Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The potential of Thymus vulgaris aqueous extract to protect against delayed gastric emptying and colonic constipation in rats
Kaïs Rtibi *a,
Slimen Selmia,
Dalanda Wannesa,
Mourad Jridib,
Lamjed Marzoukia and
Hichem Sebaia
*a,
Slimen Selmia,
Dalanda Wannesa,
Mourad Jridib,
Lamjed Marzoukia and
Hichem Sebaia
aLaboratory of Functional Physiology and Valorization of Bio-resources-Higher Institute of Biotechnology of Beja, University of Jendouba, B. P. 382, 9000 Beja, Tunisia. E-mail: rtibikais@yahoo.fr; Fax: +216 72 590 566; Tel: +216 97 479 135
bLaboratory of Enzymatic Engineering and Microbiology, National School of Engineers of Sfax, University of Sfax, B. P. 1173, 3038 Sfax, Tunisia
First published on 2nd July 2019
Abstract
Thyme is a rich source of bioactive phytochemicals and it is frequently used in folk-medicine to treat gastroenteritis irritations. The current study was performed to examine the potential of Thymus vulgaris aqueous extract (TV-AE) to protect against delayed gastric emptying (DGE) and colonic constipation in rats. Stomach ulcer was caused by a single oral dose administration of indomethacin (INDO) (30 mg kg−1 of body weight). Constipation was induced following intravenous intoxication of rats with vinblastine (VINB) (2 mg kg−1 of body weight) for one week. The effect of TV-AE at two graduated doses (100 and 200 mg kg−1) on DGE, gastrointestinal transit (GIT) and constipated rats and biochemical parameters was estimated using phenol red, charcoal meal test and colorimetric methods, respectively. The phytochemical-profile of TV-AE was explored using high-performance liquid chromatography coupled with photodiode array detection and electrospray ionization-mass spectrometry (HPLC-PDA/ESI-MS). INDO and VINB caused a significant reduction in (P < 0.05) DGE and GIT and colonic motility dysfunction. TV-AE consumption remarkably (P < 0.05) attenuated the DGE (from 58.56% to 69.871%) and difficulty in evacuating stools (from 48.5 to 55.5 fecal pellets per rat) and balanced the GIT (65% to 71%). These GI-ameliorative effects were accompanied by reversed INDO/VINB-related oxidative changes, lipid-metabolism alteration and intracellular pathway moderation. HPLC-PDA/ESI-MS-analysis identified several chemical constituents including rosmarinic acid, quinic acid, luteolin-7-o-glucoside, protocatechuic acid and trans-cinnamic acid. Thus, TV-AE bioactive components may be used as medicinal substances to regulate/attenuate gastrointestinal–physiological activities and disturbances, which support its pharmacological use.
1. Introduction
Natural bioactive components are considered to be beneficial elements designed to improve human health.1,2 Plant products have the ability to transfer physiological benefits, including reduction of cancer risks, inflammatory states, cardiovascular diseases, diabetes and low-glycemic responses, neurodegenerative disorders, high blood cholesterol levels and reactive oxygen species and free radical overproduction.3Thymus vulgaris L., an aromatic herbaceous plant, is a medicinal herb belonging to the Lamiaceae family. It is commonly cultivated and used in many countries mainly as a food seasoning and a source of essential oil that are used in the food industry, in perfumery as a cosmetic and for medical purposes.4 In traditional medicine, thyme is usually used in folk remedy in the prevention/treatment of a variety of disorders such as gastroenteric and broncho-pulmonary disruptions.5 As reported by several researchers, thyme contains numerous phenolic compounds, especially thymol and carvacrol, which are found in its essential oil. Additionally, in wild-thyme, many other abundant phenolic compounds have been found such as caffeic and rosmarinic acid derivatives.6
Thymus vulgaris extract has different pharmacological activities, which were confirmed via in vivo and in vitro experiments, including immunomodulatory activity, anti-inflammatory effect, antibacterial ability, anthelmintic action, anti-oxidant activity, anti-thrombin capacity and potent antihypertensive ability. The therapeutic potential of Thymus vulgaris is based on its content of aliphatic phenols, flavonoids, thymol, carvacrol, and eugenol, in addition to luteolin and saponins.7–9
Functional gastrointestinal disorders (FGIDs) are defined by the perturbation of gastrointestinal tract sections such as gastroenteritis, ulcerative colitis, functional constipation and delayed gastric emptying (DGE). FGIDs, also known as disturbances of the gut–brain interaction, contain a number of separate idiopathic disruptions, which alter different regions of the GI-tract and involve visceral motility disorders related to an oxidative stress installation.10,11 Excessive ROS generation may contribute to cell injuries by lipid-protein-DNA oxidation in various tissues.12 In the GI tract, the cellular redox potential may even determine the fate of the differentiating cells. Wnt/β-catenin and notch signaling pathways are the major determinants of cell commitment for specialization. There may be a direct association between NADPH oxidase 1 (NOX-1)-dependent ROS fabrication, differential provocation of Wnt/β-catenin or notch signaling pathways, and intestinal proliferation. Modifications in the redox capacities of GSH/GSSG and cysteine/cystine (Cys/CySS) have been integrated with intestinal phenotypic transitions. The reducing abilities may favor cell proliferation, but oxidizing efficiencies can lead to cell growth reduction or inhibition.13,14
There are no published reports in the literature about the effect of the extract of T. vulgaris on gastric emptying and functional constipation. Based on these considerations, this study was designed to characterize the various TV-AE phenolic components and to investigate the potentiality of TV-AE to protect the GI tract against gastric delayed emptying and colonic constipation in rats.
2. Materials and methods
2.1. Chemicals
Clonidine, red phenol, NaCl, NaOH, charcoal meal, gum arabic, and haematoxylin/eosin were purchased from Sigma Chemical Co. (Sigma-Aldrich GmbH, Steinheim, Germany). Indomethacin and vinblastine were purchased from a local central pharmacy. All other reagents used were of analytical grade.2.2. TV-AE preparation
T. vulgaris leaves were collected from the region of Jendouba (North-West of Tunisia) during the season of spring (2017). Voucher specimens were identified and authenticated by a taxonomist in the Higher Institute of Biotechnology of Beja Tunisia and deposited at the herbarium in the Faculty of Sciences, University of El Manar, Tunisia. The leaves were dried at 40 °C in air, ground, and extracted with bi-distilled water. The obtained leaf extracts were dehydrated at the same temperature under vacuum and finally freeze dried.2.3. TV-AE bioactive compound identification by liquid chromatography-high-resolution electrospray ionization mass spectrometry (LC-HRESIMS) assay
100 mg of TV-AE was dissolved in 100 mL of 10% methanol, filtered and then 1 mL was added to LC-MS vials. A reverse-phase column (Pursuit XRs ULTRA 2.8, C18, 100 × 2 mm, Agilent Technologies, UK) was used to perform HPLC examinations. 20 mL of the sample was injected at a column temperature set at 30 °C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). A gradient program was used for separation at a flow rate of 1 mL min−1. The mobile phase consisted of an initial composition of 100% solvent A, with a gradient of 100% solvent B over 20 min, held at 100% solvent B for 5 min and 100% solvent A for 25 min. The drying gas flow rate was 1 mL min−1 at 320 °C. MS was operated in the positive ion mode in the mass range of 100–2000 m/z. High-resolution mass spectral data was obtained on a Thermo Instruments ESI-MS system (LTQ XL/LTQ Orbitrap Discovery, UK) connected to a Thermo Instruments HPLC system (Accela PDA Detector, Accela PDA Autosampler and Accela Pump).152.4. Animals
Male Wistar rats (180 and 220 g) were used at 13 weeks of age. The animals were obtained from the Society of Pharmaceutical Industries of Tunisia (SIPHAT, Ben-Arours, Tunisia), and isolated into different groups and acclimatized for two weeks with a standard pellet diet (standard pellet diet Badr-Utique-TN) and water ad libitum (22 ± 2 °C; 12 h dark per light cycle). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tunis University and approved by the Animal Ethics Committee of National Institute of Health.162.5. Experimental design
| Peristaltic index = (distance travelled by charcoal meal/length of small-intestine) × 100 |
| % of inhibition = mean distance travelled by the control − mean distance travelled by the test group/mean distance travelled by the control. |
After 1 h, the animals were anesthetized and euthanized. Their stomach and its contents were combined with 100 mL of 0.1 N NaOH. The supernatants were mixed with 4 mL of 0.5 N NaOH and the absorbance of the samples was read at 560 nm. Phenol red collected immediately from stomach organs after test meal administration was used as the standard (0% of GE).20
GE (%) was calculated according to the following formula:
| Gastric emptying rate (%) = (1 − absorbance of treated/absorbance of standard) × 100. |
Lipid oxidation caused by ROS and free radicals in the UG/UC rats led to MDA production, which was estimated with double heating technique adapted by Draper et al.21 The expression of the results was reported as the mean of MDA nmol mg−1 of proteins. Thiol group (–SH) content was determined using Ellman's procedure22 and the data was expressed as nmol of –SH per mg of proteins. Carbonyl residue generation after protein oxidation was examined using the method by Levine et al.23 The results were expressed as μmol carbonyl residue per mg protein.
Superoxide dismutase action was determined using the nicotinamide adenine dinucleotide (reduced) phenazine methosulphate nitroblue tetrazolium reaction system method.24 The results were calculated and expressed in units of SOD per mg proteins. Catalase (CAT) activity was estimated using the procedure by Aebi25 and the results were expressed as nmol min−1 mg−1 protein. GPx activity was analysed using the Flohé and Günzler design26 and the data was expressed as nmol GSH min−1 mg−1 protein.
Gastric and colonic mucosal H2O2 levels were determined according to the method by Dingeon et al.27 and the data was expressed as μmol of H2O2 per mg protein. Intracellular mediators (calcium and non-haem iron) levels were measured using commercial kits purchased from Biomaghreb, Tunisia.28,29 The results were expressed as nmol of these parameters per mg protein.
2.6. Statistical analysis
A one-way analysis of variance test was used to determine the significance between the different groups of all animals. Statistical analyses were calculated using StatView statistical software. The data is representative of six to eight distinct observations. Differences were stated as mean ± SEM and designated significant when the values of p were <0.05.3. Results and discussion
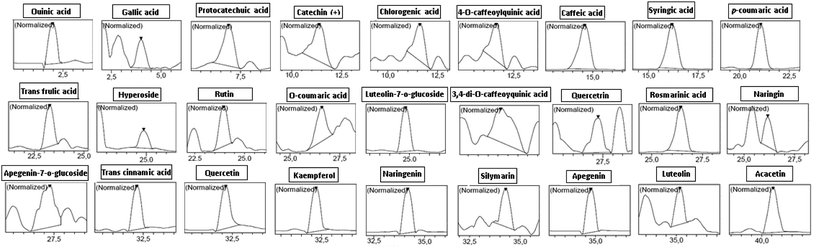
3.1. Phytochemical composition
It has been reported that thyme has been considered for long time as a good source of phenolic compounds.30 In this respect, HPLC-PDA/ESI-MS analysis revealed the presence of 27 phenolic-compounds in TV-AE, including rosmarinic acid, quinic acid, luteolin-7-o-glucoside, protocatechuic acid, trans cinnamic acid, caffeic acid, naringenin, kaempferol, quercetin, apigenin, rutin, 3,4-di-O-caffeoylquinic acid, syringic acid, p-coumaric acid, naringin, acacetin, hyperoside (quercetin-3-o-galactoside), quercetrin (quercetin-3-o-rhamonosid), gallic acid, trans ferulic acid, apigenin-7-o-glucoside, 4-O-caffeoylquinic acid, chlorogenic acid, silymarin, catechin(+), luteolin and o-coumaric acid (Fig. 1 and Table 1). Moreover, according to previous research, the phenolic profile of thyme obtained with HPLC-PDA/ESI-MS analysis showed diverse phenolic compounds such as rosmarinic acid, quinic acid, luteolin-7-O-glucoside, protocatechuic acid, trans cinnamic acid, caffeic acid, naringenin, kaempferol, quercetin, apigenin, rutin, 3,4-di-O-caffeoylquinic acid, syringic acid, p-coumaric acid, naringin, acacetin, hyperoside (quercetin-3-o-galactoside), quercetrin (quercetin-3-o-rhamonosid), gallic acid, trans ferulic acid, apigenin-7-o-glucoside, 4-O-caffeoylquinic acid, chlorogenic acid, silymarin, catechin(+), luteolin and o-coumaric acid.31 Importantly, many researchers (in vitro, in silico and in vivo) have proven the structure–activity relationship of bioactive compounds such as phenolic acids and flavonoids with their biological properties. In this respect, the bioactivity of these compounds depends mostly on the number of hydroxyl groups linked to the aromatic ring and their relative orientation in the molecular structure.32| Namea | Molecular formula | PubChem CID | [M]−Hb m/z | Retention time | Concentration (ppm) |
|---|---|---|---|---|---|
| a The compounds are suggested according to the dictionary of natural products and the characteristic fragmentation pattern.b The formulas were deduced from the quasi molecular ion peak [M + H]+. | |||||
| Quinic acid | C7H12O6 | 6508 | 191.00 | 1.978 | 364.647 |
| Gallic acid | C7H6O5 | 370 | 169.00 | 3.989 | 6.688 |
| Protocatechuic acid | C7H6O4 | 72 | 153.00 | 6.890 | 46.790 |
| Catechin(+) | C15H14O6 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
289.00 | 11.382 | 1.311 |
| Chlorogenic acid | C16H18O9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794 794![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
353.00 | 11.615 | 2.493 |
| 4-O-Caffeoylquinic acid | C16H18O9 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 281 281![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
353.00 | 11.612 | 2.558 |
| Caffeic acid | C9H8O4 | 689![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 043 |
179.00 | 14.603 | 37.468 |
| Syringic acid | C9H10O5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742 742 |
197.00 | 16.190 | 24.471 |
| p-Coumaric acid | C9H8O3 | 637![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542 |
163.00 | 21.061 | 17.003 |
| trans Ferulic acid | C10H10O4 | 445![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 858 858 |
193.00 | 23.276 | 3.501 |
| Hyperoside (quercetin-3-o-galactoside) | C21H20O12 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 281 281![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643 643 |
463.00 | 24.864 | 10.149 |
| Rutin | C27H30O16 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 805 805 |
609.00 | 23.941 | 28.653 |
| o-Coumaric acid | C9H8O3 | 637![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
163.00 | 26.598 | 1.258 |
| Luteolin-7-o-glucoside | C21H20O11 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637 637 |
447.00 | 24.800 | 46.983 |
| 3,4-Di-O-caffeoylquinic acid | C25H24O12 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 281 281![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
515.00 | 25.146 | 24.494 |
| Quercetrin (quercetin-3-o-rhamnoside) | C21H20O11 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939 |
447.00 | 27.267 | 7.219 |
| Rosmarinic acid | C18H16O8 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 315![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615 |
359.00 | 26.434 | 5066.278 |
| Naringin | C27H32O14 | 442![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
579.00 | 26.168 | 11.984 |
| Apigenin-7-o-glucoside | C21H20O10 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933 933![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926 |
431.00 | 27.291 | 3.393 |
| trans Cinnamic acid | C9H8O2 | 444![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539 539 |
147.00 | 32.184 | 41.109 |
| Quercetin | C15H10O7 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
301.00 | 32.175 | 35.730 |
| Kampherol | C15H10O6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863 |
285.00 | 32.218 | 35.972 |
| Naringenin | C15H12O5 | 439![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246 246 |
271.00 | 34.164 | 37.425 |
| Silymarin | C25H22O10 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553 553 |
481.00 | 34.290 | 1.547 |
| Apigenin | C15H10O5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 443 |
269.00 | 34.768 | 35.701 |
| Luteolin | C15H10O6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 445 445 |
285.00 | 35.175 | 1.270 |
| Acacetin | C16H12O5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442 442 |
283.00 | 40.566 | 11.362 |
3.2. TV-AE consumption accelerates GIT in healthy and constipated rats
Chemotherapy-induced constipation (CIC) is a persistent problem in the treatment of different types of cancer, and is among the primary contributors to dose decreases, delays and inefficient treatment.33 In the VINB rats, the overall gastrointestinal motor activity analysed by the progression of the test meal through the small-bowel was significantly (P < 0.05) reduced (65.12 ± 1.66%) compared to those in the healthy animals (70.65 ± 3.63%). However, TV-AE administration at both doses resulted in a significant (P < 0.05) improvement in gastrointestinal transit in both the healthy (77.92 ± 3.45 and 83.74 ± 2.88%, respectively) and VINB rats (68.90 ± 2.18 and 71.33 ± 3.46%, respectively) (Table 2). Furthermore, the rats receiving VINB showed several signs, including severe difficulty in evacuating stools, which was observed in the CON model compared with the healthy group. This effect was confirmed by the faecal parameter disorders noticed in the VINB group at day 5, including faecal pellets and water content diminution (48.64 ± 3.21 fecal pellets and 27.66 ± 2.63% water content) in comparison with the control-group (56.23 ± 3.00 fecal pellets and 39.08 ± 3.10% water content) (Table 3). These alterations were combined with histopathological-injuries,34,35 which provoked loss of fluidity/permeability. These eruptions were accompanied with equilibrium disruption of the absorption/secretion of water, electrolytes and nutrients, which led to diverse variations in the gastrointestinal physiological functions, such as GIT. TV-AE administration removed these disruptions, and thus allowed the construction of the mucosa, as shown by the histological study and the facilitation of intestinal and colonic contents advancement.36,37| Group | Dose | Gastro-intestinal motility (%) | Reduction activity (%) | Reinforcement (%) |
