Open Access Article
Open Access ArticleGC-MS-based identification and statistical analysis of liposoluble components in the rhizosphere soils of Panax notoginseng†
Yi-Jun Qiaoab,
Jia-Jiao Zhangc,
Jia-Huan Shangab,
Hong-Tao Zhua,
Dong Wanga,
Chong-Ren Yanga and
Ying-Jun Zhang *a
*a
aState Key Laboratory of Phytochemistry and Plant Resources of West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, People's Republic of China. E-mail: zhangyj@mail.kib.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
cState Key Laboratory of Hybrid Rice, College of Life Sciences, Wuhan University, Wuhan 430072, People's Republic of China
First published on 2nd July 2019
Abstract
Continuous cropping obstacle, mainly caused by microorganisms and organic components in soil, has become a serious problem for the plantation of Panax notoginseng (Araliaceae) due to the rapidly increased demands of this famous herbal medicine in recent decades. The rhizosphere soils cultivated with 3-year-old healthy and ill notoginseng were chemically investigated by gas chromatography-mass spectrometry (GC-MS) and compared with the corresponding soils without the plantation of notoginseng. Totally 47 liposoluble components were identified. Furthermore, the multiple statistical analysis showed that these constituents were qualitatively and quantitatively associated with the differences between the cultivated soil with P. notoginseng and the uncultivated soil. Among them, neophytadiene (4), D-α-tocopherol (38), (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), (3β,24R)-ergost-5-en-3-ol (40), stigmasta-5,22-dien-3-ol (41), stigmast-4-en-3-one (44) and (5α)-stigmastane-3,6-dione (47) contributed most to the significant differences between the cultivated and uncultivated soils, whereas cyclopentadecane (3), octadecanoic acid methyl ester (16), docosanoic acid ethyl ester (31), nonacosane (34), 38 and 39 were found in much higher amount in the soils with ill P. notoginseng as compared to the case of those with the healthy P. notoginseng. On the other hand, liposoluble components in different cultivation areas were of great diversity; however, they were able to remain relatively consistent across the overall trend of differential substances.
Introduction
Continuous cropping obstacle (CCO), mainly caused by the changes in the microbial community and organic components in the cultivated soil, is a common problem in the cultivation of many crops and herbs. Panax notoginseng (Burk.) F. H. Chen (Araliaceae), a famous herbal medicine in the Ginseng family with various pharmaceutical and health beneficial effects, has been historically cultivated in Wenshan district of Yunnan province and its adjacent areas of Guangxi province, China, for over 400 years.1–4 Due to the rapidly increased demands and planting scales in recent decades, CCO has become a serious problem for notoginseng cultivation, resulting in the lack of suitable land plots for the plantation of P. notoginseng.5Previous studies have shown that allelochemicals contribute significantly to the CCO problem.6 Phenolic acids and organic acids in root exudates could accelerate the formation of CCO. Benzoic acid, 2,2-di(4-hydroxyphenyl)propane and palmitic acid showed stronger allelopathic effects on the radicle or hypocotyl growth of the P. ginseng seeds.7 Ferulic acid, total saponin and root extract of P. notoginseng could inhibit the growth of the plant itself.8 Moreover, the seed germination rate of P. notoginseng was reduced by the degradation of its leaf residues in the soil.9 The soil extracts of notoginseng also displayed different allelopathic effects on its root length, seedling height, fresh weight and nitrate reductase activity, as well as on radish and lettuce seedlings.10–12 Recently, 29 liposoluble components have also been identified from the rhizosphere soil of continuously cultivated P. notoginseng; among these, p-hydroxybenzoic acid and phthalic acid diisobutylester have an allelopathic effect on the growth of the P. notoginseng pathogen.13 Another study has reported 26 volatiles originated from the cultivated soil of P. notoginseng, with high content of allelochemicals such as diisobutyl phthalate.14
As a part of our research to reveal the formation mechanism of CCO for P. notoginseng, in this study, the liposoluble components in the rhizosphere soils cultivated with 3-year-old healthy or ill notoginseng obtained from five different plantations were studied by GC-MS analysis, combining with multiple statistical analysis. The innovation of this research is that for the first time, the combination method of instrumental analysis and multivariate statistical analysis was used to study the liposoluble components in different soil samples of Panax notoginseng, by which the different key substances were more accurately and efficiently identified.
Results and discussion
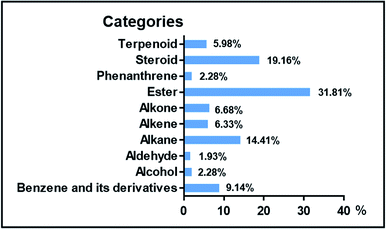
Herein, ten rhizosphere soil samples obtained from five different plantations [A-San-Long (A), Lao-Mu-Shao (L), Ba-Tang-Chong (W-1), Zhai-Tou (W-2), and Ba-Zi (W-3)] in Yunnan province, China, each with one healthy (H) and one illness (I) growing status of 3-year-old P. notoginseng, were obtained for study; moreover, one soil sample (M) without the cultivation of notoginseng was obtained from the adjacent field of each plantation.A total of 47 different liposoluble components with more than 80% matching value were identified from the soil samples based on the GC-MS analysis, and the results are listed in Table 1. The identified components were of 10 types: 15 esters (6, 8, 10–13, 16–20, 25, 29, 31, and 32), 9 steroids (35–37, 39–41, 43, 44, and 47), 7 alkanes (1, 3, 15, 22, 24, 33, and 34), 4 benzene derivatives (2, 9, 30, and 38), 3 alkones (5, 26, and 28), 3 alkenes (4, 7, and 27), 3 terpenoids (42, 45, and 46), 1 phenanthrene (21), 1 alcohol (14) and 1 aldehyde (23). The relative peak area percentage of identified compounds and their distributions normalized based on the total ion current (TIC) in the soil samples are provided in Tables S1 and S2,† respectively.
| Peaks | Compounds | Mol. formula | RT (min) | Qualifier ions (m/z) | Classification |
|---|---|---|---|---|---|
| a The identification criteria based on mass spectra matching with the Wiley7n.l library and NIST14 library which resulted in level 2 identifications.15 | |||||
| 1 | 10-Methylnonadecane | C20H64 | 27.920 | 57, 71, 85 | Alkane |
| 2 | Phenol, 2,4-bis(1,1-dimethylethyl)- | C14H22O | 28.448 | 191, 206, 57 | Benzene derivative |
| 3 | Cyclopentadecane | C15H30 | 32.995 | 149, 223, 104 | Alkane |
| 4 | Neophytadiene | C20H38 | 35.618 | 95, 68, 82 | Alkene |
| 5 | 2-Pentadecanone, 6,10,14-trimethyl- | C18H36O | 35.751 | 58, 71, 85 | Alkone |
| 6 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl)ester | C16H22O4 | 36.275 | 149, 57, 223 | Ester |
| 7 | Neophytadiene, isomer I | C20H38 | 36.494 | 81, 82, 95 | Alkene |
| 8 | Pentadecanoic acid, ethyl ester | C17H34O2 | 36.724 | 88, 101, 157 | Ester |
| 9 | Dibutyl phthalate | C16H22O25 | 38.145 | 149, 223, 104 | Benzene derivative |
| 10 | Ethyl 9-hexadecenoate | C18H34O2 | 38.294 | 55, 69, 88 | Ester |
| 11 | E-11-Hexadecenoic acid, ethyl ester | C18H34O3 | 38.487 | 55, 69, 88 | Ester |
| 12 | Hexadecanoic acid, ethyl ester | C18H36O2 | 38.700 | 88, 101, 55 | Ester |
| 13 | Heptadecanoic acid, ethyl ester | C19H38O2 | 39.892 | 88, 101, 57 | Ester |
| 14 | 1-Octadecanol | C18H38O | 40.431 | 82, 57, 96 | Alcohol |
| 15 | Heneicosane | C21H44 | 40.661 | 57, 71, 85 | Alkane |
| 16 | Octadecanoic acid, methyl ester | C19H38O2 | 41.104 | 74, 87, 143 | Ester |
| 17 | Heptadecanoic acid, 15-methyl-, ethyl ester | C20H40O2 | 41.350 | 88, 101, 57 | Ester |
| 18 | Linoleic acid, ethyl ester | C20H36O2 | 41.804 | 81, 67, 55 | Ester |
| 19 | Ethyl oleate | C20H38O2 | 41.911 | 55, 69, 41 | Ester |
| 20 | Octadecanoic acid, ethyl ester | C20H40O2 | 42.370 | 88, 101, 312 | Ester |
| 21 | Phenanthrene, 1-methyl-7-(1-methylethyl)- | C18H18 | 42.931 | 219, 234, 204 | Phenanthrene |
| 22 | Cycloeicosane | C20H40 | 43.888 | 55, 69, 83 | Alkane |
| 23 | Tetradecanal | C14H28O | 44.032 | 57, 82, 96 | Aldehyde |
| 24 | Tricosane | C23H48 | 44.165 | 57, 71, 85 | Alkane |
| 25 | 2-Propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester | C18H26O3 | 44.598 | 178, 161, 133 | Ester |
| 26 | 4,8,12,16-Tetramethylheptadecan-4-olide | C21H40O2 | 45.122 | 99, 43, 55 | Alkone |
| 27 | 1,21-Docosadiene | C22H42 | 46.313 | 95, 68, 55 | Alkene |
| 28 | 1-Docosanal | C22H44O | 47.344 | 82, 97, 111 | Alkone |
| 29 | Docosanoic acid, methyl ester | C23H46O2 | 47.916 | 41, 96, 82 | Ester |
| 30 | Bis(2-ethylhexyl)phthalate | C24H38O4 | 48.209 | 149, 167, 279 | Benzene derivative |
| 31 | Docosanoic acid, ethyl ester | C24H48O2 | 48.904 | 88, 97, 157 | Ester |
| 32 | Tricosanoic acid, methyl ester | C24H48O2 | 49.457 | 88, 101, 55 | Ester |
| 33 | Heptacosane | C27H56 | 50.416 | 57, 71, 85 | Alkane |
| 34 | Nonacosane | C29H60 | 53.231 | 57, 71, 85 | Alkane |
| 35 | Stigmasta-3,5,22-trien | C29H46 | 56.212 | 394, 145, 69 | Steroid |
| 36 | Stigmasta-3,5-dien | C29H48 | 56.602 | 396, 147, 81 | Steroid |
| 37 | Cholesterol | C27H46O | 57.094 | 386, 275, 107 | Steroid |
| 38 | D-α-Tocopherol | C29H50O2 | 57.382 | 430, 165, 69 | Benzene derivative |
| 39 | (3β,22E,24S)-Ergosta-5,22-dien-3-ol | C28H46O | 58.093 | 398, 69, 207 | Steroid |
| 40 | (3β,24R)-Ergost-5-en-3-ol | C28H48O | 59.487 | 81, 95, 400 | Steroid |
| 41 | Stigmasta-5,22-dien-3-ol | C29H48O | 60.374 | 55, 271, 412 | Steroid |
| 42 | Hop-22(29)-en-3β-ol | C30H50O | 61.517 | 189, 205, 175 | Terpenoid |
| 43 | γ-Sitosterol | C29H50O | 61.880 | 414, 329, 303 | Steroid |
| 44 | Stigmast-4-en-3-one | C29H48O | 66.095 | 124, 55, 229 | Steroid |
| 45 | (3α)-D:A-Friedooleanan-3-ol | C36H62O2 | 67.917 | 95, 169, 65 | Terpenoid |
| 46 | Friedelin | C30H50O | 68.830 | 69, 109, 95 | Terpenoid |
| 47 | (5α)-Stigmastane-3,6-dione | C29H48O2 | 72.816 | 428, 245, 207 | Steroid |
The 10 types of liposoluble components were detected from all five plantations, with a slight difference in proportions, whereas a noticeable difference in contents. Esters, steroids and alkanes composed more than half of the total components (Fig. 1), with 31.81% of esters ranking the most. Compared to the case of the soils without the cultivation of P. notoginseng, the steroids 39–41 and 47 were present in higher contents in the cultivated soils. These results are slightly different from those obtained in a previous study, in which alkanes, alkenes, aldehydes, organic acids, esters and acetylenic alcohols have been reported as major liposoluble components in the cultivated soil.16 Note that some dominant components, such as cyclopentadecane (3), nonacosane (34), D-α-tocopherol (38), (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), stigmasta-5,22-dien-3-ol (41), and (5α)-stigmastane-3,6-dione (47), present in the cultivated soils were not detected in the uncultivated soils.
The GC-MS results were analysed by two different algorithms: the PCA and the PLS-DA models. As shown in Fig. 2 and 3, the soil samples obtained from three different plantations (W-1, W-2 and W-3) in Wenshan county were nearer to each other than the other two sites (A and L). Moreover, it was found that only in Wenshan plantations (W-1, W-2 and W-3), the concentrations of some components, such as octadecanoic acid methyl ester (16), cycloeicosane (22) and (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), were changed by the growing status and the cultivation of P. notoginseng. However, this was not observed in the soil samples obtained from the other two regions (Tables 2 and 3).
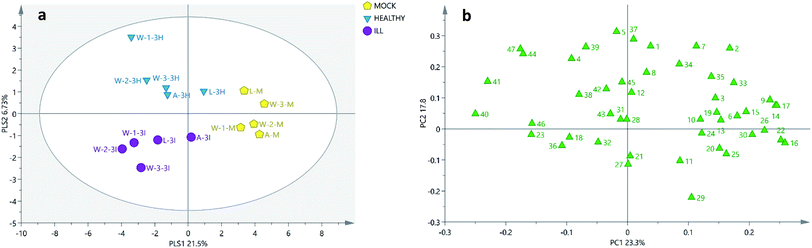 | ||
| Fig. 2 PCA score plots and loading plots of 15 soil samples. (a) PCA score plot of the soil samples. (b) PCA loading plot of the soil samples. A, L and W: the sites of sample collection (A: A-San-Long; L: Lao-Mu-Shao; W: Wenshan); three sites for sample collection in Wenshan county were numbered as W-1, W-2 and W-3.; M: mock group, used as a blank control. I and H: the growing status of 3-year-old P. notoginseng (I: ill, H: healthy); the numbers in the loading plot of all the soil samples correspond to the values provided in Table 1. | ||
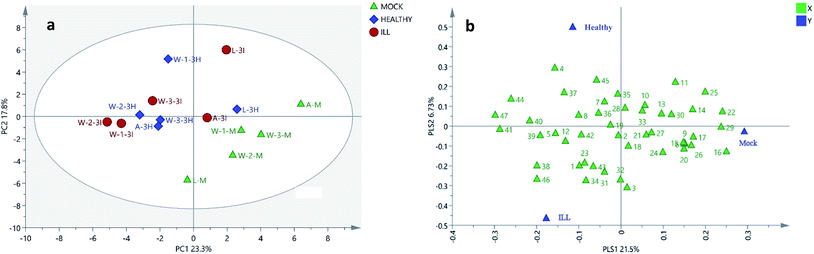 | ||
| Fig. 3 PLS-DA score plots and loading plots of 15 soil samples. (a) PLS-DA score plot of the soil samples. (b) PLS-DA loading plot of the soil samples. A, L and W: the sites of sample collection (A: A-San-Long; L: Lao-Mu-Shao; W: Wenshan); three sites for sample collection in Wenshan county were numbered as W-1, W-2 and W-3.; M: mock group, used as a blank control. I and H: the growing status of 3-year-old P. notoginseng (I: ill, H: healthy); and the numbers in the loading plot of all the soil samples correspond to the values presented in Table 1. | ||
| Components | AM/AI | LM/LI | W-1M/W-1I | W-2M/W-2I | W-3M/W-3I |
|---|---|---|---|---|---|
| Benzene derivative | |||||
| D-α-Tocopherol (38) | 0.012↑ | 0.002↑ | 0.087 | 0.003↑ | 0.001↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Aldehyde | |||||
| Tetradecanal (23) | 1.000 | 0.827 | 0.001↑ | 0.001↑ | 0.012↓ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Alkanes | |||||
| 10-Methylnonadecane (1) | 0.951 | 0.001↑ | 0.045↑ | 1.000 | 0.047↑ |
| Cyclopentadecane (3) | 0.058 | 0.013↑ | 0.605 | 0.002↓ | 0.059 |
| Neophytadiene (4) | 0.223 | 0.003↑ | 0.524 | 0.008↑ | 0.067 |
| Cycloeicosane (22) | 0.037↓ | 0.210 | 0.006↓ | 0.001↓ | 0.001↓ |
| Nonacosane (34) | 0.894 | 0.007↑ | 0.392 | 0.373 | 0.002↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Esters | |||||
| E-11-Hexadecenoic acid, ethyl ester (11) | 0.240 | 0.964 | 0.004↓ | 0.008↓ | 0.135 |
| Octadecanoic acid, methyl ester (16) | 0.172 | 0.001↑ | 0.001↓ | 0.002↓ | 0.018↓ |
| 2-Propenoic acid, 3-(4-methoxy-phenyl)-, 2-ethylhexyl ester (25) | 0.004↓ | 0.017↓ | 0.003↓ | 0.045↓ | 1.000 |
| Docosanoic acid, methyl ester (29) | 0.002↓ | 0.002↓ | 0.140 | 0.000↓ | 0.625 |
| Docosanoic acid, ethyl ester (31) | 0.143 | 0.006↓ | 0.091 | 0.001↑ | 0.002↑ |
| Tricosanoic acid, methyl ester (32) | 0.200 | 0.001↑ | 0.392 | 0.005↓ | 0.002↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Steroids | |||||
| (3β,22E,24S)-Ergosta-5,22-dien-3-ol (39) | 0.001↓ | 0.001↑ | 0.002↑ | 0.004↑ | 0.001↑ |
| (3β,24R)-Ergost-5-en-3-ol (40) | 1.000 | 0.023↑ | 0.001↑ | 0.001↑ | 0.002↑ |
| Stigmasta-5,22-dien-3-ol (41) | 0.015↑ | 0.001↑ | 0.001↑ | 0.005↑ | 0.002↑ |
| Stigmast-4-en-3-one (44) | 0.003↑ | 0.001↑ | 0.058 | 0.008↑ | 0.001↑ |
| (5α)-Stigmastane-3,6-dione (47) | 0.125 | 0.002↑ | 0.010↑ | 0.002↑ | 0.001↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Terpenoids | |||||
| (3α)-D:A-Friedooleanan-3-ol (45) | 0.003↑ | 0.005↑ | 0.005↓ | 1.000 | 0.189 |
| Friedelin (46) | 0.012↑ | 0.048↑ | 0.226 | 0.001↑ | 0.001↑ |
| Metabolites | AH/AI | LH/LI | W-1H/W-1I | W-2H/W-2I | W-3H/W-3I |
|---|---|---|---|---|---|
| Benzene derivatives | |||||
| D-α-Tocopherol (38) | 0.012↑ | 0.234 | 0.003↑ | 0.160 | 0.474 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Aldehyde | |||||
| Tetradecanal (23) | 0.005↓ | 0.003↑ | 0.150 | 0.001↑ | 0.044↓ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Alkane | |||||
| 10-Methylnonadecane (1) | 0.045↑ | 0.001↑ | 0.001↓ | 1.000 | 0.164 |
| Cyclopentadecane (3) | 0.454 | 0.028↑ | 0.007↑ | 1.000 | 0.001↑ |
| Neophytadiene (4) | 0.002↓ | 0.064 | 0.008↓ | 0.212 | 0.001↓ |
| Cycloeicosane (22) | 0.026↑ | 0.001↓ | 0.002↓ | 0.062 | 0.809 |
| Nonacosane (34) | 0.026↑ | 0.021↑ | 0.001↓ | 0.004↑ | 0.003↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Ester | |||||
| E-11-Hexadecenoic acid, ethyl ester (11) | 0.001↓ | 0.008↓ | 1.000 | 0.212 | 0.003↓ |
| Octadecanoic acid, methyl ester (16) | 0.011↑ | 0.028↑ | 0.063 | 1.000 | 1.000 |
| 2-Propenoic acid, 3-(4-methoxy-phenyl)-, 2-ethylhexyl ester (25) | 0.840 | 0.045↑ | 0.160 | 0.042↓ | 0.001↓ |
| Docosanoic acid, methyl ester (29) | 0.953 | 0.609 | 0.004↑ | 0.003↓ | 0.988 |
| Docosanoic acid, ethyl ester (31) | 0.007↑ | 0.307 | 0.006↑ | 0.074 | 0.695 |
| Tricosanoic acid, methyl ester (32) | 0.016↓ | 0.006↑ | 0.001↑ | 0.715 | 0.002 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Steroid | |||||
| (3β,22E,24S)-Ergosta-5,22-dien-3-ol (39) | 0.205 | 0.093 | 0.986 | 0.175 | 0.010↑ |
| (3β,24R)-Ergost-5-en-3-ol (40) | 0.012↓ | 0.048↑ | 0.013↑ | 0.789 | 0.929 |
| Stigmasta-5,22-dien-3-ol (41) | 0.014↓ | 0.005↑ | 0.010↑ | 0.922 | 0.999 |
| Stigmast-4-en-3-one (44) | 0.449 | 0.119 | 0.005↓ | 0.128 | 0.015↑ |
| (5α)-Stigmastane-3,6-dione (47) | 0.995 | 0.027↑ | 0.037↓ | 0.339 | 0.208 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Terpenoid | |||||
| (3α)-D:A-Friedooleanan-3-ol (45) | 0.002↑ | 0.270 | 0.001↓ | 0.003↓ | 0.090 |
| Friedelin (46) | 0.704 | 0.046↑ | 0.001↑ | 0.185 | 0.409 |
Moreover, as shown in the PCA score plot (Fig. 2a), the clusters of the cultivated soils were separated from those of the uncultivated soils by combining PC1 (23.3%) and PC2 (17.8%), and the uncultivated soils located relatively closer. Among the samples W-1, W-2, and W-3 obtained from Wenshan county, the cultivated and uncultivated soils were separated from each other by PC1. By combining these results with those of the loading plot (Fig. 2b), the top ten variables with greatest impact on the classification were found (Table S3†) to be octadecanoic acid methyl ester (16), cycloeicosane (22), stigmasta-5,22-dien-3-ol (40), 15-methyl-heptadecanoic acid ethyl ester (17), 1-octadecanol (14), dibutyl phthalate (9), (3β,24R)-ergost-5-en-3-ol (41), 4,8,12,16-tetra-methylheptadecan-4-olide (26), bis(2-ethylhexyl)phthalate (30) and heneicosane (15). Among these, the contents of the compounds 40 and 41 in the cultivated soils (both healthy and ill samples) were more than 2-fold higher than those in the uncultivated soils.
However, in the other two plantations A and L, the cultivated soils were separated from the uncultivated soils by PC2, and the top ten variables responsible for the discrimination were found in the loading plot (Fig. 2b): 2-pentadecanone (5), 6,10,14-trimethyl-cholesterol (37), 10-methylnonadecane (1), neophyte-diene isomer I (7), (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), phenol 2,4-bis(1,1-dimethyl-ethyl)- (2), (5α)-stigmastane-3,6-dione (47), stigmast-4-en-3-one (44), neophytadiene (4) and docosanoic acid methyl ester (29). Overall, in the cultivated soils, the contents of the compounds 44 and 47 in the soil obtained from the A-San-Long plantation (A) as well as those of 2, 4, 5, 7, 37, 39, 44 and 47 in the soil obtained from the Lao-Mu-Shao plantation (L) were found to increase when compared with the case of their corresponding uncultivated samples.
The PLS-DA analysis is the most commonly used classification method in metabolomics data analysis and has been conducted to investigate the discriminatory components. It combines the regression models with dimensionality reduction and discriminates the regression results with certain discriminant thresholds. As shown in Fig. 3a, the cultivated and the uncultivated soils were clearly separated by PC1 and PC2 in the score plot. Based on the VIP value (Table S4†) and the loading plot (Fig. 3b), 20 compounds, including esters, steroids, alkanes, aldehyde, the derivative of benzene and terpenoid, were found to play key roles in the classification.
Moreover, one-way ANOVA was applied to investigate lipids in different soil samples, and the lipid levels changed significantly in the cultivated soils as compared to those of the uncultivated soils. Variables with p < 0.05 were considered of remarkable difference between every two groups (Tables 2 and 4). After planting P. notoginseng for 3 years, it was observed that the contents of neophytadiene (4), D-α-tocopherol (38), (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), (3β,24R)-ergost-5-en-3-ol (40), stigmasta-5,22-dien-3-ol (41), stigmast-4-en-3-one (44), and (5α)-stigmastane-3,6-dione (47) increased significantly in the soils regardless of the growing status (healthy or ill) of P. notoginseng.
| Metabolites | AM/AH | LM/LH | W-1M/W-1H | W-2M/W-2H | W-3M/W-3H |
|---|---|---|---|---|---|
| a The numbers are the p-value calculated by ANOVA with Fisher's LSD; ↑, significant increase compared with control (p < 0.05); ↓, significant decrease compared with control (p < 0.05); A, L and W: the sites of sample collection; A: A-San-Long; L: Lao-Mu-Shao; W: Wenshan; three sites in Wenshan county were numbered W-1, W-2 and W-3, resp.; M: mock group, used as blank control; I and H: the growing status of P. notoginseng; I: ill, H: healthy. | |||||
| Benzene derivatives | |||||
| D-α-Tocopherol (38) | 1.000 | 0.006↑ | 0.001 | 0.001↑ | 0.002↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Aldehyde | |||||
| Tetradecanal (23) | 0.005↑ | 0.003 | 0.006↑ | 0.872 | 0.357 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Alkane | |||||
| 10-Methylnonadecane (1) | 0.007 | 0.426 | 0.023↑ | 1.000 | 0.396 |
| Cyclopentadecane (3) | 0.177 | 0.015↑ | 0.013 | 0.002 | 0.004 |
| Neophytadiene (4) | 0.001↑ | 0.049↑ | 0.004↑ | 0.002↑ | 0.006↑ |
| Cycloeicosane (22) | 0.029 | 0.002↑ | 0.235 | 0.009 | 0.001 |
| Nonacosane (34) | 0.031 | 0.003↑ | 0.002↑ | 0.002 | 0.630 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Ester | |||||
| E-11-Hexadecenoic acid, ethyl ester (11) | 0.002↑ | 0.007↑ | 0.004 | 0.002 | 0.025↑ |
| Octadecanoic acid, methyl ester (16) | 0.002 | 0.011↑ | 0.011 | 0.002 | 0.018 |
| 2-Propenoic acid, 3-(4-methoxy-phenyl)-, 2-ethylhexyl ester (25) | 0.003 | 0.001 | 0.023 | 0.202 | 0.001↑ |
| Docosanoic acid, methyl ester (29) | 0.002 | 0.003 | 0.001 | 0.049 | 0.615 |
| Docosanoic acid, ethyl ester (31) | 0.001 | 0.023 | 0.001 | 0.006↑ | 0.003↑ |
| Tricosanoic acid, methyl ester (32) | 0.023↑ | 0.135 | 0.002 | 0.004 | 1.000 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Steroid | |||||
| (3β,22E,24S)-Ergosta-5,22-dien-3-ol (39) | 0.003 | 0.005↑ | 0.002↑ | 0.001↑ | 0.043↑ |
| (3β,24R)-Ergost-5-en-3-ol (40) | 0.011↑ | 0.587 | 0.029↑ | 0.002↑ | 0.002↑ |
| Stigmasta-5,22-dien-3-ol (41) | 0.001↑ | 0.140 | 0.023↑ | 0.005↑ | 0.002↑ |
| Stigmast-4-en-3-one (44) | 0.001↑ | 0.004↑ | 0.001↑ | 0.001↑ | 0.060 |
| (5α)-Stigmastane-3,6-dione (47) | 0.126 | 0.060 | 0.001↑ | 0.001↑ | 0.002↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Terpenoid | |||||
| (3α)-D:A-Friedooleanan-3-ol (45) | 0.701 | 0.020↑ | 0.079 | 0.003↑ | 0.607 |
| Friedelin (46) | 0.008↑ | 0.980 | 0.002↓ | 0.004↑ | 0.002↑ |
Comparison of the liposoluble components in the cultivated soils with different growing statuses
Usually, P. notoginseng grows for 3 years before harvest. During the planting process, some of the plants might get affected by diseases, resulting in changes in their chemical compositions in the rhizosphere soils. The differences between the liposoluble components of soils with different growing statuses (healthy or ill) of P. notoginseng were analyzed by one-way ANOVA. As shown in Table 3, the concentrations of six liposoluble components, i.e. cyclopentadecane (3), octadecanoic acid methyl ester (16), docosanoic acid ethyl ester (31), nonacosane (34), D-α-tocopherol (38) and (3β,22E,24S)-ergosta-5,22-dien-3-ol (39), were found to be changed significantly and much higher in the soils with ill notoginseng. Although other compounds with values of VIP > 1 did not change consistently, they also contributed to the three clusters of variances in the PLS-DA analysis (Fig. 3a).These compounds may be produced by the pathogenic microorganisms after the plants get infested by diseases. Since the differences between the cultivation areas and plant statuses are not obvious and the changes in the contents of these compounds are basically consistent in the same planting area, attention should be paid mainly to the differences between the liposoluble components of the cultivated soils with the healthy status of plant and the uncultivated soils.
Origins and biosynthetic pathways of liposoluble compounds identified in the soils
The liposoluble components that could significantly affect the classification in the PCA and PLS-DA analysis were of different types. Among them, alkanes, esters, steroids and terpenoids account for more than half of all the compounds identified in the cultivated soils.The origins of alkanes in soils and sediments are very complex. The input paths mainly include direct input of mineral oil, sedimentation of atmospheric particulate matter, input through an aqueous medium, industrial solid, municipal and domestic waste, as well as biochemical degradation products of natural organics. There are some differences in the composition of hydrocarbon pollutants obtained from different sources. Based on these differences, the sources of hydrocarbon pollutants in the environment can be identified.17 Unlike the phthalate esters that mainly originate from chemical fertilizers and pesticides,18 the esters identified in this study are fatty acid esters. They are more likely to originate from industrial chemicals, which may be used as solvents in skin care products. The steroids can be isolated, extracted and purified from natural resources. In vivo, acetic acid can form squalene by head-to-head contact of farnesyl pyrophosphate under the action of an enzyme. The cyclization of the 2,3-epoxide of squalene produces lanosterol. Lanosterol undergoes a series of transformations in the body to form steroidal substances such as cholesterol and hormones. Terpenoids widely exist in advanced plants in the form of volatile oils. They can be divided into monoterpene, diterpene, sesquiterpenes, triterpene and polyterpenoid according to their structures. As the main type of allelochemicals, monoterpene and sesquiterpene possess strong bioactivities. After synthesis, most of the terpenoids enter the soil through plant volatilization or root secretion, which can affect the growth and development of the host plant and the neighboring plants.19,20
Experimental
Materials and methods
| No. | Longitude | Latitude | Altitude (m) | Sources |
|---|---|---|---|---|
| a All the samples were collected in Yunnan Province, China; the samples (each 5.0 kg) were obtained from five separate plantations at A-San-Long village of Honghe county (A), Lao-Mu-Shao village of Shilin county (L), and Ba-Tang-Chong (W-1), Zhai-Tou (W-2), and Ba-Zi (W-3) in Bai-Yun-Yan village of Wenshan county, resp.; 3H: 3-year-old healthy P. notoginseng; 3I: 3-year-old ill P. notoginseng. | ||||
| A-M | 103°37′40.19′′ | 23°47′59.34′′ | 1466 | Uncultivated |
| A-3H | 103°37′37.91′′ | 23°47′57.34′′ | 1462 | 3H |
| A-3I | 103°37′39.89′′ | 23°47′57.41′′ | 1465 | 3I |
| L-M | 103°26′13.57′′ | 24°54′34.12′′ | 1968 | Uncultivated |
| L-3H | 103°26′14.84′′ | 24°54′36.59′′ | 1968 | 3H |
| L-3I | 103°26′16.15′′ | 24°54′34.67′′ | 1968 | 3I |
| W-1-M | 104°17′22′′ | 23°29′53′′ | 1598 | Uncultivated |
| W-1-3H | 104°17′22′′ | 23°29′54′′ | 1601 | 3H |
| W-1-3I | 104°17′21′′ | 23°29′55′′ | 1597 | 3I |
| W-2-M | 104°18′16′′ | 23°29′47′′ | 1522 | Uncultivated |
| W-2-3H | 104°18′18′′ | 23°29′47′′ | 1521 | 3H |
| W-2-3I | 104°18′19′′ | 23°29′46′′ | 1521 | 3I |
| W-3-M | 104°18′18′′ | 23°30′11′′ | 1517 | Uncultivated |
| W-3-3H | 104°18′22′′ | 23°29′56′′ | 1519 | 3H |
| W-3-3I | 104°18′19′′ | 23°30′00′′ | 1522 | 3I |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split injection ratio. The ionization mode was electron impact at 70 eV. An HP-5MS capillary column (0.25 mm × 30 m, 0.25 μm) with helium as a carrier gas at 1.0 mL min−1 was used to analyze the samples. The injected volume was 2.0 μL for each sample. The oven temperature was initially held at 40 °C, firstly ramped to 80 °C at 3 °C min−1, and then to the target temperature of 280 °C at 5 °C min−1 with the duration of 30 min. The temperatures of injector, the ion source and the quadrupole were maintained at 250 °C, 230 °C and 150 °C, respectively. The obtained mass range m/z 35–500 was acquired using the full scan monitoring mode. The solvent delay time was set at 2.4 min.21–26
1 split injection ratio. The ionization mode was electron impact at 70 eV. An HP-5MS capillary column (0.25 mm × 30 m, 0.25 μm) with helium as a carrier gas at 1.0 mL min−1 was used to analyze the samples. The injected volume was 2.0 μL for each sample. The oven temperature was initially held at 40 °C, firstly ramped to 80 °C at 3 °C min−1, and then to the target temperature of 280 °C at 5 °C min−1 with the duration of 30 min. The temperatures of injector, the ion source and the quadrupole were maintained at 250 °C, 230 °C and 150 °C, respectively. The obtained mass range m/z 35–500 was acquired using the full scan monitoring mode. The solvent delay time was set at 2.4 min.21–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were rejected. Peak width was set at 0.1 s, and the threshold was set at 14.0. The compounds were identified by searching NIST98 and Wiley7n.l library, with the assistance of their qualifier ions. Further, to minimize the number of missing values, compounds with less than 80% matching value were discarded from the total peak list. Peak alignment was performed by manually comparing the retention times with the values present in a reference chromatogram, which had the most of the peaks among all samples. In the end, the relative content (%) of each compound in a sample was normalized based on total ion current (TIC) and subjected to further statistical analysis.27–29
000 were rejected. Peak width was set at 0.1 s, and the threshold was set at 14.0. The compounds were identified by searching NIST98 and Wiley7n.l library, with the assistance of their qualifier ions. Further, to minimize the number of missing values, compounds with less than 80% matching value were discarded from the total peak list. Peak alignment was performed by manually comparing the retention times with the values present in a reference chromatogram, which had the most of the peaks among all samples. In the end, the relative content (%) of each compound in a sample was normalized based on total ion current (TIC) and subjected to further statistical analysis.27–29Conclusions
In conclusion, the liposoluble components in the soils obtained from 5 different plantations and the growing statuses of P. notoginseng were analyzed and compared with the case of the uncultivated soils by GC-MS analysis combined with the multivariate statistical analysis (PCA and PLS-DA). In total, 47 liposoluble components belonging to 10 different types were identified and put together for comprehensive analysis. Alkanes, i.e. cyclopentadecane (3) and 10-methylnonadecane (1), alkene, i.e. neophytadiene (4), esters, i.e. E-11-hexadecenoic acid ethyl ester (11) and octadecanoic acid methyl ester (16), benzene derivatives, i.e. D-α-tocopherol (38), and steroids, i.e. (3β,22E,24S)-ergosta-5,22-dien-3-ol (39) and (3β,24R)-ergost-5-en-3-ol (40), were revealed as discriminatory components by statistical analysis, which contributed significantly to the discrimination of the cultivated and uncultivated soils. Moreover, a comparison of liposoluble components in the soils obtained from different planting areas and plant growing statuses were carried out, resulting in the demonstration of the components that were of notable differences.Studies on the chemical substances in rhizosphere soil of P. notoginseng were mainly focused on the water-soluble components and the bioactivities of different solvent extracts.33,34 Liposoluble components in the cultivated soil of P. notoginseng have been identified and reported in only two studies in recent years, and only some of the common allelochemicals, which have been found to exert an allelopathic effect on other plants, have been considered in their studies.13,14 The differences between the cultivated and uncultivated soils had not been discussed to date. Therefore, by decently and statistically comparing the liposoluble components of different soils, this study provides a reference value for the subsequent research on the changes of the chemical composition of the soil and the key chemical substances leading to continuous cropping obstacle of P. notoginseng. Furthermore, by analyzing and discussing the possible origins and transformation pathways of these compounds, this study explains their potential role as allelochemicals and provides a theoretical basis for the further study of their allelopathic effects on P. notoginseng and solutions to solve the problem of continuous cropping obstacle.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Major Science and Technique Programs in Yunnan Province (2016ZF001-001), the Science and Technology Planning Project of Yunnan Province (2013FC008) and the Yung-Chi Cheng Academician Workstation of Yunnan Provincial Academy of Science and Technology (2015IC017).Notes and references
- Y.-J. Qiao, J.-H. Shang, D. Wang, H.-T. Zhu, C.-R. Yang and Y.-J. Zhang, Nat. Prod. Bioprospect., 2018, 8, 245–263 CrossRef CAS PubMed.
- C.-R. Yang, Res. Pract. Chin. Med., 2015, 29, 83–86 Search PubMed.
- T. Wang, R.-X. Guo, G.-H. Zhou, X. Zhou, Z.-Z. Kou, S. Feng, C. Li, L.-Y. Tang and Z.-J. Wang, J. Ethnopharmacol., 2016, 188, 234–258 CrossRef CAS PubMed.
- J.-W. Lu, Yunnan Agriculture, 2016, 11, 28–30 Search PubMed.
- Z.-J. Chen, J. Zeng, Y. Wang, Y.-Q. Sun and G.-Q. Feng, J. Chin. Med. Mater., 2002, 25, 387–389 Search PubMed.
- E.-L. Rice, Allelopathy, Academic Press, New York, 2nd edn, 1984 Search PubMed.
- X.-F. Huang, Y. Li and W.-L. Ding, Seed, 2009, 28, 4–7 Search PubMed.
- M.-L. Wei, Y.-Q. Sun, T.-W. Huang, X.-M. Cui, Z.-J. Chen and B.-Y. Wang, Special Wild Economic Animal and Plant Research, 2010, 32, 32–34 Search PubMed.
- Y. Zhu, L. Yang, X.-M. Cui, Y.-Q. Sun, L.-P. Guo and L.-Q. Huang, Spec. Wild Econ. Anim. Plant Res., 2013, 2, 40–42 Search PubMed.
- P.-J. You, W.-Q. Wang, Y. Zhang, Z.-L. Zhang, Y.-X. Pang and X.-M. Cui, Southwest China Journal of Agricultural Sciences, 2009, 22, 308–310 Search PubMed.
- P.-J. You, Y. Zhang, W.-Q. Wang, Z.-L. Zhang, J.-Z. Yang and L.-M. Yin, Mod. Chin. Med., 2009, 11, 12–13 Search PubMed.
- K.-M.-M. Dakshini, On laboratory bioassays in allelopathy, Bot. Rev., 1995, 61, 28–44 CrossRef.
- J. Zhao, X.-D. Zhang, L.-C. Wang, L.-J. Zheng and Z.-Y. Wang, Chin. J. Microecol., 2018, 30, 146–149 Search PubMed.
- L. Zhu, N. Ma, X.-M. Cui, Y. Zhu, J.-M. Zhou, L.-P. Guo and L.-Q. Huang, Res. Pract. Chin. Med., 2014, 28, 3–5 CAS.
- L.-W. Sumner, A. Amberg, D. Barrett, M.-H. Beale, R. Beger, C.-A. Daykin, T.-W. Fan, O. Fiehn, R. Goodacre and J.-L. Griffin, Metabolomics, 2007, 3, 211–221 CrossRef CAS PubMed.
- J.-H. Liu, C.-S. Lee, K.-M. Leung, Z.-K. Yan, B.-H. Shen, Z.-Z. Zhao and Z.-H. Jiang, J. Agric. Food Chem., 2007, 55, 8830–8835 CrossRef CAS PubMed.
- J.-W. Wong, M.-K. Hennessy, D.-G. Hayward, A.-J. Krynitsky and I. Cassias andF.-J. Schenck, J. Agric. Food Chem., 2007, 55, 1117–1128 CrossRef CAS PubMed.
- S. Chan, M.-F. Kong, Y.-C. Wong, S.-K. Wong and D.-W.-M. Sin, J. Agric. Food Chem., 2007, 55, 3339–3345 CrossRef CAS PubMed.
- G.-X. Xie, Y.-P. Qiu, M.-F. Qiu, X.-F. Gao, Y.-M. Liu and W. Jia, J. Pharm. Biomed. Anal., 2007, 43, 920–925 CrossRef CAS PubMed.
- X.-G. Yang, T.-S. Liu, B. Chen, F.-Y. Li, S.-S. Chu and L.-M. Gong, Chinese Agricultural Science Bulletin, 2013, 29, 173–177 Search PubMed.
- J. Ju, M.-Z. Zhu, J.-R. Zhang and L.-Z. Xia, Chin. Hosp. Pharm. J., 2007, 27, 1076–1078 CAS.
- J. Zhang, D.-S. Yang, M.-X. Li and L.-X. Shi, PLoS One, 2016, 11, e0159622 CrossRef PubMed.
- R. Guo, L. Shi, C. Yang, C. Yan, X. Zhong, Q. Liu, X. Xia and H. Li, Front. Plant Sci., 2016, 7, 1–9 Search PubMed.
- H.-Z. Sun, B. Wang, J.-K. Wang, H.-Y. Liu and J.-X. Liu, J. Anim. Sci. Biotechnol., 2016, 7, 49 CrossRef PubMed.
- M.-A. Nemeth, Multi- and megavariate data analysis, Technometrics, 2003, 45, 362 CrossRef.
- D.-L. Massart, B.-G.-M. Vandeginste, S.-N. Deming, Y. Michotte and L. Kaufman, Chemometrics: a textbook, ed. B.-G.-M. Vandeginste and L. Kaufman, Elsevier, 1988, pp. 104–105 Search PubMed.
- C.-F. Ke, T. Zhang, X.-Y. Wu and K. Li, Chinese Journal of Health Statistics, 2014, 31, 357–365 Search PubMed.
- J.-Y. Aa, Chin. J. Clin. Pharmacol. Ther., 2010, 15, 481–489 Search PubMed.
- Z.-H. Zhang, S. Tao, B.-X. Ye, Z.-Q. Peng and J.-P. Yuan, Chin. J. Soil Sci., 2004, 35, 793–798 CAS.
- B.-L. Zhou, F. Chen, N. Liu, Q. Wu and B. Lu, Acta Agric. Boreali-Occident. Sin., 2010, 19, 179–183 Search PubMed.
- Q.-J. Zhang, A.-H. Zhang, J.-B. Sun and L.-X. Zhang, J. Ecol. Environ. Sci., 2012, 21, 187–193 Search PubMed.
- X.-X. Sun, H.-B. Wang, H.-B. He, J.-C. Lu and W.-X. Lin, Chin. J. Eco-Agric., 2007, 22, 806–812 Search PubMed.
- J.-M. Zhou, W.-B. Zhang and J.-Z. Yang, Res. Pract. Chin. Med., 2012, 26, 14–16 CAS.
- L.-J. Wu, J. Liu and W.-Y. Wang, World Science and Technology-Modernization of Traditional Chinese Medicine and Materia Medica, 2014, 4, 825–829 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: GC-MS total ion chromatograms (TIC) of the soil sample L-3I, relative peak area percentage of total (%) for 47 identified compounds in the soil samples, the sources of identified compounds in the collected soils of P. notoginseng, contribution of variances identified in the cultivated and uncultivated soils and compounds responsible for separation of cultivated and uncultivated soils. See DOI: 10.1039/c9ra02110h |
| This journal is © The Royal Society of Chemistry 2019 |