Open Access Article
Open Access ArticleEffects of the combined pollution of cadmium, lead and zinc on the phytoextraction efficiency of ryegrass (Lolium perenne L.)
Jun Zhangab,
Ningning Yanga,
Yani Geng *a,
Jinhong Zhoua and
Ji Leia
*a,
Jinhong Zhoua and
Ji Leia
aShaanxi Key Laboratory of Disaster Monitoring and Mechanism Simulation, College of Geography and Environment, Baoji University of Arts and Sciences, Baoji 721013, P. R. China. E-mail: zhangjun1190@126.com; gyn1977@126.com
bKey Laboratory of Subsurface Hydrology and Ecological Effect in Arid Region of Ministry of Education, Chang'an University, Xi'an 710054, China
First published on 2nd July 2019
Abstract
The effects of cadmium (Cd), lead (Pb) and zinc (Zn) combined pollution on the phytoextraction efficiency of ryegrass (Lolium Perenne L.) were investigated in this work. Orthogonal experimental design was adopted in pot test (composition and interaction). The results showed that, with the increase of heavy metal concentration, the accumulation of elements in ryegrass was increased. The order of enrichment in root was Cd > Pb > Zn, was Zn > Pb > Cd in the stem and leaf, and the order of total EF was Cd > Zn > Pb. Ryegrass revealed the strongest enrichment effect on soil Cd and a strong ability to transfer Zn. Besides, ryegrass showed good potential in phytoextraction heavy metal Cd pollution and Cd × Zn combined pollution.
1 Introduction
Soil heavy metal pollution is often not caused by a single element pollutant, but by a variety of heavy metal (such as cadmium, lead, zinc, copper, and arsenic)1,2 pollutants and their interaction;3 studies have shown that the mechanism of action of combined pollution is extremely complex and usually related to the species and concentration of heavy metals.4,5 Moreover, the interaction between heavy metals has been manifested as synergistic, additive or antagonistic.6–8 At present, studies on the soil chemistry and phytoremediation of heavy metals mainly focus on a single heavy metal, and only few studies have been conducted on the effect of the interaction of multiple heavy metal complexes on the phytoremediation efficiency;9,10 thus, it is important to study the effect of soil heavy metal combined pollution on the phytoremediation efficiency of plants to further understand the interaction between different heavy metals in the soil.Considering the heavy metal complex pollution, the current study mainly focuses on the accumulation, enrichment, phytoextraction and interaction mechanism of heavy metals in plants. Studies have shown that the effect of heavy metal combined pollution on phytoremediation is related to the characteristics of heavy metals,11,12 plant factors and environmental factors.13,14 The main mechanism of interaction of combined heavy metal pollution are listed below, compete the adsorption point, activate the complex protease,15,16 interfere with the normal physiological and biochemical functions of plants, change cell structure and function, chelation or coprecipitation,17,18 interferes with the structural and functional absorption of plant biological macromolecules.19–22 Based on the abovementioned studies, significant progress has been made in the phytoremediation of soil heavy metal combined pollution; however, heavy metal combined pollution is not a simple addition of pollutions caused by single elements, and its effect on the phytoextraction efficiency is related to not only physical and chemical properties, species, concentration and proportion of heavy metals, but also the species and location of the plant and method and time.23,24
Ryegrass (Lolium perenne L.) is a fast growing, high biomass and drought-resistant forage grass widely grown in northern China.25–27 According to the literature, ryegrass shows a certain absorption capacity for cadmium, lead and zinc in soil and is a potential heavy metal-enriched plant.12 The species,28 growth status,20,29 enrichment site,30 physiological and biochemical characteristics31 and other factors of ryegrass show significant effects on the distribution of different heavy metals in soil-plant tissues32–34 as well as the absorption, accumulation and enrichment capacity of various heavy metals at different sites. Since ryegrass is a potential heavy metal-enriched plant, it is necessary to improve and regulate its phytoextraction ability under heavy metal combined pollution.
An orthogonal experiment is a scientific and effective method to design experiments by the mathematical statistics theory and the orthogonality principle to simplify experiments and obtain data.35–37 Due to the complexity and interactivity of the effects of the combined pollution of different heavy metals on the efficiency of ryegrass restoration, an orthogonal experimental design was adopted in this study. It not only retains the advantages of the traditional single or combined experimental design method, but also represents the effect of the interaction between combined and interactive pollution on the investigation target.
In this study, the heavy metals cadmium, lead and zinc, which had caused serious pollution in Shaanxi province, were selected as the sources of pollution. Ryegrass was taken as the pot experiment object, and combined and interactive orthogonal experimental methods were adopted to study the effects of different types and concentrations of heavy metals on the phytoextraction efficiency of ryegrass. Thus, this study can provide a theoretical and practical basis for understanding the effect of soil heavy metal combined pollution on the heavy metal phytoremediation mechanism of ryegrass.