|---|---|---|---|---|
| a Rats were intoxicated with VINB (2 mg kg−1) for one week. Then, the animals were treated with two increasing doses of TV-AE or reference molecule (clonidine, 1 mg kg−1). 2 h later, the rats were given the standard charcoal meal (10% charcoal in 5% gum arabic) or vehicle (NaCl, 0.9%). Data are expressed as mean ± standard error (n = 7); *P < 0.05 versus control group, #P < 0.05 versus VINB-group. | ||||
| Control (NaCl) | (5 mL kg−1) | 70.65 ± 3.63 | — | — |
| Clonidine | (1 mg kg−1) | 28.23 ± 1.77* | 60.04 | — |
| VINB | (2 mg kg−1) | 65.12 ± 1.66* | 07.82 | — |
| TV-AE alone | (100 mg kg−1) | 77.92 ± 3.45*# | — | 10.29 |
| (200 mg kg−1) | 83.74 ± 2.88*# | — | 18.52 | |
| VINB/TV-AE | (100 mg kg−1) | 68.90 ± 2.18# | — | 5.80 |
| (200 mg kg−1) | 71.33 ± 3.46# | — | 09.53 | |
| Rat group | Fecal parameters on day 5 (collection for 24 h) | Gastric mucosa injury indicators | |||||
|---|---|---|---|---|---|---|---|
| Fecal pellet (n) | Wet weight (g/24 h/rat) | Dry weight (g/24 h/rat) | Water content (%) | Ulcer index | Gastric juice volume (mL) | Gastric juice pH | |
| a VINB/INDO alterations reflected by indicated parameters and assessed in healthy rats and rats treated with VINB (2 mg kg−1) or INDO (30 mg kg−1). Data are expressed as mean ± standard error (n = 7); *P < 0.05 versus control group, #P < 0.05 versus VINB/INDO groups. | |||||||
| CONT (NaCl) | 56.23 ± 3.00 | 6.77 ± 4.64 | 3.21 ± 0.33 | 39.08 ± 3.10 | 0.23 ± 0.04 | 2.04 ± 0.14 | 3.46 ± 0.09 |
| VINB (2 mg kg−1) | 48.64 ± 3.21* | 4.23 ± 0.21* | 2.22 ± 0.70* | 27.66 ± 2.63* | — | — | — |
| INDO (30 mg kg−1) | — | — | — | — | 19.77 ± 1.56 | 4.44 ± 0.16 | 2.31 ± 0.06 |
| TV-AE (100 mg kg−1) | 51.11 ± 2.90* | 4.93 ± 0.55* | 2.97 ± 0.61* | 31.17 ± 3.55* | 10.64 ± 1.20* | 2.62 ± 0.37*# | 2.87 ± 0.12*# |
| TV-AE (200 mg kg−1) | 55.50 ± 1.72# | 5.64 ± 0.81# | 3.24 ± 0.10# | 37.44 ± 2.85# | 3.00 ± 0.06* | 2.12 ± 0.22# | 3.72 ± 0.10# |
Actually, the exact mechanisms of the action of CIC remain unclear, but are believed to result from the association of intersecting processes, including GI dysmotility, secretory dysfunction, inflammation and alterations in GI innervation.33
TV-AE may contain many bioactive compounds that behave as purgatives/stimulate, which activates the gut musculature by acting on the nerve endings of the colon and causing movements that promote defecation. They also act on the walls of the intestine and increase mineral/liquid production, while lessening the absorption of sodium/chlorine.38
In addition, interstitial cells of Cajal (ICCs) appear to play crucial roles in the physiological GI motility modulation and act as the pacemaker of gastrointestinal movement. In this respect, we think that TV-AE can depolarize the membrane potentials of ICCs. This excitation may lead to smooth muscle cells via a gap junction. These last cells may respond to this depolarization with the activation of voltage-dependent Ca2+ channels.39 Therefore, this action of pacemaker potential depolarization may produce intensification of the intestinal motility rate. For this point, many recent research showed that diverse traditional herbal medicines such as Schisandra chinensis,40 Ge-Gen-Tang,41 Liriope platyphylla42 and Citrus unshiu43 demonstrated elicit excitatory/inhibitory effects on the ICC pacemaker activity, which supports the notion that ICCs are critical in the control of smooth muscle motility in the GI tract.
Direct assessments of calcium currents in single smooth muscle cells by the whole cell voltage clamp methods in the rat and mouse colons showed a 50–70% decrease in Ca2+ ion flux after an inflammatory response. Akbarali et al.44 established that in addition to the down-regulation of Ca2+ currents, the response to the ATP-sensitive K+ channel agonist lemakalim improved in the inflamed smooth muscle cells. Likewise, colonic irritation results in contracted contractions of colonic muscle strips in both human/animal models of colitis to depolarizing K+ solutions. In particular, recent studies indicated that transient receptor potential vanilloid-4 (TRPV4) activation in the GI tract induces intracellular calcium accumulation, chemokine release enhancement, and colitis reinforcement. In contrast, anti-inflammatory compounds significantly diminished TRPV4-expression in the upper-intestine.45,46
3.3. TV-AE consumption facilitates the emptying process in healthy and INDO group
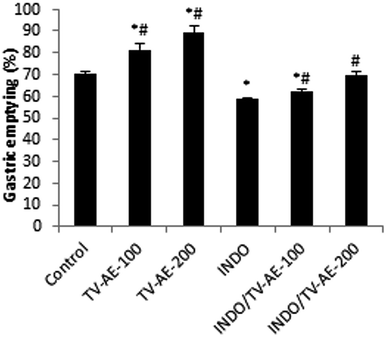
NSAIDs stimulate gastric ulcers via the suppression of prostaglandins, which are cytoprotective constituents to the gastric mucosal tissues, specifically due to the COX pathway disturbance of arachidonic acid metabolism, resulting in the massive generation of leukotrienes and other factors such as that of the 5-lipoxygenase enzyme metabolic pathway.47INDO administration at a dose of 30 mg kg−1 produced acute ulcerative gastritis in the animals. This damage was manifested by an increase in the ulcer index and gastric juice volume and a significant reduction in pH. These disruptive actions were accompanied with a delayed gastric emptying effect.17 TV-AE at both concentrations induced a significant (P < 0.05) acceleration of normal gastric emptying (81.52 ± 2.67% and 89.05 ± 3.44%, respectively) when the results are compared to that found in the healthy animals (70.25 ± 1.45%). In the ulcerative gastritis situation, the acquired data showed that the gastric emptying time analysed by the red phenol method strongly (P < 0.05) decreased in the gastric ulcer situation (58.56 ± 0.78%). In contrast, comparatively with the ulcerative gastritis group, TV-AE pre-treatment attenuated UG and remarkably accelerated (P < 0.05) the gastric emptying time (62.40 ± 0.99% and 69.87 ± 1.66%, respectively) (Fig. 2).
Natural small molecules produced from medicinal plants have been used for a long time to treat and prevent diverse ailments. Recently, the use of natural medicine in the treatment of various pathologies like peptic ulcer is an absolute requirement. Therefore, an alternative advance in recent days is the research of phytomedicaments serving as a tool in the prevention of peptic ulcers.48 Several phyto-components such as flavonoids, tannins, terpenoids, and saponin have been reported in distinct anti-ulcer findings as possible gastro-protective agents. Rosmarinic acid is known to be facilely absorbed from the gastrointestinal tract into the blood. It activates prostaglandin E2 generation, diminishes leukotriene B4 production in human leucocytes, and inhibits the complement system. It is also proven that caffeic acid and derivatives such as rosmarinic acid have therapeutic potential in the treatment/prevention of spasmogenic disruptions, peptic ulcers, and inflammatory disorders.49
3.4. TV-AE consumption relieves and ameliorates oxidative and antioxidative balance
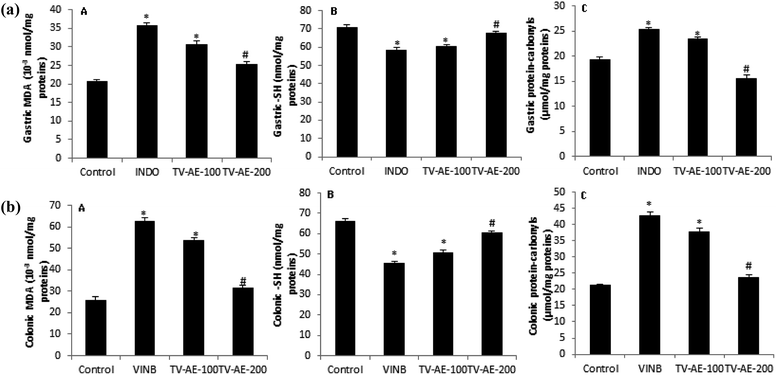
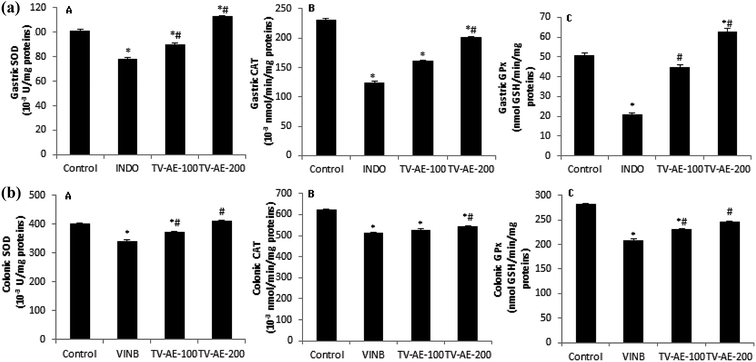
In both INDO/VINB groups, the obtained results showed an oxidative stress installation, which is related with an enzymatic antioxidant disruption following a massive ROS/free radical generation. These injuries were confirmed by the increase in MDA production and carbonyl protein formation in the UG/UC conditions, and associated with the depletion of enzyme activities, such as SOD, CAT and GPx. However, TV-AE consumption at both different doses (100 and 200 mg kg−1) relieved oxidative damage and ameliorated/stimulated the antioxidant profile (Fig. 3 and 4).A recent study clearly exhibited the participation of oxidative stress/inflammation in gastrointestinal problems such as ulcers and constipation.50 This contribution was checked by the augmentation of intracellular ROS production, which induces damage to lipids, proteins and DNA and inflammatory indicators. Iron catalyzes hydroxyl radical-mediated oxidative injury through its implication in the Fenton pathway, which leads to the reduction of this element in oxidative stress condition. Many authors showed that the phenolic compounds of thyme extract have greater antioxidant activity compared to α-tocophenol and butylated-hydroxylanisole. Rosmarinic acid (RA), the most important component of the thyme extract, has strong antioxidative activity since it acts as a free radical scavenger.51 Further research suggested other possible mechanisms for the protection of RA against lipid peroxidation, including the scavenging effect of numerous radical species, interaction with peroxyl-radicals and partitioning into the LDL particle and terminating chain reactions of oxidative degradation of lipids by scavenging lipid radicals.52 An alternative hypothesis is that antioxidant supplements modulate endogenous mechanisms that either decrease ROS production or increase the enzymes activities that decompose ROS.53
3.5. TV-AE consumption improves lipid metabolisms/intracellular mediators disruptions in intoxicated rats by drugs
To investigate the cell level damage and modifications in lipid metabolism, we studied the effect of drugs on lipidemia indicators as well as intracellular mediators. Accordingly, we observed a significant (P < 0.05) increase in H2O2 production in both eruptions, which was accompanied by a depletion of calcium and free iron. TG, TC and HDL cholesterol were significantly (P < 0.05) altered in the GU condition. However, the bioactive compounds and mineral constituents in TV-AE resulted in protection against all these disruptions (Table 4).| Group | Dose | H2O2 (μmol mg−1 protein) | Free iron (nmol mg−1 protein) | Calcium (10−3 nmol mg−1 protein) |
|---|---|---|---|---|
| a VINB/INDO/TV-AE effects were reflected by intracellular mediators assessed in healthy rats and rats treated with VINB (2 mg kg−1) or INDO (30 mg kg−1). Results are expressed as mean ± SEM; n = 7 in each group. Data was analyzed by Statview ANOVA. Data are expressed as mean ± standard error (n = 7); *P < 0.05 versus control group, #P < 0.05 versus VINB/INDO-groups. | ||||
| CONT (NaCl) | (5 mL kg−1) | 09.05 ± 0.33 | 11.34 ± 0.06 | 33.67 ± 3.57 |
| VINB | (2 mg kg−1) | 25.77 ± 2.13* | 7.04 ± 0.17* | 19.47 ± 2.07* |
| VINB/TV-AE | (100 mg kg−1) | 24.70 ± 2.11*# | 9.24 ± 0.21 | 22.99 ± 0.84* |
| (200 mg kg−1) | 11.98 ± 1.22# | 10.24 ± 0.06# | 28.90 ± 1.34# | |
| INDO | (30 mg kg−1) | 37.33 ± 2.29* | 04.28 ± 0.07* | 21.38 ± 1.01* |
| INDO/TV-AE | (100 mg kg−1) | 19.15 ± 1.37*# | 07.52 ± 0.20*# | 27.10 ± 0.74# |
| (200 mg kg−1) | 12.65 ± 0.93# | 09.35 ± 0.13# | 30.80 ± 2.21# | |
Previous studies demonstrated that RA exhibited substantial H2O2 scavenging action and inhibited H2O2-induced intracellular ROS generation.54 The data also showed that the lipid components such as triglyceride, total cholesterol, and HDL cholesterol contents were remarkably different among the major groups (Table 5). A recent study reported that many bioactive components such as gallic acid, catechin, and epicatechin have cholesterol-lowering activities by reducing pancreatic cholesterol esterase activity, binding of bile acids, and inhibiting solubility of cholesterol in micelles, which may result in delayed cholesterol absorption. These flavonoids may also reduce cholesterol absorption via interaction with cholesterol carriers and transporters across the brush border membrane in rats.55 Abdulkarimi et al.4 showed that thyme extract consumption decreased the plasma triglyceride, total cholesterol, LDL-c and VLDL-c, which in turn lowered the liver and abdominal lipids, and reduced the proportional liver and abdominal fat weights. These actions were exerted by the lower activity of the HMG-CoA reductase enzyme, diminished fat absorption from the gastrointestinal tract or the lipid catabolism for the gluconeogenesis process.
| Group | Dose | Triacylglycerol (mg dL−1) | Total cholesterol (mg dL−1) | HDL-cholesterol (mg dL−1) |
|---|---|---|---|---|
| a VINB/INDO/TV-AE effects were reflected by serum lipid metabolism assessed in healthy rats and rats treated with VINB (2 mg kg−1) or INDO (30 mg kg−1). Results are expressed as mean ± SEM; n = 7 in each group. Data was analyzed by Statview ANOVA. Data are expressed as mean ± standard error (n = 7); *P < 0.05 versus control group, #P < 0.05 versus VINB/INDO-groups. | ||||
| CONT (NaCl) | (5 mL kg−1) | 80.00 ± 5.28 | 61.34 ± 4.51 | 32.13 ± 2.45 |
| VINB | (2 mg kg−1) | 78.40 ± 6.81 | 62.03 ± 5.12 | 30.40 ± 2.30 |
| VINB/TV-AE | (100 mg kg−1) | 77.37 ± 4.02 | 61.45 ± 3.59 | 33.12 ± 0.93 |
| (200 mg kg−1) | 79.80 ± 2.87 | 60.23 ± 5.10 | 26.06 ± 1.21*# | |
| INDO | (30 mg kg−1) | 67.12 ± 3.50* | 78.22 ± 2.15* | 35.46 ± 1.57 |
| INDO/TV-AE | (100 mg kg−1) | 70.59 ± 4.21* | 75.68 ± 3.17* | 40.16 ± 3.55*# |
| (200 mg kg−1) | 81.35 ± 5.32# | 63.27 ± 4.22# | 44.86 ± 2.22*# | |
4. Conclusion
The current study demonstrated that TV-AE represents a source of bioactive functional components and may contribute an improvement in gastrointestinal physiological actions and their associated disorders in rats via various mechanisms. Consequently, this investigation supports the therapeutic potential and strong possibility of TV-AE as a functional food in phytomedicine, which should be further explored in clinical studies.Financial disclosures
None declared.Conflicts of interest
Only the authors are responsible for the content of this paper.Abbreviations
| CAT | Catalase |
| CIC | Chemotherapy induced constipation |
| CON | Constipation |
| COX | Cyclooxygenase |
| DGE | Delayed gastric emptying |
| FGID | Functional-gastrointestinal-disorders |
| GE | Gastric emptying |
| GET | Gastric emptying time |
| GIT | Gastrointestinal transit |
| GPx | Glutathione peroxidase |
| GU | Gastric ulcer |
| HDL | High density lipoproteins |
| HPLC-PDA/ESI-MS | Liquid chromatography-photodiode-array-mass spectrometry |
| ICCs | Interstitial cells of Cajal |
| INDO | Indomethacin |
| MDA | Malondialdehyde |
| NOX-1 | NADPH-oxidase 1 |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TG | Triglycerides |
| TRPV4 | Transient receptor potential vanilloid-4 |
| TV-AE | Thymus vulgaris aqueous extract |
| UC | Ulcerative colitis |
| UG | Ulcerative gastritis |
| VINB | Vinblastine |
Acknowledgements
Financial support of Tunisian Ministry of Higher Education and Scientific Research is gratefully acknowledged.References
- T. Lang, Functional foods, Br. Med. J., 2007, 334, 1015–1016 CrossRef PubMed.
- A. Santini, S. M. Cammarata, G. Capone, A. Ianaro, G. C. Tenore, L. Pani and E. Novellino, Nutraceuticals: opening the debate for a regulatory framework, Br. J. Clin. Pharmacol., 2018, 84, 659–672 CrossRef PubMed.
- C. Xu, R. Zhang and Z. Wen, Bioactive compounds and biological functions of sea cucumbers as potential functional foods, J. Funct. Foods, 2018, 49, 73–84 CrossRef CAS.
- R. Abdulkarimi, M. Daneshyar and A. Aghazadeh, Thyme (Thymus vulgaris) extract consumption darkens liver, lowers blood cholesterol, proportional liver and abdominal fat weights in broiler chickens, Ital. J. Anim. Sci., 2011, 10, e20 CrossRef.
- E. M. A. Dauqan and A. Abdullah, Medicinal and Functional Values of Thyme (Thymus vulgaris L.) Herb, J. Appl. Biol. Biotechnol., 2017, 5, 017–022 CAS.
- A. Komaki, F. Hoseini, S. Shahidi and N. Baharlouei, Study of the effect of extract of Thymus vulgaris on anxiety in male rats, J. Tradit. Complement. Med., 2016, 6, 257–261 CrossRef PubMed.
- H. G. D. Dorman and S. G. Deans, Antimicrobial Plants: Antibacterial Activity of Plant, Appl. Microbiol., 2000, 88, 308–316 CrossRef CAS.
- M. Khani, P. Motamedi, M. R. Dehkhoda, S. Dabagh-Nikukheslat and P. Karimi, Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats, J. Int. Soc. Sports Nutr., 2017, 14, 11 CrossRef PubMed.
- Z. Amirghofran, H. Ahmadi and M. H. Karimi, Immunomodulatory activity of the water extract of Thymus vulgaris, Thymus daenensis, and Zataria multiflora on dendritic cells and T cells responses, J. Immunoassay Immunochem., 2012, 33, 388–402 CrossRef CAS PubMed.
- D. A. Drossman, Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV, Gastroenterology, 2016, 150, 1262–1279 CrossRef PubMed.
- D. Liu, L. Gao, J. Zhang, X. Huo, H. Ni and L. Cao, Anti-inflammatory and Anti-oxidant Effects of Licorice Flavonoids on Ulcerative Colitis in Mouse Model, Chin. Herb. Med., 2017, 9, 358–368 CrossRef.
- Y. S. Bae, H. Oh, S. G. Rhee and Y. D. Yoo, Regulation of Reactive Oxygen Species Generation in Cell Signaling, Mol. Cell, 2011, 32, 491–509 CrossRef CAS PubMed.
- W. H. Watson, J. D. Ritzenthaler and J. Roman, Lung extracellular matrix and redox regulation, Redox Biol., 2016, 8, 305–315 CrossRef CAS PubMed.
- I. Khan, S. E. Samson and A. K. Grover, Antioxidant Supplements and Gastrointestinal Diseases: A Critical Appraisal, Med. Prin. Pract., 2017, 26, 201–217 CrossRef PubMed.
- N. Hajji, M.-A. Jabri, H. Tounsi, D. Wanes, I. Ben El Hadj Ali, A. Boulil, L. Marzouki and H. Sebai, Phytochemical analysis by HPLC-PDA/ESI-MS of Globularia alypum aqueous extract and mechanism of its protective effect on experimental colitis induced by acetic acid in rat, J. Funct. Foods, 2018, 47, 220–228 CrossRef CAS.
- National Research Council, Guide for the Care and the Use of Laboratory Animals, National Institute of Health, Bethesda, 1985, vol. 20, p. 85 Search PubMed.
- I. J. Oluwabunmi and T. Abiola, Gastroprotective effect of methanolic extract of Gomphrena celosioides on indomethacin induced gastric ulcer in Wistar albino rats, Int. J. Appl. Basic Med. Res., 2015, 5, 41–45 CrossRef PubMed.
- K. Rtibi, D. Grami, S. Selmi, M. Amri, H. Sebai and L. Marzouki, Vinblastine, an anticancer drug, causes constipation and oxidative stress as well as others disruptions in intestinal tract in rat, Toxicol. Rep., 2017, 4, 221–225 CrossRef CAS PubMed.
- K. Rtibi, S. Selmi, M. A. Jabri, G. Mamadou, N. Limas-Nzouzi, H. Sebai, J. El-Benna, L. Marzouki, B. Eto and M. Amri, Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice, RSC Adv., 2016, 6, 44345–44353 RSC.
- K. Rtibi, S. Selmi, K. Saidani, D. Grami, M. Amri, H. Sebai and L. Marzouki, Reverse effect of Opuntia ficus-indica L. juice and seeds aqueous extract on gastric emptying and small-bowel motility in rat, J. Food Sci., 2018, 83, 205–211 CrossRef CAS PubMed.
- H. H. Draper and M. Hadley, Malondialdehyde determination as index of lipid peroxidation, Methods Enzymol., 1990, 186, 421–431 CAS.
- G. E. Ellman, Tissue sulfhydryl groups, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS PubMed.
- L. R. Levine, D. Garland, C. N. Oliver, A. Amici, I. Climent, A. G. Lenz, B. W. Ahn, S. Shaltiel and E. R. Stadtman, Determination of carbonyl content in oxidatively modified proteins, Methods Enzymol., 1990, 186, 464–478 Search PubMed.
- P. Kakkar, B. Das and P. N. Viswanathan, Modified spectrophotometric assay of SOD, Indian J. Biochem. Biophys., 1984, 2, 130–132 Search PubMed.
- H. Aebi, Catalase, in Methods in Enzymatic Analysis, ed. H. U. Bergmeyer, Academic Press Inc., New York, 1974, pp. 673–686 Search PubMed.
- L. Flohé and W. A. Günzler, Assays of glutathione peroxidase, Methods Enzymol., 1984, 105, 114–121 Search PubMed.
- B. Dingeon, J. P. Ferry and A. Roullet, Auto analysis of blood sugar by Trinder's method, Ann. Biol. Clin., 1975, 33, 3–13 CAS.
- A. Leardi, M. Caraglia, C. Selleri, S. Pepe, C. Pizzi, R. Notaro, A. Fabbrocini, S. De Lorenzo, M. Musico, A. Abbruzzese, A. R. Bianco and P. Tagliaferri, Desferrioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells, Br. J. Haematol., 1998, 102, 746–752 CrossRef CAS PubMed.
- J. Stern and W. H. Lewis, The colorimetric estimation of calcium in serum with ocresolphthalein-complexone, Clin. Chim. Acta, 1957, 2, 576–580 CrossRef CAS.
- A. A. El-Nekeety, S. R. Mohamed, A. S. Hathout, N. S. Hassan, S. E. Aly and M. A. Abdel-Wahhab, Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats, Toxicon, 2011, 57, 984–991 CrossRef CAS PubMed.
- M. H. H. Roby, M. A. Sarhan, K. A. H. Selim and K. I. Khalel, Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts, Ind. Crops Prod., 2013, 43, 827–831 CrossRef CAS.
- D. L. Ambriz-Pérez, N. Leyva-López, E. P. Gutierrez-Grijalva and J. B. Heredia, Phenolic compounds: natural alternative in inflammation treatment a review, Cogent Food Agric., 2016, 2, 1131412 Search PubMed.
- M. M. Rachel, S. Vanesa, A. Raquel, C. B. Joel and N. Kulmira, Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments, Front. Pharmacol., 2016, 7, 414 Search PubMed.
- S. H. Han, K. Park, E. Y. Kim, S. H. Ahn, H. S. Lee and H. J. Suh, Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats, BMC Complementary Altern. Med., 2017, 17, 49 CrossRef PubMed.
- A. Jaja-Chimedza, B. L. Graf, C. Simmler, Y. Kim, P. Kuhn, G. F. Pauli and I. Raskin, Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract, PLoS One, 2017, 12, e0182658 CrossRef PubMed.
- S. Roth, M. R. Spalinger, C. Gottier, L. Biedermann, J. Zeitz, S. Lang, A. Weber, G. Rogler and M. Scharl, Bilberry-Derived Anthocyanins Modulate Cytokine Expression in the Intestine of Patients with Ulcerative Colitis, PLoS One, 2016, 11, e0154817 CrossRef PubMed.
- S. Sabiu and O. T. Ashafa, Toxicological implications and laxative potential of ethanol root extract of Morella serrata in loperamide-induced constipated Wistar rats, Pharm. Biol., 2016, 54, 2901–2908 CrossRef PubMed.
- P. Holzer, Opioid receptors in the gastrointestinal tract, Regul. Pept., 2009, 155, 11–17 CrossRef CAS PubMed.
- G. D. S. Hirst and F. R. Edwards, Role of Interstitial Cells of Cajal in the Control of Gastric Motility, J. Pharmacol. Sci., 2004, 96, 1–10 CrossRef CAS.
- T. S. Ahn, D. G. Kim, N. R. Hong, H. S. Park, H. Kim, K. T. Ha, J. H. Jeon, I. So and B. J. Kim, Effects of Schisandra chinensis extract on gastrointestinal motility in mice, J. Ethnopharmacol., 2015, 169, 163–169 CrossRef PubMed.
- S. Lee, H. Gim, J. H. Shim, K. H. Jung, J. R. Lee, S. C. Kim, Y. K. Kwon, K. T. Ha, I. So and B. J. Kim, The traditional herbal medicine, Ge-Gen-Tang, inhibits pacemaker potentials by nitric oxide/cGMP dependent ATP-sensitive K(+) channels in cultured interstitial cells of Cajal from mouse small intestine, J. Ethnopharmacol., 2015, 170, 201–209 CrossRef PubMed.
- H. J. Kim, S. Y. Park, D. G. Kim, S. H. Park, H. Lee, D. Y. Hwang, M. H. Jung, K. T. Ha and B. J. Kim, Effects of the roots of Liriope platyphylla Wang Et tang on gastrointestinal motility function, J. Ethnopharmacol., 2016, 26(184), 144–153 Search PubMed.
- J. H. Shim, S. J. Lee, H. Gim, H. J. Kim, T. Han, J. G. Kim, E. Y. Lim, Y. T. Kim and B. J. Kim, Regulation of the pacemaker activities in cultured interstitial cells of Cajal by Citrus unshiu peel extracts, Mol. Med. Rep., 2016, 14, 3908–3916 CrossRef CAS PubMed.
- H. I. Akbarali, E. G. Hawkins, G. R. Ross and M. Kang, Ion channel remodeling in gastrointestinal inflammation, Neurogastroenterol. Motil., 2010, 22, 1045–1055 CrossRef CAS PubMed.
- J. Fichna, A. Mokrowiecka, A. I. Cygankiewicz, P. K. Zakrzewski, E. Małecka-Panas, A. Janecka, W. M. Krajewska and M. A. Storr, Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment?, Neurogastroenterol. Motil., 2012, 24, e557–e560 CrossRef CAS PubMed.
- N. Vergnolle, TRPV4: new therapeutic target for inflammatory bowel diseases, Biochem. Pharmacol., 2014, 89, 157–161 CrossRef CAS PubMed.
- R. S. Pandian, C. V. Anuradha and P. Viswanathan, Gastroprotective effect of fenugreek seeds (Trigonella foenum-graecum) on experimental gastric ulcer in rats, J. Ethnopharmacol., 2002, 81, 393–397 CrossRef PubMed.
- D. Sasmal, S. Das and S. P. Basu, Phytoconstituents and therapeutic potential of Nyctanthes arbortristis Linn, Pharmacol. Rev., 2007, 1, 344–349 CAS.
- M. Petersen and M. S. Simmonds, Rosmarinic acid, Phytochemistry, 2003, 62, 121–125 CrossRef CAS PubMed.
- K. Rtibi, D. Grami, D. Wannes, S. Selmi, M. Amri, H. Sebai and L. Marzouki, Ficus carica aqueous extract alleviates delayed gastric emptying and recovers ulcerative colitis-enhanced acute functional gastrointestinal disorders in rats, J. Ethnopharmacol., 2018, 224, 242–249 CrossRef CAS PubMed.
- S. Maqsood, S. Benjakul, A. Abushelaibi and A. Alam, Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: a detailed review, Compr. Rev. Food Sci. Food Saf., 2014, 13, 1125–1140 CrossRef CAS.
- H. Ahmadvand, A. Khosrobeigi, L. Nemati, M. Boshtam, N. Jafari, R. H. Hosseini, Y. Pournia, H. Ahmadvand, A. Khosrobeigi, L. Nemati, M. Boshtam, N. Jafari, R. H. Hosseini and Y. Pournia, Rosmarinic acid prevents the oxidation of low density lipoprotein (LDL) in vitro, J. Biol. Sci., 2012, 12, 301–307 CrossRef CAS.
- H. J. Forman, K. J. Davies and F. Ursini, How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo, Free Radical Biol. Med., 2014, 66, 24–35 CrossRef CAS PubMed.
- H. J. Hahn, K. B. Kim, I. S. An, K. J. Ahn and H. J. Han, Protective effects of rosmarinic acid against hydrogen peroxide-induced cellular senescence and the inflammatory response in normal human dermal fibroblasts, Mol. Med. Rep., 2017, 16, 9763–9769 CrossRef CAS PubMed.
- S. Ngamukote, K. Mäkynen, T. Thilawech and S. Adisakwattana, Cholesterol-lowering activity of the major polyphenols in grape seed, Molecules, 2011, 16, 5054–5061 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |