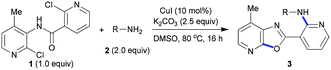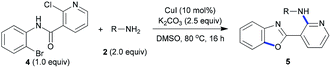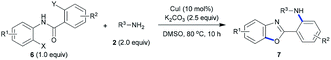Open Access Article
Open Access ArticleCopper-catalyzed synthesis of 2-aminopyridylbenzoxazoles via domino reactions of intermolecular N-arylation and intramolecular O-arylation†
Ju-You Lu *
*
Laboratory of Green Catalysis and Reaction Engineering of Haikou, Hainan Provincial Fine Chemical Engineering Research Center, School of Chemical Engineering and Technology, Hainan University, Haikou 570228, China. E-mail: lujy@hainanu.edu.cn
First published on 1st May 2019
Abstract
A simple and general approach to nitrogen-containing heterocycles via copper-catalyzed domino reaction has been developed, and the corresponding 2-aminopyridylbenzoxazole derivatives were obtained in good to excellent yields using the readily available starting materials. This method possesses unique step economy features, and is of high tolerance towards various functional groups in the substrates.
Introduction
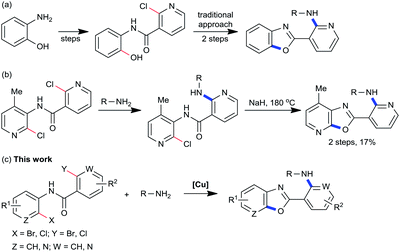
Nitrogen-containing heterocycles are ubiquitous subunits of a variety of biologically active substances,1 and they have been assigned as privileged structures in drug discovery.2 The 2-aminopyridylbenzoxazole derivatives have attracted much attention for their wide applications as enzyme inhibitors,3 activators,4 and fluorescence sensors.5 The development of new methods and strategies for the direct formation of several C-heteroatom bonds is an ongoing subject for organic chemists. The C-heteroatom reaction has played a prominent role in this context due to its suitability for complex structure formation, and featured in dozens of syntheses.6 Despite many attempts to improve the efficiency and practicality of the synthesis of benzoxazoles, the majority of conditions still require stepwise formation (such as 4 steps),3a and use extra additives (such as polyphosphoric acid),3b or elevated temperature.4 Additionally, it is commonplace that costly 2-aminophenol substrates are required to access the 2-halo-N-(2-hydroxyphenyl)nicotinamides employed in these procedures (Scheme 1a).4 Another approach to benzoxazole derivatives involves the oxidative cyclization of bisaryloxime ethers.7 Herein, a simple method is disclosed wherein domino reactions of readily available 2-halo-N-(2-haloaryl)nicotinamides with amines are performed well under mild conditions.Copper-catalyzed domino reactions, through the incorporation of several distinct transformations into one single sequence, are one of the most powerful synthetic tools in modern organic chemistry.8 Their application to the construction of N-heterocycles has been reported by us9 and other research groups.10 Recently, research efforts in this laboratory have explored the utility of 2-halo-N-(2-halophenyl)benzamide and 2-(2-halophenyl)benzoimidazole derivatives as starting materials in a variety of domino C-heteroatom reactions, including C–C/C–N formation,11 C–N/C–N formation,12 and C–C/C–N/C–N formation.13 The success of these domino processes prompted the investigation of amines as suitable reagents towards forging C–N/C–O bonds. The development of such a reaction was motivated by several useful amines that are extremely laborious to access.14 An illustrative example is depicted in Scheme 1b, wherein an 2-aminopyridylbenzoxazole derivative was accessed from a 2-halo-N-(2-haloaryl)nicotinamide in two steps.15 Unfortunately, the 2-aminopyridylbenzoxazole product was isolated in only 17% yield. In contrast, a mild domino C-heteroatom strategy could dramatically simplify the pathway to 2-aminopyridylbenzoxazoles because the in situ nicotinamide group could be viewed as a directing group for C–N formation. This method would provide convenient access to 2-aminopyridylbenzoxazole derivatives without addition of any ligands or additives (Scheme 1c).
Results and discussion
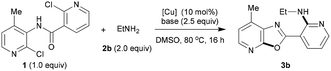
As shown in Table 1, 2-chloro-N-(2-chloro-4-methylpyridin-3-yl)nicotinamide (1) and ethanamine (2b) were chosen as the model substrates to optimize reaction conditions including catalysts, and bases under nitrogen atmosphere. Compound 1 was prepared from ortho-haloarylamines and acyl chlorides.16 Previously, we demonstrated that a combination of weak coordination from the heteroatom directing group of the substrate on the Cu centre could accelerate C(sp2)–X activation.11–13 We therefore began screening of copper catalysts in the presence of 2.5 equiv. of K2CO3 as base, and DMSO as solvent at 80 °C. To our delight, when we introduced copper powder into the reaction mixture, we obtained the desired reaction product in 40% yield (entry 1). Further screening of copper catalysts revealed that Cu(I) gave a higher yield (entries 2–9). For example, the CuCl2 gave a yield of 63%, but the CuCl improved the yields to 72%. In normal copper(I) promoted reactions, the ease of introduction of a halogen from the copper salts follows the order Cl > Br > I. CuI provided the highest yield because of the reduced possibility of the iodide anion interfering with the desired reaction.17 The control experiment carried out in the absence of a copper catalyst gave no target product (entry 10). Encouraged by these results, we proceeded to optimize the reaction conditions using CuI as catalyst, and found that the use of mild bases, such as potassium salts, was crucial to the reactivity. In general, the usefulness of bases may be attributed to their good solubility and ionization ability in organic solvents.18 Among the bases screened, carbonates were found to be optimal, with 2.5 equiv. K2CO3 affording the highest yield of 82% (compare entries 4, 11–13). Further comprehensive screening data are presented in the ESI.†| Entry | [Cu] | Base | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: 1 (0.5 mmol), 2b (1 mmol), catalyst (0.05 mmol), base (1.25 mmol), DMSO (2 mL), 80 °C, 16 h in a Schlenk tube under nitrogen atmosphere.b Isolated yield.c Cu2O (0.025 mmol).d In the absence of catalyst. DMSO = dimethylsulfoxide. | |||
| 1 | Cu | K2CO3 | 40 |
| 2 | CuCl | K2CO3 | 72 |
| 3 | CuBr | K2CO3 | 75 |
| 4 | CuI | K2CO3 | 82 |
| 5 | Cu2O | K2CO3 | 65c |
| 6 | CuCl2 | K2CO3 | 63 |
| 7 | CuO | K2CO3 | 60 |
| 8 | CuSO4 | K2CO3 | 54 |
| 9 | Cu(OAc)2 | K2CO3 | 65 |
| 10 | — | K2CO3 | 0d |
| 11 | CuI | Na2CO3 | 54 |
| 12 | CuI | Cs2CO3 | 38 |
| 13 | CuI | K3PO4 | 46 |
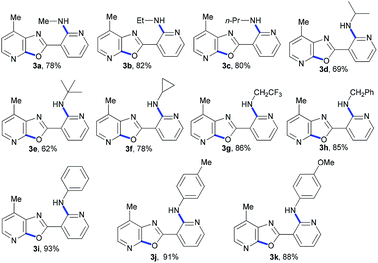
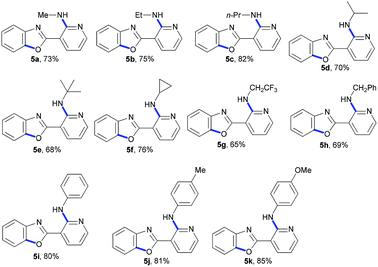
Under optimal conditions, we examined the scope of copper-catalyzed domino C-heteroatom reaction of 2-chloro-N-(2-chloro-4-methylpyridin-3-yl)nicotinamide (1) with amines (2). As shown in Table 2, the tested substrates afforded good to excellent yields. The domino C-heteroatom reactions did not need addition of any additional ligand and additive which exhibited ortho-substituent effect of amide group in 1 during N-arylation. In the previous copper-catalyzed reactions, aryl chlorides were weak substrates, and the results also showed the ortho-substituent effect. A variety of amines were tested, aliphatic and aromatic amines all gave good yields. In general, the electronic properties of amines did not have much influence on the reaction, as both electron-donating and electron-withdrawing groups were well tolerated. As expected, the electron deficient 2,2,2-trifluoroethanamine underwent domino C-heteroatom reaction giving good isolated yield (3g). Notably, the presence of MeO substituent in products (3k) allows for further functionalization of the aromatic ring. A challenging problem in this domino C-heteroatom processes is the combination of 1 with sterically hindered amine substrates. To our delight, the domino reaction with isopropylamine proceeded smoothly and product 3d was obtained in moderate yield. Remarkably, the highly hindered tert-butylamine could be coupled under these optimal conditions affording the corresponding product 3e in 62% yield. In addition, the high-strain molecule cyclopropylamine was also proved to be suitable substrate, indicating a broad scope of amines.
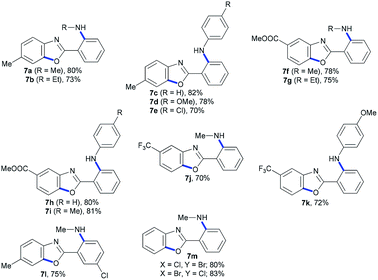
We next extended the scope of 2-halo-N-(2-haloaryl)nicotinamide substrates under the standard conditions. Interestingly, N-(2-bromophenyl)-2-chloronicotinamide (4) (Table 3) and 2-halo-N-(2-halophenyl)benzamide (6) (Table 4) also proceeded smoothly, and various 2-aminopyridylbenzoxazoles were synthesized in good to excellent yields. The results showed that our method was of wide application for construction of nitrogen-containing heterocycles. The copper-catalyzed domino C-heteroatom reaction of 2-halo-N-(2-haloaryl)nicotinamides with amines access to 2-aminopyridylbenzoxazoles could tolerate various functional groups including ester, ether, CF3 group, C–Cl bond, and benzyl group.
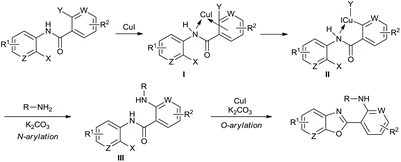
In the domino reactions above, no ligand or additive were required, and the result showed ortho-substituent effect of nicotinamide group.19 Therefore, a possible mechanism for synthesis of 2-aminopyridylbenzoxazole derivatives was proposed in Scheme 2. Firstly, coordination of 2-halo-N-(2-haloaryl)nicotinamide with CuI gives I, and oxidative addition of I leads to II. Intermolecular N-Arylation of II with amine provides intermediate III, and the intramolecular O-Arylation of III affords the target product.
Conclusions
In conclusion, we have developed a simple and general process for 2-aminopyridylbenzoxazole derivatives via copper-catalyzed domino reaction. The approach possesses unique step economy features, and uses the readily available substituted 2-halo-N-(2-haloaryl)nicotinamides or 2-halo-N-(2-halophenyl)benzamides and amines as the starting materials, inexpensives CuI as the catalyst, no any ligand and additive was required, and the corresponding 2-aminopyridylbenzoxazoles were obtained in good to excellent yields. This protocol is of high tolerance towards various functional groups in the substrates. Therefore, the method will attract much attention in academic and industrial researches. Further details of the mechanism and application of this methodology is being pursued in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Scientific Research Fund Project of Hainan University (KYQD(2R)1958), National Natural Science Foundation of China (21403163) and Young Technology Stars Project in Shaanxi Province of China (2018KJXX-048) for financial support.Notes and references
- R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473 CrossRef CAS PubMed.
- P. D. Leeson and B. Springthorpe, Nat. Rev. Drug Discovery, 2007, 6, 881 CrossRef CAS PubMed.
- (a) S. Y. Cho, S. Han, J. D. Ha, J. W. Ryu, C. O. Lee, H. Jung, N. S. Kang, H. R. Kim, J. S. Koh and J. Lee, Bioorg. Med. Chem. Lett., 2010, 20, 4223 CrossRef CAS PubMed; (b) J. Lee, S. Han, H. Jung, J. Yang, J. Choi, C. H. Chae, C. H. Park, S. U. Choi, K. Lee, J. D. Ha, C. O. Lee, J. W. Ryu, H. R. Kim, J. S. Koh and S. Y. Cho, Bioorg. Med. Chem. Lett., 2012, 22, 4044 CrossRef CAS PubMed; (c) S. Y. Cho, B. H. Lee, H. Jung, C. S. Yun, J. D. Ha, H. R. Kim, C. H. Chae, J. H. Lee, H. W. Seo and K. Oh, Bioorg. Med. Chem. Lett., 2013, 23, 6711 CrossRef CAS PubMed.
- J. E. Bemis, C. B. Vu, R. Xie, J. J. Nunes, P. Y. Ng, J. S. Disch, J. C. Milne, D. P. Carney, A. V. Lynch, L. Jin, J. J. Smith, S. Lavu, A. Iffland, M. R. Jirousek and R. B. Perni, Bioorg. Med. Chem. Lett., 2009, 19, 2350 CrossRef CAS PubMed.
- (a) S. C. Liang, H. Wang, Z. M. Zhang and H. S. Zhang, Anal. Bioanal. Chem., 2005, 381, 1095 CrossRef CAS PubMed; (b) Y. Wu, X. Peng, J. Fan, S. Gao, M. Tian, J. Zhao and S. Sun, J. Org. Chem., 2007, 72, 62 CrossRef CAS PubMed.
- For selected examples, see: (a) R. B. Bedford, Chem. Commun., 2003, 15, 1787 RSC; (b) A. S. Kashin and V. P. Ananikov, J. Org. Chem., 2013, 78, 11117 CrossRef CAS PubMed; (c) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC; (d) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922 RSC; (e) V. Ritleng, M. Henrion and M. J. Chetcuti, ACS Catal., 2016, 6, 890 CrossRef CAS; (f) J. Muzart, Tetrahedron, 2013, 69, 6735 CrossRef CAS; (g) M. Drusan and R. Sebesta, Tetrahedron, 2014, 70, 759 CrossRef CAS.
- M. M. Guru, M. A. Ali and T. Punniyamurthy, Org. Lett., 2011, 13, 1194 CrossRef CAS PubMed.
- For recent reviews on copper-catalyzed domino reactions, see: (a) S. Hassan and T. J. J. Müller, Adv. Synth. Catal., 2015, 357, 617 CrossRef CAS; (b) X. Zeng, Chem. Rev., 2013, 113, 6864 CrossRef CAS PubMed; (c) G. C. Tsui and M. Lautens, Angew. Chem., Int. Ed., 2012, 51, 5400 CrossRef CAS PubMed; (d) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (e) T. Piou, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 3760 CrossRef CAS PubMed; (f) S. Cai, F. Wang and C. Xi, J. Org. Chem., 2012, 77, 2331 CrossRef CAS PubMed; (g) Z. Galeštoková and R. Šebesta, Eur. J. Org. Chem., 2012, 6688 CrossRef; (h) T. Liu and H. Fu, Synthesis, 2012, 44, 2805 CrossRef CAS; (i) H. Rao and H. Fu, Synlett, 2011, 745 CAS; (j) S. G. Newman, J. K. Howell, N. Nicolaus and M. Lautens, J. Am. Chem. Soc., 2011, 133, 14916 CrossRef CAS PubMed; (k) Y. Liu and J. Wan, Org. Biomol. Chem., 2011, 9, 6873 RSC; (l) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC.
- For selected examples, see: (a) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., 2009, 121, 354 (Angew. Chem., Int. Ed., 2009, 48, 348) CrossRef; (b) D. Yang, H. Liu, H. Yang, H. Fu, L. Hu, Y. Jiang and Y. Zhao, Adv. Synth. Catal., 2009, 351, 1999 CrossRef CAS; (c) X. Yang, H. Fu, R. Qiao, Y. Jiang and Y. Zhao, Adv. Synth. Catal., 2010, 352, 1033 CrossRef CAS; (d) X. Gong, H. Yang, H. Liu, Y. Jiang, Y. Zhao and H. Fu, Org. Lett., 2010, 12, 3128 CrossRef CAS PubMed; (e) C. Huang, Y. Fu, H. Fu, Y. Jiang and Y. Zhao, Chem. Commun., 2008, 6333 RSC; (f) D. Yang, H. Fu, L. Hu, Y. Jiang and Y. Zhao, J. Org. Chem., 2008, 73, 7841 CrossRef CAS PubMed; (g) F. Wang, H. Liu, H. Fu, Y. Jiang and Y. Zhao, Org. Lett., 2009, 11, 2469 CrossRef CAS PubMed; (h) H. Zhao, H. Fu and R. Qiao, J. Org. Chem., 2010, 75, 3311 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Liu, L. Xu and Y. Wei, J. Org. Chem., 2019, 84, 1596 CrossRef CAS PubMed; (b) J. Xiong, G. Zhong and Y. Liu, Adv. Synth. Catal., 2019, 361, 550 CAS; (c) C. Xu, S. Jiang, Y. Wu, F. Jia and A. Wu, J. Org. Chem., 2018, 83, 14802 CrossRef CAS PubMed; (d) A. K. Panday, R. Mishra, A. Jana, T. Parvin and L. H. Choudhury, J. Org. Chem., 2018, 83, 3624 CrossRef CAS PubMed; (e) V. Kavala, Z. Yang, A. Konala, T. Yang, C. Kuo, J. Ruan and C.-F. Yao, Eur. J. Org. Chem., 2018, 1241 CrossRef CAS; (f) C. J. Ball, J. Gilmore and M. C. Willis, Angew. Chem., 2012, 124, 5816 (Angew. Chem., Int. Ed., 2012, 51, 5718) CrossRef; (g) J. Li, S. Bénard, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 5980 CrossRef CAS PubMed.
- J.-Y. Lu and H. Fu, J. Org. Chem., 2011, 76, 4600 CrossRef CAS PubMed.
- S. Xu, J.-Y. Lu and H. Fu, Chem. Commun., 2011, 47, 5596 RSC.
- J.-Y. Lu, X. Gong, H. Yang and H. Fu, Chem. Commun., 2010, 46, 4172 RSC.
- V. Kavala, D. Janreddy, M. J. Raihan, C. Kuo, C. Ramesh and C.-F. Yao, Adv. Synth. Catal., 2012, 354, 2229 CrossRef CAS.
- S. Bernard, D. Defoy, Y. L. Dory and K. Klarskov, Bioorg. Med. Chem. Lett., 2009, 19, 6127 CrossRef CAS PubMed.
- G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802 CrossRef CAS PubMed.
- (a) J. Lindley, Tetrahedron, 1984, 40, 1433 CrossRef CAS; (b) J. H. Clark and C. W. Jones, J. Chem. Soc., Chem. Commun., 1987, 18, 1409 RSC.
- C. Yang, Y. Fu, Y. Huang, J. Yi, Q. Guo and L. Liu, Angew. Chem., Int. Ed., 2009, 48, 7398 CrossRef CAS PubMed.
- (a) T. Kotipalli, V. Kavala, D. Janreddy, C. Kuo, T. Kuo, H. Huang, C. He and C.-F. Yao, RSC Adv., 2014, 4, 2274 RSC; (b) A. Modi, W. Ali, P. R. Mohanta, N. Khatun and B. K. Patel, ACS Sustainable Chem. Eng., 2015, 3, 2582 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01908a |
| This journal is © The Royal Society of Chemistry 2019 |