2 Materials and methods
2.1 Test soil and plant
The tested soil was obtained from the loess of Sickle Bay area in North Shaanxi. After the collected soil samples are naturally air-dried, plant residues, stones and other debris are picked out, ground and then screened by 2 mm sieve. CO(NH2)2 (400 mg kg−1), KH2PO4 (200 mg kg−1) and K2SO4 (300 mg kg−1) were mixed thoroughly with the soil sample as the basal fertilizer. Moreover, Cd, Zn and Pb were added to the soil in the form of CdCl2·2.5H2O, Zn(NO3)2·6H2O and Pb(NO3)2 aqueous solutions, which were fully stirred and preserved. The concentrations of three kinds of heavy metals were in accordance with the Chinese soil environmental quality standard (GB 15618-2008).The total nitrogen (TN, salicylic acid method), total phosphorus (TP, sulfuric acid–perchloric acid heating digestion method), and organic matter content (OM, potassium dichromate method), pH value (potentiometry), cation exchange capacity (CEC, EDTA–ammonium salt method) and background values of the three metal ions in the tested soil were determined and are listed in Table 1.
| Indicators | TN/g kg−1 | TP/g kg−1 | OM/% | pH | CEC/cmol kg−1 | Background values/mg kg−1 | ||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | ||||||
| Values | 1.78 | 2.53 | 3.1 | 8.3 | 8.9 | 0.62 | 12.2 | 54.1 |
The tested plant was ryegrass, and the experiment was carried out between July 2017 and August 2017. The full-grain ryegrass seeds were selected, rinsed with deionized water and soaked for 24 hours before potting. The number of seeds was limited to about 30. The pot was placed in a place that had uniform illumination and ventilation, and the moisture content in the soil was maintained at 60%. After a week, the seeds in the pot sprouted; when the growth was stable, the plant and soil samples were obtained on the 42nd day for investigation.
The plant roots, root soils and plant leaves were dried, cut into 0.1 g samples and placed in a microwave digestion tank for digestion, and set constant volume. After passing the samples through a 0.45 μm filter membrane, ICP-MS (NexION 350X, PE, USA) was used to determine the heavy metal content of the samples. In the experiment, the quality control was carried out using the Chinese national standard soil sample (gss-25) and plant standard sample (GB07603), and the element recovery rate was controlled between 92 and 105%. Instrument quality control was performed using a standard solution (PE#: N9303837) supplied by PE with the error range of ±5%. Each sample was measured three times, and the relative standard deviation (RSD) of each heavy metal element was less than 10%. The reagents used in the experiment were of excellent grades.
2.2 Orthogonal experiment design
The orthogonal experiment design (OED) method is regarded as a modern approach to optimize and characterize experimental operation in many research areas. The orthogonal test method was adopted for test design and analysis in this study. It can reduce the number of tests, arrange the test methods scientifically and rationally and finally provide reliable test results. In this experiment, the number of heavy metal factors was 3 (Cd, Pb and Zn), and the level of each factor was 4 (different levels of concentration).Bioconcentration factor (BCF), translocation factor (TF) and extraction efficiency (EF) were selected as indicators. Factors and levels were designed as shown in Table 2. The orthogonal experiment is described as La (bc), where L stands for orthogonal design symbol, a is the number of experimental designs, b is the number of factor levels, and c is the number of factors. The combined effect of Cd, Zn and Pd on three phytoextraction indices was determined using the orthogonal table of 5 factors 4 level L16 (45). The 5 factors of the orthogonal table design could include 3 factors set in this experiment, the design level of the orthogonal table was 4, the orthogonal design was 16 experiments, and each experiment was repeated three times, i.e., a total of 48 pot experiments were performed. After analyzing the leading influence of the combined orthogonal experiment, three factors, i.e. Cd, Pd and Zn, were selected to rearrange the orthogonal interaction table of L8 (27) to investigate the influence of the interactions of Cd, Pb and Zn on the phytoextraction efficiency. The level of the interaction table design was 2. The orthogonal design of interaction was 8 experiments, and each experiment was repeated three times, i.e., a total of 24 pot experiments were performed. A total of 48 + 24 = 72 pot experiments were designed for the combined experiment and interactive experiment. The data obtained by the orthogonal experiment were analyzed by the SPSS software for variance (ANOVA) and significance of difference (Duncan, P < 0.05).
| Treatment levels | L16(45)/mg kg−1 | Treatment levels | L8(27)/mg kg−1 | ||||
|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd | Pb | Zn | ||
| Level 1 | 0 | 0 | 0 | Level 1 | 20 | 300 | 250 |
| Level 2 | 10 | 100 | 150 | Level 2 | 40 | 700 | 450 |
| Level 3 | 30 | 500 | 350 | ||||
| Level 4 | 50 | 900 | 550 | ||||
2.3 Phytoextraction efficiency evaluation factors
The phytoextraction efficiency of ryegrass was evaluated by three factors, i.e., BCF, TF and EF, as shown in the formula (1)–(3):38,39| BCF = Croot/Csoil | (1) |
| TF = Cshoot/Croot | (2) |
| EF = Cshoot/Csoil | (3) |
3 Discussion
3.1 Analysis of the effect of combined action
| Treatments | Heavy metal elements | Heavy metal content in root/mg kg−1 | Heavy metal content in stem and leaf/mg kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd | Pb | Zn | Cd | Pb | Zn | |
| T1 | 1 | 1 | 1 | 0.84k ± 0.01 | 4.78g ± 0.08 | 20.70k ± 0.31 | 0.04m ± 0.01 | 4.78h ± 0.02 | 3.41n ± 0.05 |
| T2 | 1 | 2 | 2 | 0.14m ± 0.00 | 32.56d ± 0.34 | 41.94g ± 0.51 | 0.02m ± 0.00 | 1.68k ± 0.02 | 5.48m ± 0.11 |
| T3 | 1 | 3 | 3 | 0.14m ± 0.00 | 27.36de ± 0.33 | 66.10e ± 0.48 | 0.02m ± 0.00 | 20.98d ± 0.28 | 29.09d ± 0.43 |
| T4 | 1 | 4 | 4 | 0.49l ± 0.01 | 50.82b ± 1.10 | 79.24c ± 0.73 | 0.12l ± 0.00 | 21.58c ± 0.23 | 22.09g ± 0.39 |
| T5 | 2 | 2 | 1 | 0.50l ± 0.10 | 20.90ef ± 0.22 | 13.43m ± 0.05 | 0.40j ± 0.01 | 1.90k ± 0.02 | 5.33m ± 0.07 |
| T6 | 2 | 1 | 2 | 3.04g ± 0.02 | 3.63g ± 0.01 | 32.73i ± 0.41 | 0.26k ± 0.00 | 0.78l ± 0.03 | 7.95l ± 0.08 |
| T7 | 2 | 4 | 3 | 1.63j ± 0.01 | 20.10ef ± 0.22 | 26.26j ± 0.41 | 0.66i ± 0.01 | 18.20e ± 0.23 | 17.63i ± 0.52 |
| T8 | 2 | 3 | 4 | 2.23i ± 0.02 | 17.19f ± 0.26 | 64.97e ± 1.11 | 1.69e ± 0.05 | 16.90f ± 0.43 | 52.26a ± 1.23 |
| T9 | 3 | 3 | 1 | 3.25g ± 0.29 | 41.63c ± 0.17 | 15.88l ± 0.23 | 1.95d ± 0.03 | 22.98a ± 0.33 | 16.60j ± 0.19 |
| T10 | 3 | 4 | 2 | 11.81c ± 0.11 | 30.49d ± 0.36 | 32.41i ± 0.52 | 2.59b ± 0.04 | 22.12b ± 0.26 | 25.25e ± 0.27 |
| T11 | 3 | 1 | 3 | 9.07d ± 0.10 | 2.19g ± 0.04 | 36.20h ± 0.82 | 1.46g ± 0.01 | 2.09k ± 0.03 | 42.45b ± 0.53 |
| T12 | 3 | 2 | 4 | 4.04f ± 0.15 | 19.04f ± 17.28 | 118.67a ± 3.03 | 1.21h ± 0.01 | 2.60j ± 0.05 | 20.88h ± 0.54 |
| T13 | 4 | 4 | 1 | 8.16e ± 0.02 | 79.68a ± 1.19 | 13.89m ± 0.24 | 1.22h ± 0.02 | 20.95d ± 0.34 | 9.74k ± 0.29 |
| T14 | 4 | 3 | 2 | 19.70a ± 0.36 | 27.79de ± 0.42 | 55.76f ± 1.23 | 2.21c ± 0.05 | 16.26g ± 0.41 | 23.71f ± 0.37 |
| T15 | 4 | 2 | 3 | 15.16b ± 0.02 | 5.79g ± 0.03 | 19.39k ± 0.28 | 1.59f ± 0.02 | 3.28i ± 0.02 | 20.80h ± 0.21 |
| T16 | 4 | 1 | 4 | 2.47h ± 0.17 | 5.71g ± 0.04 | 94.34b ± 2.59 | 3.24a ± 0.08 | 1.95k ± 0.05 | 36.91c ± 0.38 |
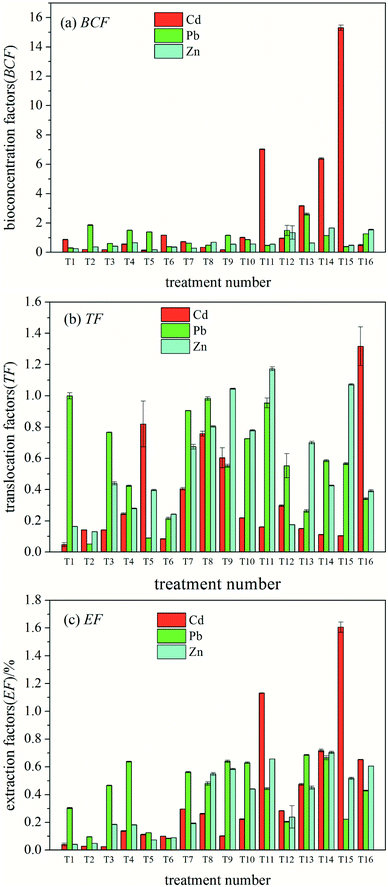
The overall TF of ryegrass increased with an increase in heavy metal concentration. Compared to the case of the control (T1), the order of the growth degree of the three heavy metal ions was Zn > Pb > Cd (Fig. 1(b)). The results show that among the exogenous heavy metals, Zn is more easily transferred to the ground of ryegrass and best absorbed by the stems and leaves of ryegrass.
As shown in Fig. 1(c), with an increase in heavy metal concentration, the EF of ryegrass towards three heavy metal ions increased as compared to that in the case of the control (T1), and the degree of growth was Cd > Zn > Pb. The results showed that among the three heavy metal ions, the efficiency of extracting Cd from ryegrass was highest.
The results of the variance analysis of the orthogonal test for Cd in the combined action are shown in Table 4. At the 95% confidence interval, Cd presented a significant influence on its own BCF and EF. Zn revealed a significant effect on the Cd EF (P < 0.05) and an insignificant effect on the BCF and TF of Cd. Pb did not show a significant effect on the phytoextraction factor of Cd.
| Sources of variation | Degrees of freedom | Sum of squared differences (Cd) | Significant (Cd) | ||||
|---|---|---|---|---|---|---|---|
| BCF | TF | η (%) | BCF | TF | η (%) | ||
| Cd | 3 | 42.92 | 0.14 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323.93 323.93 |
P < 0.05 | P < 0.05 | |
| Pb | 3 | 3.03 | 0.37 | 1435.22 | |||
| Zn | 3 | 2.25 | 0.80 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170.21 170.21 |
P < 0.05 | ||
| Errors | 3 | 14.76 | 0.08 | 3424.50 | |||
| Amount | 12 | ||||||
The orthogonal experiment for the heavy metal Pb in the combined action was performed via the variance analysis (Table 5). Pb had a significant impact on its own EF (P < 0.05). Moreover, the three factors did not show a significant influence on the BCF and TF of Pb. This indicates that the EF of Pb is mainly related to its own concentration and not affected by Zn and Cd.
| Sources of variation | Degrees of freedom | Sum of squared differences (Pb) | Significant (Pb) | ||||
|---|---|---|---|---|---|---|---|
| BCF | TF | η (%) | BCF | TF | η (%) | ||
| Cd | 3 | 1.29 | 0.09 | 1290.90 | |||
| Pb | 3 | 1.34 | 0.61 | 6344.73 | P < 0.05 | ||
| Zn | 3 | 1.93 | 0.00 | 149.92 | |||
| Errors | 3 | 1.36 | 0.76 | 499.64 | |||
| Amount | 12 | ||||||
Similarly, the results of Zn variance analysis are shown in Table 6. Cd demonstrated a significant effect on the BCF and EF of Zn (P < 0.05), and no significant influence on other factors. This shows that the BCF and EF of Zn are mainly affected by Cd.
| Sources of variation | Degrees of freedom | Sum of squared differences (Zn) | Significant (Zn) | ||||
|---|---|---|---|---|---|---|---|
| BCF | TF | η (%) | BCF | TF | η (%) | ||
| Cd | 3 | 1.26 | 2.51 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493.88 493.88 |
P < 0.05 | P < 0.05 | |
| Pb | 3 | 0.21 | 2.04 | 6973.62 | |||
| Zn | 3 | 1.02 | 0.55 | 1241.55 | |||
| Errors | 3 | 0.45 | 2.00 | 4362.46 | |||
| Amount | 12 | ||||||
3.2 Analysis of the effect of interaction
| Treatments | Heavy metal elements | Heavy metal content in root/mg kg−1 | Heavy metal content in stem and leaf/mg kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd | Pb | Zn | Cd | Pb | Zn | |
| S1 | 1 | 1 | 1 | 5.56f ± 0.23 | 30.63f ± 0.03 | 35.20h ± 0.76 | 7.42a ± 0.19 | 27.50b ± 0.80 | 58.76b ± 1.26 |
| S2 | 1 | 2 | 1 | 4.23g ± 0.10 | 39.64d ± 0.59 | 94.47b ± 1.00 | 2.78d ± 0.00 | 14.30e ± 0.17 | 45.29e ± 0.51 |
| S3 | 1 | 1 | 2 | 1.85h ± 0.01 | 33.99e ± 0.48 | 38.78g ± 0.47 | 1.14e ± 0.02 | 9.54f ± 0.12 | 31.68h ± 0.33 |
| S4 | 1 | 2 | 2 | 12.55c ± 0.26 | 31.54f ± 0.72 | 81.77c ± 1.05 | 1.19e ± 0.01 | 27.87b ± 0.47 | 37.99f ± 0.38 |
| S5 | 2 | 1 | 1 | 15.11b ± 0.10 | 43.62c ± 0.29 | 73.00e ± 0.77 | 2.73d ± 0.03 | 10.20f ± 0.07 | 35.58g ± 0.63 |
| S6 | 2 | 2 | 1 | 10.00d ± 0.24 | 54.34a ± 0.64 | 76.42d ± 0.78 | 5.16b ± 0.02 | 15.02d ± 0.16 | 55.63c ± 1.63 |
| S7 | 2 | 1 | 2 | 9.09e ± 0.09 | 10.31g ± 0.15 | 65.64f ± 0.68 | 5.03b ± 0.10 | 17.76c ± 0.53 | 68.01a ± 0.96 |
| S8 | 2 | 2 | 2 | 33.10a ± 0.56 | 50.53b ± 0.78 | 105.57a ± 1.17 | 4.86c ± 0.02 | 31.79a ± 0.20 | 51.04d ± 0.22 |
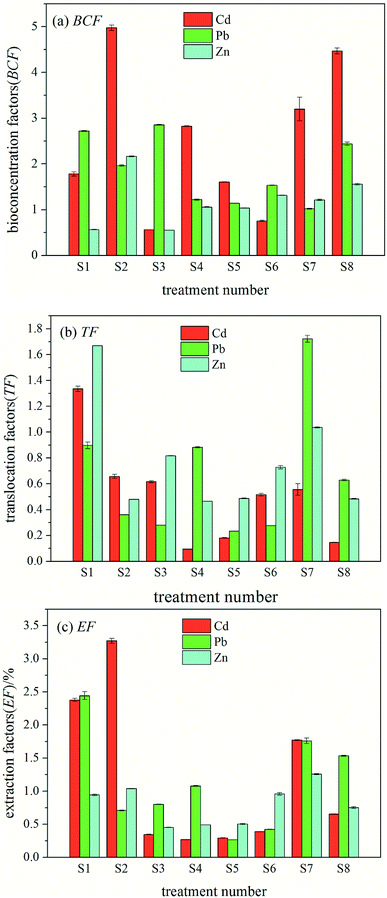
The variance analysis of the impact of cadmium in the orthogonal experiment of interaction is shown in Table 8. At the 95% confidence interval, Zn and Cd × Zn showed significant effects on the EF of Cd (P < 0.05), Cd × Pb and Zn × Pb showed an effect on the EF of Cd (P < 0.1), and the sources of variation had no significant effect on other phytoextraction factors. The variance analysis of Pb and Zn interaction showed that there was no significant difference between the impacts of each heavy metal ion on the three phytoextraction factors; therefore, the interaction variance analysis of Pb and Zn is not listed in Table 8.
| Sources of variation | Degrees of freedom | Sum of squared differences | Significant | ||||
|---|---|---|---|---|---|---|---|
| BCF | TF | η (%) | BCF | TF | η (%) | ||
| Cd | 1 | 0.002 | 0.215 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470.942 470.942 |
|||
| Zn | 1 | 0.470 | 0.000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533.415 533.415 |
P < 0.05 | ||
| Cd × Zn | 1 | 9.418 | 0.208 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575.818 575.818 |
P < 0.05 | ||
| Pb | 1 | 4.322 | 0.000 | 51.308 | |||
| Cd × Pb | 1 | 3.200 | 0.160 | 4201.694 | P < 0.1 | ||
| Zn × Pb | 1 | 0.180 | 0.041 | 5960.136 | P < 0.1 | ||
| Errors | 1 | 1.170 | 0.312 | 76.385 | |||
| Amount | 7 | ||||||
4 Discussion
4.1 The main effects of Cd, Pb and Zn on the phytoextraction efficiency of ryegrass
The main effect analysis selected a series of heavy metal pollution factors (different concentrations of the same contaminant) as the research object in the orthogonal experimental results to make phytoextraction factor change curve with heavy metal concentration as shown in Fig. 3(a)–(c), and the contents of the other two pollution factors were fixed in the selected data. This process can be used to determine the effect of the concentration of a single pollution factor on the restoration of ryegrass and does not require repeated impact experiments of individual factors.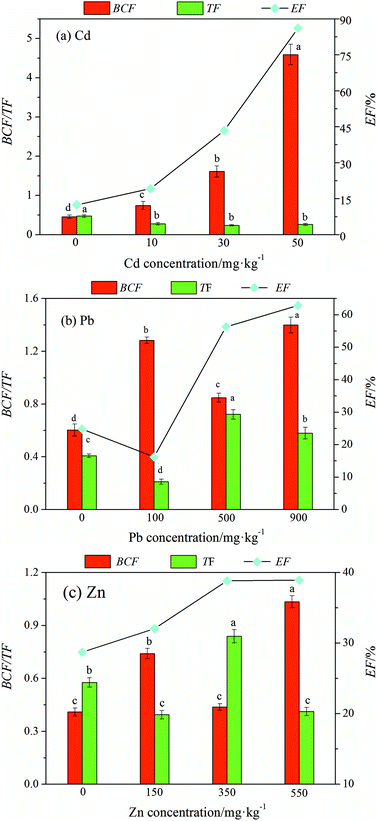 | ||
| Fig. 3 The three phytoextraction factors varied with the content of heavy metal elements (Cd, Pb, and Zn) in soil. | ||
As shown in Fig. 3(a), the BCF and EF increased with an increase in the cadmium concentration and the TF decreased; however, there was no significant difference among the concentrations. When the Cd concentration was 50 mg kg−1, the BCF and EF reached a maximum, which was 9.18 times and 8.21 times that of the control, respectively.
As shown in Fig. 3(b), the Pb phytoextraction factor fluctuated with an increase in lead concentration. The BCF and EF were maximum when the Pb concentration was 900 mg kg−1, which was 2.33 times and 3.49 times that of the control, respectively. Overall, Pb had no significant effect on the phytoextraction efficiency.
As shown in Fig. 3(c) the BCF of Zn showed an upward trend except that it decreased when the zinc concentration was 350 mg kg−1. The TF fluctuated with an increase in the Zn concentration. Moreover, compared with the case of the control, the EF increased with an increase in Zn concentration, and the maximum value was 1.34 times that of the control, indicating that the change in Zn concentration presented a certain impact on the EF.
4.2 Regression analysis of the ryegrass elemental accumulation by combined action
The results of the multiple regression equation of the effect of cadmium, lead and zinc on the element accumulation in ryegrass are listed in Table 9. It can be seen from the multiple regression equation of Cd that Cd shows a positive correlation with its own BCF, whereas Pb and Zn present a slight influence on the BCF of Cd. In addition, cadmium, lead and zinc had no effect on the TF of Cd, and cadmium was positively correlated with its own EF. As can be seen from the multiple regression equation of the lead element, lead had a significant influence on its EF, whereas other factors had no significant influence on its phytoextraction factor. According to the multiple regression equation of the zinc element, cadmium had a significant influence on the BCF and EF of Zn, whereas other heavy metals did not have a significant influence on the phytoextraction factor of Zn. In general, this is consistent with the results of the orthogonal test variance analysis of the combined effects of cadmium, lead and zinc.| Phytoextraction factors | The regression equations | The correlation coefficients | |
|---|---|---|---|
| Cd | BCF | YBCF = 0.118 + 0.08(Cd2+) + 0.001(Pb2+) − 0.001(Zn2+) | 0.804 |
| TF | YTF = 0.238 − 0.003(Cd2+) | 0.375 | |
| EF | Yη = 8.206 + 1.474(Cd2+) + 0.027(Pb2+) − 0.042(Zn2+) | 0.704 | |
| Pb | BCF | YBCF = 0.274 + 0.015(Cd2+) + 0.001(Pb2+) + 0.001(Zn2+) | 0.633 |
| TF | YTF = 0.365 + 0.002(Cd2+) | 0.342 | |
| EF | Yη = 4.653 + 0.441(Cd2+) + 0.008(Pb2+) + 0.085(Zn2+) | 0.871 | |
| Zn | BCF | YBCF = 0.187 + 0.014(Cd2+) | 0.747 |
| TF | YTF = 0.601 + 0.016(Cd2+) + 0.001(Pb2+) + 0.001(Zn2+) | 0.489 | |
| EF | Yη = 12.798 + 1.872(Cd2+) + 0.022(Pb2+) + 0.023(Zn2+) | 0.805 | |
The phytoextraction efficiency of ryegrass can be affected by the heavy metal combined pollution.11,21 In this study, it was found that under the combined pollution of cadmium, lead and zinc, the enrichment capacity of cadmium in ryegrass roots was higher than that of lead and zinc, and the BCF increased significantly with an increase in cadmium concentration. This may be because cadmium is most active in soil, exists in a water soluble and exchangeable state, and has a strong migration capacity. Moreover, the physical and chemical properties of cadmium and zinc are similar, and the atomic radius is close; furthermore, zinc can reduce the toxicity of cadmium towards ryegrass, increase the enrichment of cadmium in the roots of ryegrass, and promote the absorption and accumulation of cadmium in ryegrass. However, due to the different physical and chemical properties of lead and cadmium, lead is easily absorbed by soil and loses its activity. Therefore, lead presented a slight effect on the accumulation of cadmium and zinc in ryegrass. These findings are consistent with the results reported in the literature.20,40
The interaction of heavy metal elements is related to the absorption, transport, distribution, accumulation and physiological activity of elements in plants. Some elements (such as zinc) can alleviate the toxic effects of heavy metals in plants, thereby increasing the ability of plants to accumulate other heavy metals.33,34,41 This study showed that the concentration of zinc in ryegrass was much higher than that of cadmium and lead. It is suggested that ryegrass has the potential to enrich zinc under the interaction effect; this may be because zinc is an essential nutrient element for plant growth, and it is a component or auxiliary of many important enzymes in plants, which can effectively cooperate or antagonize the absorption of other heavy metals and change the absorption intensity of heavy metals in plants.
The variance analysis of interaction orthogonal experiment of Cd showed that Zn and Cd × Zn had significant effects on the EF of Cd (P < 0.05), Cd × Pb and Zn × Pb had significant effects on the EF of Cd (P < 0.1), and Cd and Pb had no significant effect on the EF of Cd. This may be because there is a competitive relationship between cadmium and zinc as well as a synergistic effect. When the concentrations of Cd and Zn are close, the synergy between them becomes the dominant effect, and ryegrass shows certain differences in absorption and enrichment according to their concentration ratio. When the difference between the cadmium and zinc concentrations was greater, the accumulation of cadmium and zinc was inhibited by ryegrass. When the concentrations of Cd and Zn were close, a synergistic effect of ryegrass on the enrichment of Cd and Zn was observed. This is consistent with the results reported in the literature.30 In conclusion, the degree of inhibition or promotion of the restoration efficiency of ryegrass varied with the species, concentration and interaction treatment of heavy metals. This is related to the comprehensive influence of heavy metal-added quality fraction, pollutant factors, biological factors and environmental factors on the environmental effects of the combined pollution.
5 Conclusions
(1) Under the complexation of Cd, Pb and Zn, the accumulation of elements in ryegrass increased with an increase in exogenous heavy metal concentration; the order of root enrichment was Cd > Pb > Zn, the order of stem and leaf absorption was Zn > Pb > Cd, and the order of total extraction efficiencies of the elements was Cd > Zn > Pb. Moreover, ryegrass represented strong enrichment and absorption capacity for cadmium and zinc. When Cd was 50 mg kg−1, the ryegrass roots represented largest adsorption capacity, and the enrichment was 23.45 times that of the control group. When Zn was 550 mg kg−1, the uptake of ryegrass reached maximum value, which was 15.33 times that of the control group.(2) Under the combined action of Cd, Pb and Zn, Zn had a significant effect on the EF of Cd, and Cd had a significant effect on the BCF and EF of Zn; Zn could reduce the toxicity of Cd towards ryegrass and promote the absorption and accumulation of Cd in ryegrass; the maximum enrichment coefficient and extraction efficiency of ryegrass for Cd were 15.3 and 1.6 times that of the control group, respectively. Cd can activate Zn in soil and promote the enrichment and extraction of Zn by ryegrass. The maximum enrichment coefficient of Zn in ryegrass was 1.54, and the maximum transfer coefficient was 1.17.
(3) Under the interaction of Cd, Pb and Zn, Zn and Cd × Zn showed significant effects on the EF of Cd in ryegrass (P < 0.05). The maximum extraction efficiency coefficient of ryegrass for Cd was 1.6. Cd × Pb and Zn × Pb had an effect on the EF of Cd in ryegrass (P < 0.1). The interaction had no significant effect on the phytoremediation efficiency of Zn and Pb.
Funding
This work was supported by the Program of National Natural Science Foundation of China (41771215), the Open Fund of Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region (Ministry of Education) (No. 310829151140), and the Open Fund of Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region (Ministry of Education) (No. 310829151141).Conflicts of interest
There are no conflicts to declare.References
- B. L. Clabeaux, D. A. Navarro, D. S. Aga and M. A. Bisson, Ecotoxicol. Environ. Saf., 2013, 98, 236–243 CrossRef CAS PubMed.
- X. Zhai, Z. Li, B. Huang, N. Luo, M. Huang, Q. Zhang and G. Zeng, Sci. Total Environ., 2018, 635, 92–99 CrossRef CAS PubMed.
- O. V. Singh, S. Labana, G. Pandey, R. Budhiraja and R. K. Jain, Appl. Microbiol. Biotechnol., 2003, 61, 405–412 CrossRef CAS PubMed.
- R. D. Harter and R. Naidu, in Advances in Agronomy, ed. D. L. Sparks, Academic Press, 1995, vol. 55, pp. 219–263 Search PubMed.
- S. Willscher, L. Jablonski, Z. Fona, R. Rahmi and J. Wittig, Hydrometallurgy, 2017, 168, 153–158 CrossRef CAS.
- Z. J. Xu, C. H. Wu, X. Y. Qiu and H. Zhang, J. Soil Water Conserv., 2007, 1–6 Search PubMed.
- X. Pan, F. C. Shi, L. M. Liu, M. W. Cai and F. C. Liu, J. Bot., 2012, 32, 717–723 CAS.
- N. Sooksawat, M. Meetam, M. Kruatrachue, P. Pokethitiyook and K. Nathalang, J. Environ. Sci., 2013, 25, 596–604 CrossRef CAS.
- K. Mahdavian, S. M. Ghaderian and M. Torkzadeh-Mahani, J. Soils Sediments, 2015, 1–11 Search PubMed.
- M. Poniedziałek, A. Sekara, E. Jedrszczyk and J. Ciura, Folia Hortic., 2010, 22, 25–31 Search PubMed.
- A. Bernardini, E. Salvatori, V. Guerrini, L. Fusaro, S. Canepari and F. Manes, Int. J. Phytorem., 2016, 18, 16–24 CrossRef CAS PubMed.
- S. Khalid, M. Shahid, N. K. Niazi, B. Murtaza, I. Bibi and C. Dumat, J. Geochem. Explor., 2017, 182, 247–268 CrossRef CAS.
- X. W. Liu, Y. Li, Z. R. Nan, Z. J. Zhao, H. X. Ding and S. L. Wang, J. Lanzhou Univ., Nat. Sci., 2009, 45, 1–6 CAS.
- J. F. Zhu, M. H. Li, P. J. Xie and Y. L. Qiao, Chin. J. Eco-Agric., 2018, 26, 303–313 Search PubMed.
- X. Yang, Y. Feng, Z. He and P. J. Stoffella, J. Trace Elem. Med. Biol., 2005, 18, 339–353 CrossRef CAS PubMed.
- L. B. Zhou, Q. Wu and G. L. Gao, Appl. Mech. Mater., 2012, 209–211, 1116–1119 Search PubMed.
- F. P. C. Blamey, D. C. Joyce, D. G. Edwards and C. J. Asher, Plant Soil, 1986, 91, 171–180 CrossRef CAS.
- P. Rojjanateeranaj, C. Sangthong and B. Prapagdee, Chemosphere, 2017, 185, 764–771 CrossRef CAS PubMed.
- G. F. Koopmans, P. F. A. M. Römkens, M. J. Fokkema, J. Song, Y. M. Luo, J. Japenga and F. J. Zhao, Environ. Pollut., 2008, 156, 905–914 CrossRef CAS PubMed.
- Y. Zhang, Z. G. Tian, C. L. Cao, J. C. Liu and J. Q. Kang, J. Agro-Environ. Sci., 2010, 29, 2080–2086 CAS.
- Y. Y. Sun, P. Guan, S. He and J. M. Shi, Pratacult. Sci., 2016, 33, 1589–1597 Search PubMed.
- M. J. He, H. R. Shen, Z. T. Li, L. Wang, F. Wang, K. L. Zhao, X. M. Liu, O. Wendroth and J. M. Xu, Environ. Pollut., 2019, 244, 431–439 CrossRef CAS PubMed.
- S. Y. Lin and Y. B. Feng, Environ. Eng., 2017, 35, 168–173 Search PubMed.
- N. Bolan, A. Kunhikrishna, R. Thangarajan, J. Kumpiene, J. Park, T. Makino, M. B. Kirkham and K. Scheckel, J. Hazard. Mater., 2014, 266, 141–166 CrossRef CAS PubMed.
- H. Xie, L. Zhu and J. Wang, Environ. Sci. Pollut. Res., 2018, 1–11 Search PubMed.
- G. Vigliotta, S. Matrella, A. Cicatelli, F. Guarino and S. Castiglione, J. Environ. Manage., 2016, 179, 93–102 CrossRef CAS PubMed.
- R. J. Reid, J. D. Brookes, M. A. Tester and F. A. Smith, Planta, 1996, 198, 39–45 CrossRef CAS.
- P. Feng, L. Sun, X. H. Shen, C. Jiang, R. L. Li, Z. J. Li, H. Y. Zheng, H. Zhang, W. Gou, X. D. Han and Y. N. Hong, Acta Prataculturae Sinica, 2016, 25, 153–162 Search PubMed.
- A. K. Salama, K. A. Osman and A. R. Gouda, Int. J. Phytorem., 2016, 18, 364–367 CrossRef CAS PubMed.
- W. H. Xu, Z. T. Xiong, H. X. Wang, Y. R. Li, J. Z. Liu and W. Y. Li, J. Soil Water Conserv., 2005, 32–35 Search PubMed.
- J. Zhang, W. K. Wang, Y. N. Geng, X. Y. Ren, Z. F. Wang and S. M. Cao, J. Agro-Environ. Sci., 2018, 37, 1117–1124 Search PubMed.
- A. Mahar, P. Wang, A. Ali, M. K. Awasthi, A. H. Lahori, Q. Wang, R. Li and Z. Zhang, Ecotoxicol. Environ. Saf., 2016, 126, 111–121 CrossRef CAS PubMed.
- C. B. Tabelin, T. Igarashi, M. Villacorte-Tabelin, I. Park, E. M. Opiso, M. Ito and N. Hiroyoshi, Sci. Total Environ., 2018, 645, 1522–1553 CrossRef CAS PubMed.
- J. Guo, R. Feng, Y. Ding and R. Wang, J. Environ. Manage., 2014, 141, 1–8 CrossRef CAS PubMed.
- Y. Lee, J. J. Filliben, R. J. Micheals and P. Jonathon Phillips, Comput. Vis. Image Underst., 2013, 117, 532–550 CrossRef.
- J. Tang, G. Gong, H. Su, F. Wu and C. Herman, Appl. Energy, 2016, 169, 696–708 CrossRef.
- D. S. Sui and Z. S. Cui, Appl. Energy, 2009, 22, 13–21 Search PubMed.
- J. Yoon, X. Cao, Q. Zhou and L. Q. Ma, Sci. Total Environ., 2006, 368, 456–464 CrossRef CAS PubMed.
- A. S. Reza Hesami and S. M. Ghaderian, Environ. Sci. Pollut. Res., 2018, 25, 1–14 Search PubMed.
- Z. Yang, W. Wang, B. W. Li, Y. J. Guo and H. X. Wang, J. Soil Water Conserv., 2008, 83–87 Search PubMed.
- M. I. Dar, I. D. Green, M. I. Naikoo, F. A. Khan, A. A. Ansari and M. I. Lone, Sci. Total Environ., 2017, 584–585, 1221–1229 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |