. Chang, T. M. Hsiung, Y. F. Huang and C. C. Huang, Environ. Sci. Technol., 2011, 45(4), 1534–1539 CrossRef CAS PubMed.
- D. Liu, S. Wang, M. Swierczewska, X. Huang, A. A. Bhirde, J. Sun, Z. Wang, M. Yang, X. Jiang and X. Chen, ACS Nano, 2012, 6(12), 10999–11008 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |