Open Access Article
Open Access ArticleRetracted Article: Structural characterization of Mesobuthus martensii Karsch peptides and anti-inflammatory potency evaluation in human vascular endothelial cells
Man Zhenga,
Xiafeng Yana,
Fanli Bua,
Fenglei Zhanga,
Zhenhua Lia,
Jiali Cuib,
Jie liu *c and
Minya Dong*a
*c and
Minya Dong*a
aDepartment of Cardiology, Dongying People's Hospital, Dongying, Shandong 257091, China. E-mail: myddongying@126.com; Fax: +86-755-86671907; Tel: +86-755-86671907
bPeople's Hospital of Shule County, Kashi, Xinjiang 844043, China
cThe Research Center of Allergy & Immunology, School of Medicine, Shenzhen University, Shenzhen, 518060, China. E-mail: jnuoligopeptide@sina.com; Fax: +86-755-86671905; Tel: +86-755-86671905
First published on 20th June 2019
Abstract
Studies have reported that scorpion toxins have excellent anti-cancer effects; however, the anti-inflammatory activity of scorpion peptides has rarely been studied. Here, a series of Mesobuthus martensii Karsch peptides (MMKPs) were isolated and the amino acid sequence was identified. The MMKPs mitigated TNF-α-mediated inflammation in human umbilical vein endothelial cells (HUVECs). The results showed that MMKP-1 (His-Glu-Gly-His) treatment (43.0 μM) significantly attenuated the reactive oxygen species (ROS) generation and mitochondrial membrane potential collapse in HUVECs. Moreover, MMKP-1 down-regulated the intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expressions and blocked the NF-κB pathway to alleviate the damage caused by TNF-α. Of note, our study provides a good reference for the anti-inflammation research on scorpion oligopeptides.
1 Introduction
Atherosclerosis, a disease of the large arteries, is the primary cause of heart disease and stroke. In westernized societies, it is the underlying cause of about 50% of all deaths. Endothelial cell damage is a fatal factor in serious cardiovascular diseases, including atherosclerosis, which can cause serious clinical consequences such as myocardial infarction, heart failure and stroke.1–3 Studies have reported that atherosclerosis is closely related to the inflammatory and proliferative responses of endothelial cells after damage.4 During the early phase of atherosclerosis, adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), are secreted by the activated endothelial cells in atherosclerotic lesions, stimulating immune cell and monocyte recruitment and migration into the intimal area of the vascular wall.5 Tumor necrosis factor (TNF)-α is one of the most potent pro-inflammatory cytokines and its function is closely connected to the apoptosis of endothelial cells and the development of atherosclerotic lesions.6Nuclear factor-κB (NF-κB) plays a significant role in the transcriptional regulation of inflammatory proteins such as cyclooxygenase-2 (COX-2), ICAM-1, VCAM-1, and E-selectin.7–9 NF-κB exists in the cytoplasm of unstimulated cells and is bound to its inhibitory protein IκBα. IκBα phosphorylation leads to its degradation and the subsequent translocation of NF-κB to the nucleus, where it activates target gene transcription.10 During atherosclerosis, NF-κB performs as a regulator of pro-inflammatory and anti-inflammatory gene transcription and also as a regulator of cell survival and proliferation.
Arthropods, such as centipedes and scorpions, are widely used in traditional Chinese medicine. The scorpion has been used in traditional Chinese medicine for many centuries to treat various neuronal problems such as chronic pain, paralysis, apoplexy and epilepsy. Meanwhile, Mesobuthus martensii Karsch is a significant traditional Chinese medicine that has been widely used for anti-thrombosis treatment.11 Mesobuthus martensii Karsch peptides (MMKPs) are a class of molecules that show strong anticoagulant, antithrombotic, and fibrinolysis activities.12–14 Consequently, identifying the bioactive peptide in Mesobuthus martensii Karsch and exploring the anti-inflammation effect will be of great value and significance. To solve this issue, we isolated and identified MMKPs; we explored their anti-inflammatory potency in human umbilical vein endothelial cells (HUVECs) and illustrated the underlying mechanism.
2 Materials and methods
2.1 Isolation and amino acid sequence analysis of the MMKPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 (w/v) and stirred without interruption for 4 h. Iso-propanol was replaced every 1 h. After that, the supernatant was removed, and the sediment was freeze-dried and stored at −20 °C as the total protein.
7.5 (w/v) and stirred without interruption for 4 h. Iso-propanol was replaced every 1 h. After that, the supernatant was removed, and the sediment was freeze-dried and stored at −20 °C as the total protein.The total protein (40 g) was dissolved (5%, w/v) in a 0.20 M phosphate buffer solution (PBS, pH 7.5); then, an ultrasonic cleaner (Shanghai, China) with a straight probe and continuous pulse was used for ultrasonication for 4 h. After centrifugation (8000 × g, 40 min), the supernatant was collected and then fractionated by salting-out with increasing concentrations of (NH4)2SO4 (0, 0.70 and 1.40 mM); the fraction in the 1.40 mM (NH4)2SO4 resulting supernatant was freeze-dried and stored at −20 °C for further analysis.
Hydrophobic chromatography. MMKP-A was dissolved in 1.50 M (NH4)2SO4 prepared with 30 mM PBS (pH 7.5) and loaded onto a Phenyl Sepharose CL-4B hydrophobic chromatography column (2.5 cm × 150 cm), which had previously been equilibrated with the above buffer. A stepwise elution was carried out with decreasing concentrations of (NH4)2SO4 (1.50, 0.75 and 0 M) dissolved in 30 mM PBS (pH 7.5) at a flow rate of 3.0 mL min−1. Each fraction was collected at a volume of 80 mL and was monitored at 280 nm. Fractions were then freeze-dried and the anti-inflammatory activity was detected. The fraction having the strongest anti-inflammatory activity was collected and prepared for anion-exchange chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100
0.1, v/v) and acetonitrile–trifluoroacetic acid (solvent B; 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v). The peptide was separated using a gradient elution from 15% to 65% of solvent B for 40 min at a flow rate of 1.0 mL min−1. The detection wavelength was set at 280 nm and column temperature was 20 °C.
0.1, v/v). The peptide was separated using a gradient elution from 15% to 65% of solvent B for 40 min at a flow rate of 1.0 mL min−1. The detection wavelength was set at 280 nm and column temperature was 20 °C.2.2 Reagents and cell culture
The Mesobuthus martensii Karsch was acquired from the Ertiantang pharmacy (Guangzhou, China) and identified by Professor Zhou (Jinan University, Guangzhou, China). All the antibodies were purchased from Cell Signaling Technology (CST, USA). TNF-α was obtained from Sigma-Aldrich (USA); the other chemicals used in the current experiment were purchased from Aldrich or Admas and used without any further purification.HUVECs were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and were grown in a culture medium containing 20% fetal bovine serum, 2 mmol L−1 glutamine, antibiomycins (10 μmol L−1 penicillin G and 10 μmol L−1 streptomycin) (all from Sigma-Aldrich, U.S.A.) in a humidified incubator containing 5% CO2 at 37 °C.15
2.3 Cell viability and MMKP protective effect assays
HUVECs were plated in Costa 96-well plates at densities of 1 × 105 cells per milliliter. Cells were treated with 10 μL of fresh FBS-free DMEM (control group) or FBS-free DMEM containing different concentrations of MMKPs for 24 h in an incubator. Then, the plates were washed twice with PBS and treated with 10 μL of CCK-8 solution for 4 h.16 After that, the absorbance was recorded at 450 nm (BioTek, America).We detected the protective effects of the MMKPs under a non-toxic concentration, and the HUVECs were treated as follows: control group: DMEM only; proliferative control group: TNF-α at 20 ng mL−1 for 6 h; MMKPs group: TNF-α at 20 ng mL−1 for 6 h and then MMKPs at 40 μM for 24 h. After that, the CCK8 method was used to detect the cell viability.
2.4 Detection of reactive oxygen species (ROS) and mitochondrial membrane potential (Δψm)
Free radicals, such as reactive oxygen species (ROS), overproduction or antioxidant capacity decline can result in balance disorders and cause ROS accumulation and oxidative stress, which can lead to fatal damage to DNA and proteins within the cell to subsequently induce apoptosis.17 DCFH can be oxidized into dichlorofluorescein (DCF), which fluoresces, by intracellular oxidants. Therefore, the intracellular ROS level of HUVECs was measured with a Reactive Oxygen Species assay kit.The experimental groups were set up as follows: control group: DMEM only; TNF-α group: TNF-α at 20 ng mL−1 for 6 h; MMKP-1-L group: TNF-α at 20 ng mL−1 for 6 h and then MMKP-1 at 43.0 μM for 24 h; MMKP-1-H group: TNF-α at 20 ng mL−1 for 6 h and then MMKP-1 at 215 μM for 24 h. The cells were treated as described above, afterwards, the cells were collected and washed thrice with serum-free medium.15 HUVECs were incubated in 200 μL of a serum-free medium containing DCFH-DA (25 μM) for 30 min at room temperature and the serum-free medium washed thrice. The cells were collected and assayed by flow cytometric analysis (BD FACS Calibur, Franklin Lakes, CA, USA).
The change in Δψm in the HUVECs under TNF-α stimulation was detected by JC-1 staining according to the manufacturer's protocol (Beyotime, China). Briefly, 2 mL of 1 × 106/mL cells were treated as described above in 6-well plates. The cells were washed three times with cold PBS and incubated with 1 μg mL−1 of JC-1 at 37 °C for 30 min without light. The supernatant was removed and washed three times with cold PBS and then assayed by flow cytometric analysis (FACScan, CA). Each treatment was carried out in triplicate and the final results were compared with that of the control group or the TNF-α group.
2.5 Apoptosis and cell cycle flow cytometry analysis
The HUVECs were plated on a 6-well plate at a cell density of 2 × 105 cells per well and treated as the experimental design. After that, the HUVECs were harvested and collected. The apoptosis was detected by Annexin V and PI staining using flow cytometry (FACSCalibur, Franklin Lakes, USA).For cell cycle analysis, the HUVECs were treated as the experimental design. Then, HUVECs were harvested, fixed in 70% ethanol and stored at −20 °C overnight. After that, the cells were washed with PBS and the cell cycle distribution was detected with PI (propidium iodide) staining using flow cytometry (FACSCalibur, Franklin Lakes, USA).
All the tests were repeated at least 3 times.
2.6 Western blotting analysis
HUVECs were plated in 6-well dishes and treated as the experimental design. After that, HUVECs were washed with cold PBS, scraped, pelleted and lysed in a radioimmunoprecipitation assay (RIPA) buffer. After incubation for 1 h on ice, the cell lysate was centrifuged at 3000 g for 0.5 h at 4 °C.Lysate protein concentrations were determined by a BCA protein assay kit (Thermo Scientific, USA) and the lysate was adjusted with a lysis buffer.18 The protein was resolved on a 15% SDS-PAGE and transferred to immobilon polyvinyl difluoride (PVDF) membranes. The blots were blocked with a blocking buffer for 10 min at room temperature and then probed with anti-human antibodies for 1 h at room temperature. Then, the blots were incubated with a peroxidase-conjugated anti-rabbit secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 dilution) for 1 h at room temperature. The resulting images were scanned using a scanner (Epson V330 Photo, Japan).
3000 dilution) for 1 h at room temperature. The resulting images were scanned using a scanner (Epson V330 Photo, Japan).
2.7 Statistical analysis
Experiments were repeated at least three times and results are expressed as mean ± SD. Data were analyzed by Student t-test and an analysis of variance (ANOVA) test, followed by a Tukey post test to determine the significant differences between groups. Here, p < 0.05 was considered to be significant.3 Results
3.1 MMKP amino acid sequence identification and protective potency evaluation
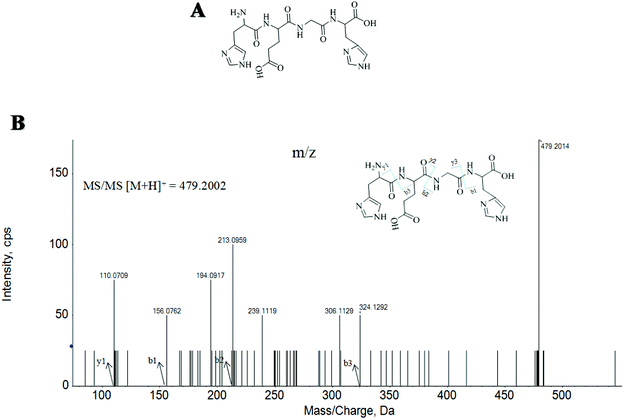
Here, twenty-seven MMKPs were isolated, and the amino acid sequences were identified by HPLC-ESI-MS. The peptide is usually protonated under ESI-MS/MS conditions, and fragmentations mostly occur at the amide bonds because it is difficult to break the chemical bonds of the side chains at such a low energy.19 Therefore, the b and y ions are the main fragment ions when the collision energy is <200 eV.The molecular mass determination and peptide characterization of MMKP-1 were analyzed by HPLC-ESI-MS. According to the result, the molecular mass of MMKP-1 was determined to be 479.2014 Da. The ion fragment m/z 156.0762 was regarded as the y1 ion and proved to be [His + H]+, while m/z 213.0959 was regarded as the b2 ion and represented the [M − His-Glu + H]+ ion. The y1 ion (m/z 110.0709) was the typical fragment [His-COOH]+ and m/z 324.1292 was the b3 ion (Fig. 1). On the basis of this, we concluded that the sequence of the peptide was HEGH. The amino acid sequences of MMKPs were identified with the method as MMKP-1; this was further confirmed with the BioTools database and the results are listed in Table 1.
| Compounds | Amino acid sequences | Cell survival rate (%) |
|---|---|---|
| MMPK-1 | HEGH | 95.9 ± 4.88 |
| MMPK-2 | DHRFLH | 85.2 ± 8.21 |
| MMPK-3 | HDRFLH | 88.7 ± 8.09 |
| MMPK-4 | YAHRGWS | 82.9 ± 8.05 |
| MMPK-5 | YAHGSWA | 81.7 ± 6.99 |
| MMPK-6 | HAGYSWA | 83.3 ± 8.22 |
| MMPK-7 | HASWEH | 90.1 ± 7.73 |
| MMPK-8 | WESHAS | 86.2 ± 8.80 |
| MMPK-9 | SHAYSH | 85.0 ± 7.83 |
| MMPK-10 | HKYRHD | 86.9 ± 7.72 |
| MMPK-11 | WGHE | 89.8 ± 8.20 |
| MMPK-12 | HKFW | 87.2 ± 7.08 |
| MMPK-13 | FWEH | 90.6 ± 5.89 |
| MMPK-14 | GAEG | 88.2 ± 6.38 |
| MMPK-15 | WHGE | 82.6 ± 6.08 |
| MMPK-16 | GEYHSHE | 83.7 ± 8.67 |
| MMPK-17 | TKFSYE | 84.6 ± 7.50 |
| MMPK-18 | YKHEWR | 91.5 ± 6.60 |
| MMPK-19 | KHGEL | 85.0 ± 8.23 |
| MMPK-20 | EGHGF | 79.9 ± 8.02 |
| MMPK-21 | HGEY | 86.5 ± 6.34 |
| MMPK-22 | YEEGAH | 85.6 ± 7.70 |
| MMPK-23 | AHEFEL | 82.5 ± 8.39 |
| MMPK-24 | DSHTS | 79.8 ± 7.88 |
| MMPK-25 | EAHGHSF | 82.4 ± 8.03 |
| MMPK-26 | EHGEYF | 79.8 ± 7.08 |
| MMPK-27 | EGFHL | 85.4 ± 6.51 |
| Control group | — | 100 ± 9.24 |
| Proliferative control group | — | 76.3 ± 6.60 |
Studies have reported that natural products can protect HUVECs against oxidative stress-induced apoptosis and reduced amyloid-β peptide-induced oxidative stress. TNF-α plays a central role in the intestinal inflammation of various inflammatory disorders.20 In the current study, cell viability decreased significantly. TNF-α attenuated the cell survival rate of the control group by 23.7%, which indicated that HUVECs suffered severe damage induced by TNF-α. Thus, experiments were conducted to investigate the protective effects of MMKPs on HUVECs under TNF-α stimulation.
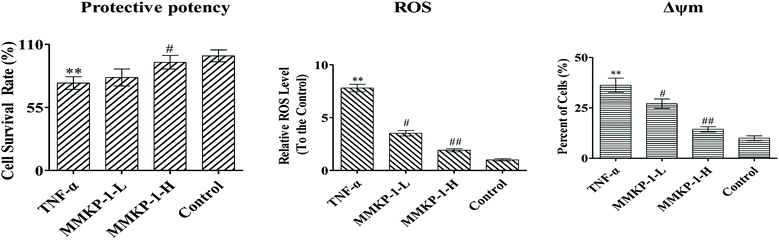
First, the cytotoxic effects of MMKPs were detected in HUVECs and the results showed that peptides exhibited no harmful effects on HUVECs at 0.8 mM. We then detected the protective effects of MMKPs under a non-toxic concentration, and the results showed that MMKP-1 has the best protective effects. Of interest, most MMKPs showed protective potency. After treatments with MMKP-1, 7, 11, 13 and 18 and compared to the result for the proliferative control group, the cell survival rates were up-regulated by 19.6%, 13.8%, 13.5%, 14.3% and 15.2%, respectively. Further studies showed that MMKP-1 significantly attenuated the damage induced by TNF-α, and the survival rates were effectively elevated by 71.1% and 93.2% with 43.0 and 215 μM MMKP-1 treatments (Fig. 2).
3.2 MMKP-1 down-regulated the ROS level in HUVECs
ROS plays a fatal role in the oxidative process as an oxidization molecule. The interaction of the cellular immune system with endogenous or exogenous inflammatory stimuli determines the generation of ROS. As reported before, TNF-α is believed to play a fatal role in ROS production and oxidative stress in HUVECs.5 Studies have also revealed that the hyperactivation of the inflammatory responses can induce oxidative stress in cells and tissues. Therefore, the intracellular ROS level can be an indicator of oxidative stress.21To investigate the ROS eliminating ability of MMKP-1, the ROS level was detected in HUVECs. We set the ROS content in the control group as 1. As shown in Fig. 2, compared with the ROS level of the control group, the ROS level increases by a factor of 7.81 with TNF-α stimulation. Interestingly, compared with the ROS level of the TNF-α group, the ROS levels decreased by 54.8%, and 75.4%, respectively, after 43.0 and 215 μM MMKP-1 treatments (Fig. 2).
Remarkably, the TNF-α-induced ROS generation was significantly attenuated by the MMKP-1 treatments. Of note, the result confirmed that MMKP-1 could reduce the intracellular oxidative stress and protect HUVECs from oxidative stress damage by scavenging ROS.
3.3 Flow cytometry analysis of cell apoptosis
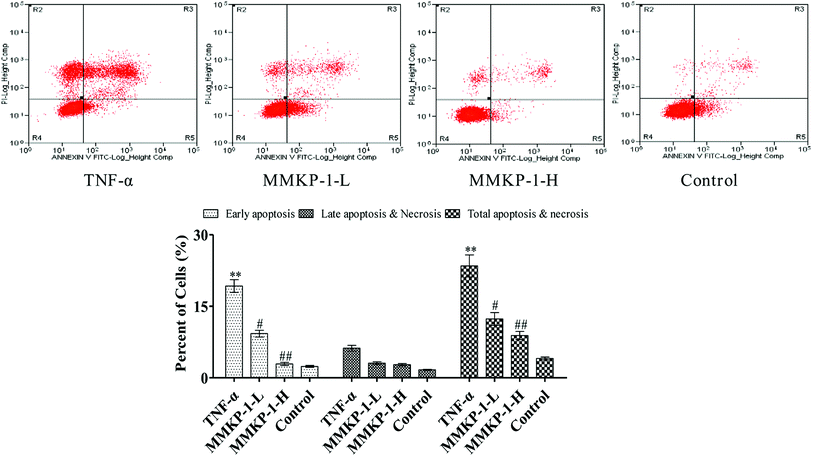
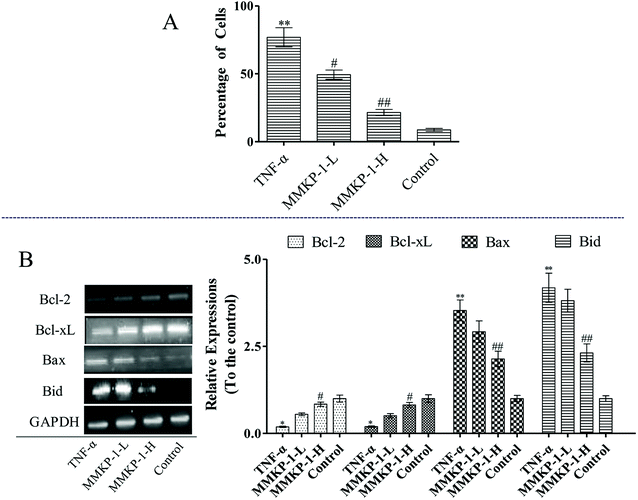
Cell apoptosis was detected with an Annexin V-FITC/PI assay using flow cytometry, and the results are shown in Fig. 3. Compared with the observation for the control group, the total apoptosis and necrosis rate decreased by about 19.4% with TNF-α stimulation. It is gratifying that the MMKP-1 treatments decreased the total apoptosis and necrosis rates by 11.0% and 14.5% and showed great protective effects. Cell apoptosis provided visual evidence for the intracellular protective potency of MMKP-1 in HUVECs. On the basis of this, the protective effects and the underlying mechanism were further investigated.3.4 Flow cytometry analysis of cell cycle arresting
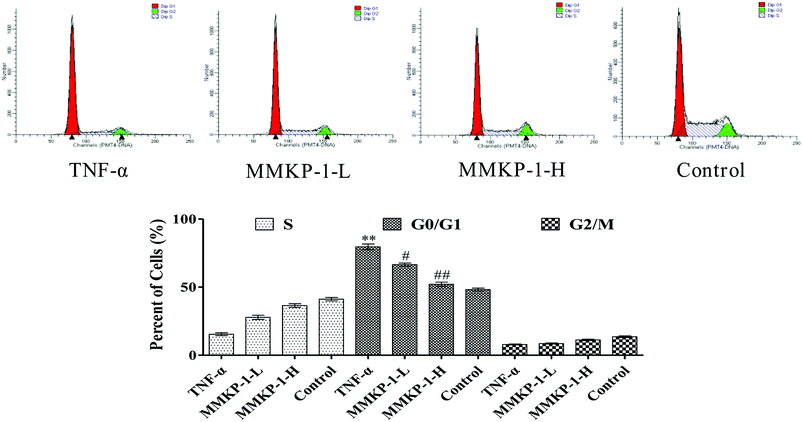
Notably, according to the content of DNA in cells, which regulates cell growth and replication, the cell cycle can be divided into three phases: G0/G1, S and G2/M. Excess ROS might cause irreversible damage to biological macromolecules, including DNA, nucleic acids, lipids, and proteins.22To further investigate the protective potency of MMKP-1 in the cell cycle phase distributions under TNF-α stimuli, the cell cycle was detected and the profiles are listed in Fig. 4. An increase of 31.4% in the G0/G1 population was observed in HUVECs after TNF-α stimulation. It is suggested that TNF-α blocked the cells in the G0/G1 phase, due to which the cells could not enter the S stage to synthesize DNA; eventually, the proliferation of HUVECs was inhibited. Interestingly, after the MMKP-1 treatments and compared to the result for the TNF-α group, the population of G0/G1 decreased by 13.1% and 27.5%, respectively. It can be concluded that cell cycle arresting also contributed to the protective potency of MMKP-1.
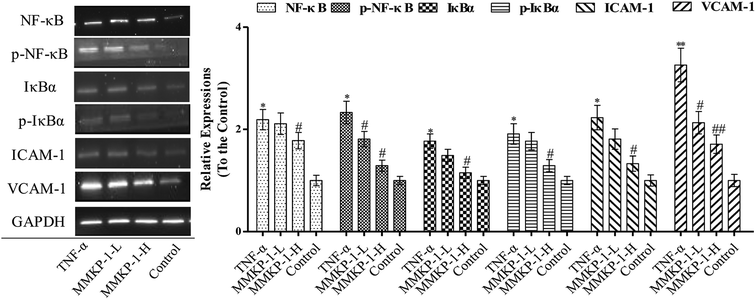
3.5 MMKP-1 attenuated ICAM-1 and VCAM-1 expressions and blocked NF-κB activation
The activation of the endothelial cells by TNF-α has been known to enhance the expressions of adhesion molecules such as ICAM-1 and VCAM-1.5 As shown in Fig. 5, TNF-α up-regulates the expressions of both ICAM-1 and VCAM-1. Interestingly, the up-regulation expressions of ICAM-1 and VCAM-1 induced by TNF-α were clearly attenuated by the MMKP-1 treatments. (Fig. 5).NF-κB plays a significant role in regulating the expressions of inflammatory proteins such as ICAM-1 and VCAM-1 in many types of cells.23 IκBα is the main regulator of NF-κB activation. Inactive NF-κB, bound to its inhibitor IκBα as a complex, is restricted to the cytoplasm. IκBα phosphorylation results in its ubiquitination and degradation; then, NF-κB is released from the NF-κB-IκBα complex and translocated to the nucleus.24 As studied above, MMKP-1 attenuated the ICAM-1 and VCAM-1 expressions; hence, we speculated that MMKP-1 might also affect NF-κB activation. Therefore, the activation of NF-κB and IκBα was further explored.
As shown in Fig. 5, ICAM-1 and VCAM-1 were up-regulated after being induced by TNF-α, which was also accompanied with NF-κB and IκBα activation. However, MMKP-1 attenuated the up-regulation of ICAM-1 and VCAM-1 and decreased the levels of phosphorylated NF-κB and IκBα in TNF-α-induced HUVECs. The results revealed that MMKP-1 inhibited the activation of NF-κB and IκBα, leading to the down-regulation of the adhesion molecule expression.
3.6 MMKP-1 attenuated the collapse of Δψm and down-regulated the Bax/Bcl-2 ratio
The Δψm balance and mitochondrial integrity are significant for the physiological function of cells. Studies have reported that the collapse of Δψm is correlated to the events of the apoptotic process. When the concentration of ROS and oxygen stress reach a certain level, Δψm can decline, resulting in the release of apoptosis factors. Once the mitochondrial membrane barrier function is lost, several factors including the loss of redox homeostasis, the metabolic consequences at the bio-energetic level, and the perturbation of ion homeostasis lead to cell death.Here, a JC-1 fluorescent probe was used to detect Δψm. As shown in Fig. 2, with the TNF-α stimuli, cells exhibiting Δψm decline increase from 10.0% to 36.3%, indicating an increase by 26.3%. Of interest, with MMKP-1 treatments and compared with the result of the TNF-α group, the cells with Δψm decline decreased by 9.20% and 21.9%, respectively. Therefore, we concluded that MMKP-1 may show a protective effect by attenuating the mitochondria damage induced by TNF-α.
The mitochondrial pathway plays a significant role in apoptotic modulation. The mitochondrial-mediated apoptotic pathway can be triggered by several factors including the expressions of B-cell lymphoma 2 (Bcl-2) family members such as Bcl-2 associated protein X (Bax) and B-cell lymphoma-extra large (Bcl-xL). This protein family can affect the permeability of the mitochondrial membrane and trigger the opening of the mitochondrial permeability transition pores in the inner mitochondrial membrane, resulting in the release of cytochrome c and mitochondrial dysfunction.
As shown in Fig. 6, up-regulations of the anti-apoptotic proteins Bcl-2 and Bcl-xL can be observed after MMKP-1 treatments. Usually, the greater the Bax/Bcl-2 ratio, the more percentage of cell apoptosis involved. Here, the TNF-α stimuli induced clear Bax/Bcl-2 ratio up-regulation. However, MMKP-1 attenuated TNF-α-induced Bax and Bid and enhanced Bcl-2 and Bcl-xL expressions. In addition, MMKP-1 attenuated the Bax/Bcl-2 ratio.
4 Discussion and conclusion
Inflammation is involved in the initiation, rupture, and thrombosis of atherosclerotic plaques.25 During the early stages of atherosclerosis, inflammatory cell recruitment plays a central role.27 TNF-α is a key cytokine and is involved in nearly every step of inflammation. Both ICAM-1 and VCAM-1 play significant roles in the initiation of early atherosclerosis, preferentially contributing to monocyte adhesion.5,26 Inhibition of the inflammatory response is widely known to be beneficial in the early stages of atherosclerosis.28 Scorpions have been used in antithrombosis treatments for years; the results showed that the expressions of ICAM-1 and VCAM-1 were up-regulated obviously in TNF-α-induced HUVECs, while this up-regulation was significantly suppressed by the MMKP-1 treatment.Research demonstrates that oxidative stress can result in adhesion function damage, cell survival and apoptosis, which is mediated through apoptosis-associated proteins such as Bax and Bcl-2.29 In the current study, TNF-α increased intracellular ROS production and caused serious apoptosis in HUVECs. However, MMKP-1 significantly attenuated TNF-α-induced oxidative damage in HUVECs by inhibiting NF-κB activation and regulating Bax and Bcl-2 expressions. Studies have reported that TNF-α can increase the binding of NF-κB to its recognition site in the VCAM-1 promoter, subsequently exhibiting monocyte adhesion to vascular endothelial cells. Here, we observed that the TNF-α-induced increase in IκBα degradation was attenuated by MMKP-1 treatment.
In summary, the results of this study demonstrate that the endothelial dysfunction induced by TNF-α, which is associated with the up-regulation of adhesion molecules, reduced viability and apoptosis in HUVECs, can be reversed by the MMKP-1 treatment. The results revealed that MMKP-1 showed a preventive effect against endothelial dysfunction, which may be via inhibiting oxidative stress, improving endothelial survival and preventing the adhesion function damage. Therefore, we inferred that some MMKPs might be used as potential agents in the prevention and treatment of endothelial cell injury-related diseases.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We thank Professor Chen (Jinan University, Guangzhou, China) for technical assistance as well as critical editing of the manuscript.References
- Y. Shan, R. Zhao, W. Geng, N. Lin, X. Wang, X. Du and S. Wang, Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage, Cardiovasc. Toxicol., 2010, 10, 139–145 CrossRef CAS PubMed.
- C. Wang, H. Li, P. Fu, S. Zhang and R. Xiu, Serum C-reactive protein and circulating endothelial cells in patients with acute myocardial infarction, Clin. Hemorheol. Microcirc., 2005, 32, 287 CAS.
- S. K. Nadar, G. Y. Lip, K. W. Lee and A. D. Blann, Circulating endothelial cells in acute ischaemic stroke, Thromb. Haemostasis, 2005, 94, 707–712 CrossRef PubMed.
- Y. H. Zhang, Y. H. Zhang, X. F. Dong, Q. Q. Hao, X. M. Zhou, Q. T. Yu, S. Y. Li, X. Chen, A. F. Tengbeh and B. Dong, ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response, Inflammation Res., 2015, 64, 253–260 CrossRef CAS PubMed.
- Y. P. Zhu, T. Shen, Y. J. Lin, B. D. Chen, Y. Ruan, Y. Cao, Y. Qiao, Y. Man, S. Wang and J. Li, Astragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human vascular endothelial cells by blocking NF-κB activation, Acta Pharmacol. Sin., 2013, 34, 1036–1042 CrossRef CAS PubMed.
- N. Vrachnis and F. M. Malamas, Immune aspects and myometrial actions of progesterone and CRH in labor, Clin. Dev. Immunol., 2011, 2012, 937618 Search PubMed.
- Y. S. Li, L. P. Wu, K. H. Li, Y. P. Liu, R. Xiang, S. B. Zhang, L. Y. Zhu and L. Y. Zhang, Involvement of nuclear factor κB (NF-κB) in the downregulation of cyclooxygenase-2 (COX-2) by genistein in gastric cancer cells, J. Int. Med. Res., 2011, 39, 2141 CrossRef CAS PubMed.
- C. Lawson, M. Ainsworth, M. Yacoub and M. Rose, Ligation of ICAM-1 on Endothelial Cells Leads to Expression of VCAM-1 via a Nuclear Factor-κB-Independent Mechanism, in International Plant and Animal Genome Conference Xx, 1999, 1999, pp. 2990–2996 Search PubMed.
- H. Liu, Z. C. Wang, Y. G. Bai, Y. Cai, J. W. Yu, H. J. Zhang, J. X. Bao, X. L. Ren, M. J. Xie and J. Ma, Simulated microgravity promotes monocyte adhesion to rat aortic endothelium via nuclear factor-κB activation, Clin. Exp. Pharmacol. Physiol., 2015, 42, 510–519 CrossRef CAS PubMed.
- S. Shishodia, S. Majumdar, S. Banerjee and B. B. Aggarwal, Ursolic Acid Inhibits Nuclear Factor-κB Activation Induced by Carcinogenic Agents through Suppression of IκBα Kinase and p65 Phosphorylation Correlation with Down-Regulation of Cyclooxygenase 2, Matrix Metalloproteinase 9, and Cyclin D1, Cancer Res., 2003, 63, 4375–4383 CAS.
- G. J. Müller, Scorpionism in South Africa. A report of 42 serious scorpion envenomations, S. Afr. Med. J., 1993, 83, 405 Search PubMed.
- Y. Ren, H. Wu, F. Lai, M. Yang, X. Li and Y. Tang, Isolation and identification of a novel anticoagulant peptide from enzymatic hydrolysates of scorpion (Buthus martensii Karsch) protein, Food Res. Int., 2014, 64, 931–938 CrossRef CAS PubMed.
- Y. M. Song, X. X. Tang, X. G. Chen, B. B. Gao, E. Gao, L. Bai and X. R. Lv, Effects of scorpion venom bioactive polypolypeptides on platelet aggregation and thrombosis and plasma 6-keto-PG F1alpha and TXB2 in rabbits and rats, Toxicon, 2005, 46, 230–235 CrossRef CAS PubMed.
- Y. G. Peng, X. U. Ai-Liang, Y. Huang, Y. Yang, D. Shi, J. Y. Zhao and Y. I. Xiao-Min, Effects of Scorpion Purified Liquid on Fibrinolysis System and Coagulation System in Rats with Thrombosis, Chin. J. Inf. Tradit. Chin. Med., 2011, 18, 47–48 Search PubMed.
- R. L. Adams, I. P. Adams, S. W. Lindow, W. Zhong and S. L. Atkin, Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium, Br. J. Cancer, 2005, 92, 1493 CrossRef CAS PubMed.
- Z. Yin, X. Chen, J. L. Chen, W. L. Shen, T. M. H. Nguyen, L. Gao and H. W. Ouyang, The regulation of tendon stem cell differentiation by the alignment of nanofibers, Biomaterials, 2010, 31, 2163 CrossRef CAS PubMed.
- W. Sangkitikomol, A. Rocejanasaroj and T. Tencomnao, Effect of Moringa oleifera on advanced glycation end-product formation and lipid metabolism gene expression in HepG2 cells, Genet. Mol. Res., 2014, 13, 723–735 CrossRef CAS PubMed.
- J. Dalmau, H. M. Furneaux, R. J. Gralla, M. G. Kris and J. B. Posner, Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer--a quantitative western blot analysis, Ann. Neurol., 1990, 27, 544–552 CrossRef CAS PubMed.
- M. A. Raji, P. Frycak, M. Beall, M. Sakrout, J. M. Ahn, Y. Bao, D. W. Armstrong and K. A. Schug, Development of an ESI-MS screening method for evaluating binding affinity between integrin fragments and RGD-based peptides, Int. J. Mass Spectrom., 2007, 262, 232–240 CrossRef CAS.
- A. M. Westbrook, B. Wei and K. Hacke, et al., The role of tumour necrosis factor-α and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity, Mutagenesis, 2012, 27(1), 77–86 CrossRef CAS PubMed.
- M. Manczak, T. S. Anekonda, E. Henson, B. S. Park, J. Quinn and P. H. Reddy, Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression, Hum. Mol. Genet., 2006, 15, 1437 CrossRef CAS PubMed.
- T. Takami and I. Sakaida, Iron regulation by hepatocytes and free radicals, J. Clin. Biochem. Nutr., 2011, 48, 103 CrossRef CAS PubMed.
- S. J. Cullen, S. Ponnappan and U. Ponnappan, Catalytic activity of the proteasome fine-tunes Brg1-mediated chromatin remodeling to regulate the expression of inflammatory genes, Mol. Immunol., 2009, 47, 600 CrossRef CAS PubMed.
- W. C. Huang and M. C. Hung, Beyond NF-κB activation: nuclear functions of IκB kinase α, J. Biomed. Sci., 2013, 20, 1–13 CrossRef PubMed.
- K. R. K. Murthy, D. As, M. Z. Abbas and L. Haghnazari, Investigations on the role of insulin and scorpion antivenom in scorpion envenoming syndrome, J. Venomous Anim. Toxins Incl. Trop. Dis., 2003, 9, 202–238 CrossRef.
- R. S. Wright, J. G. Murphy, K. A. Bybee, S. L. Kopecky and J. M. Lablanche, Statin lipid-lowering therapy for acute myocardial infarction and unstable angina: efficacy and mechanism of benefit, Mayo Clin. Proc., 2002, 77, 1085–1092 CrossRef CAS PubMed.
- C. Viedt, W. Shen, J. Fei, M. Kamimura, G. M. Hänsch, H. A. Katus and J. Kreuzer, HMG-CoA reductase inhibition reduces the proinflammatory activation of human vascular smooth muscle cells by the terminal complement factor C5b-9, Basic Res. Cardiol., 2003, 98, 353–361 CAS.
- Y. J. Li, Y. Chen, Y. You, X. G. Weng, Q. Yang, C. X. Ruan and X. X. Zhu, Effects of shenlian extracts on atherosclerosis by inhibition of the inflammatory response, J. Tradit. Chin. Med., 2011, 31, 344–348 CrossRef PubMed.
- R. Ghosh, A. Alajbegovic and A. V. Gomes, NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species, Oxid. Med. Cell. Longevity, 2015, 9–20 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |