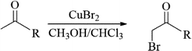Open Access Article
Open Access ArticleSynthesis of 5-aminolevulinic acid with nontoxic regents and renewable methyl levulinate†
Yuxia Zaia,
Yunchao Fenga,
Xianhai Zeng *abc,
Xing Tang
*abc,
Xing Tang abc,
Yong Sunabc and
Lu Linabc
abc,
Yong Sunabc and
Lu Linabc
aCollege of Energy, Xiamen University, Xiamen 361102, China. E-mail: xianhai.zeng@xmu.edu.cn; Fax: +86-592-2880701; Tel: +86-592-2880701
bFujian Engineering and Research Centre of Clean and High-valued Technologies for Biomass, Xiamen 361102, China
cXiamen Key Laboratory of Clean and High-valued Utilization for Biomass, Xiamen 361102, China
First published on 1st April 2019
Abstract
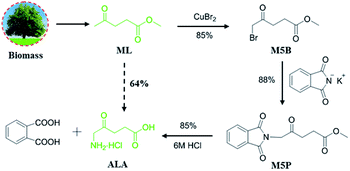
Synthesis of 5-aminolevulinic acid (5-ALA) was presented with novel bromination of biobased methyl levulinate (ML), followed by ammoniation and hydrolysis. Copper bromide (CuBr2) was employed as the bromination reagent with higher selectivity and activity instead of the conventional liquid bromine (Br2). 5-ALA was obtained in a high yield (64%) and purity (>95%) by optimum design, which is of great potential in industrialization.
5-Aminolevulinic acid (5-ALA) is generally known as an essential precursor molecule for tetrapyrrole synthesis such as porphyrin, heme, chlorophyll and vitamin B12.1 It has been widely applied in localizing and photodynamic therapy for various cancers.2–4 It has also been used as a selective biodegradable insecticide, herbicide, salt tolerance agent or plant growth regulator in agricultural fields.5
To date, 5-ALA was mainly synthesized by microbial production methods,6 but the long-time and high-cost course restrict its scaled applications. On the other side, chemical routes using 2-hydroxypyridine, tetrahydrofurfurylamine and furfurylamine as starting materials involved in numerous bottleneck including toxic intermediates and rigorous reaction conditions.7,8 Thus, to develop a new pathway for 5-ALA production is of great significance, especially one that is a green and sustainable.
Biomass is an appealing starting material in value-added chemicals synthesis because of its advantages of renewability, sustainability and availability.9 Several biomass-derived platform compounds such as 5-hydroxymethylfurfural (HMF), 5-chloromethylfurfural (CMF), levulinic acid (LA) or its esters have been reported as efficient raw materials in the production of 5-ALA.10,11 However, the industrial manufacture of furan-type HMF and CMF cannot currently be achieved easily due to the high production and environment costs.12,13 Furthermore, conversion of furan-type chemicals to 5-ALA also suffers the economic problems concerning the use of expensive oxidants in the ring-opening stage. Unlike CMF and HMF, LA and its esters can be easily produced both from hemicellulose and cellulose, and its yearly tonnage is therefore available via the acidic processing of biomass at a competitively low price.14–16 Thus, to synthesize 5-ALA from LA or its esters is exceptionally promising. Typically, 5-ALA can be effectively prepared from levulinates via a three-stage process including bromination, ammoniation and acidolysis.17 However, the bromination of levulinates with Br2 in this course has low selectivity to 5-bromo derivatives. Besides, Br2 is hazardous and environmentally unfriendly. Hence, a crucial step of the production of 5-ALA from levulinates is to explore a safe bromide agent with higher selectivity and activity.
CuBr2, a green and low toxic brominated reagent, was usually used for the synthesis of α-bromination of cyclopentenone derivatives and its closest analogues-indanone of carbonyl compounds for its advantages of short reaction times, high selectivity of the products, high yields and easily handle procedures.18,19 In this content, various unsymmetrical aliphatic ketones including levulinic acid, methyl levulinate, ethyl levulinate, 5-hydroxy-2-pentanone and 2-butanone was attempted for bromizing with CuBr2 (Table 1). Interestingly, the yields of bromination products were different, depending on the source of aliphatic ketones.
| a Reaction condition: compounds = 2.5 mmol, CuBr2 = 1.67 g, solvent = 30 mL, temperature = 40 °C, time = 4 h. |
|---|
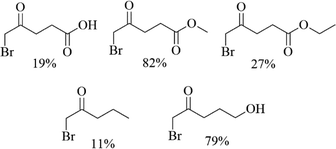 |
In this work, we present the synthesis of 5-ALA from biomass derived methyl levulinate (ML) under mild conditions using CuBr2 as a greener bromine donor, and a high yield of 5-bromolevulinate (M5B) up to 85% was achieved. Furthermore, a detailed discussion of ammoniation and acidolysis was also presented, corresponding a high total 5-ALA yield over 64% (Scheme 1).
The first attempt to screen the reaction conditions for the bromination of ML with CuBr2 are shown in Table 2. An encouraging yield of the desired product (50%) is indeed obtained using CuBr2 as bromide agent in CH3OH at 40 °C for 3 h (Table 2, entry 1). The investigation of the solvent indicated that CH3OH–CHCl3 mixed solvent was superior to ethyl acetate (EA), CHCl3, CH3OH, CH3OH–EA and EA–CHCl3 (Table 2, entry 2–6). The results may due to the fact that CH3OH can improve the selectivity of M5B and haloalkanes are favourable to halogenation.13,20,21 Based on the above, changing the volume ratio of CH3OH to CHCl3 (Table 2, entry 6–10), improved the yield of M5B to 80% (Table 2, entry 6). We then proceeded to evaluate various metal bromides, including ZnBr2, MgBr2, and AlBr3 under the identical conditions. However, no conversion was detected even after 24 h (Table 2, entry 11–13). These metal bromides were also found to be inactive in bromination as noted in previous studies.22,23 The conventional bromination agent such as Br2, 2C4H9NOHBr·Br2 and NBS can obviously improve the reaction (Table 2, entry 14–17), although they were lower than that achieved with CuBr2 (Table 2, entry 6). Experiments that screened for the bromine donors suggested that CuBr2 was the most effective for the bromination of ML (Table 2, entry 6). Note that a detailed study of the reaction conditions were discussed (Tables S1, S2 and Fig. S1†), and a high M5B yield over 85% was obtained at 40 °C for 5 h (detected by GC-MS, Fig. S3†).
| Entry | Bromine source | Solvent | M5B yield (%) |
|---|---|---|---|
| a Reaction condition: ML = 0.33 g, bromine source = 3 mole of ML, solvent = 30 mL, temperature = 40 °C, time = 3 h.b Time = 24 h. | |||
| 1 | CuBr2 | CH3OH | 50 |
| 2 | CuBr2 | CHCl3 | 7 |
| 3 | CuBr2 | EA | 13 |
| 4 | CuBr2 | CH3OH/EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
32 |
| 5 | CuBr2 | CHCl3/EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
14 |
| 6 | CuBr2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 |
| 7 | CuBr2 | CH3OH/CHCl3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
56 |
| 8 | CuBr2 | CH3OH/CHCl3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
75 |
| 9 | CuBr2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
74 |
| 10 | CuBr2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
66 |
| 11b | ZnBr2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 12b | MgBr2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 13b | AlBr3 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 14 | Br2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
55 |
| 15 | 2C4H9NOHBr·Br2 | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40 |
| 16 | NBS + BPO | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
28 |
| 17 | NBS + AIBN | CH3OH/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
18 |
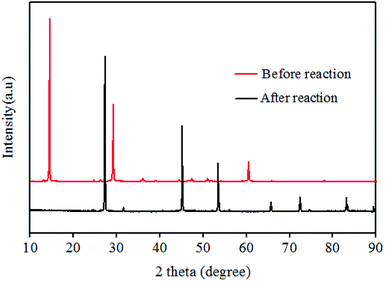
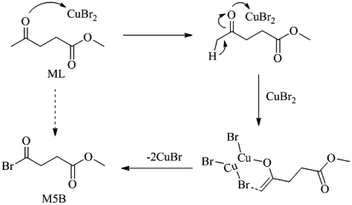
As shown in Fig. 1, XRD patterns indicated that CuBr2 was transformed into CuBr (PDF#06-0292) after the reaction. When TEMPO and 2,6-di-tert-butyl-4-methylphenol (BHT) were introduced to eliminate free radical, no M5B was detected. Based on these results, a mechanism for the current bromination process was proposed, as shown in Scheme 2. Initially, Lewis acidity of CuBr2 promoted the transformation from carbonyl keto to copper-bound enolate at the α-position.24 Subsequently, the hemolysis of enolate to get ethenyloxy radical, and the reactive group reacts with CuBr2 to generate an M5B along with an equivalent of CuBr.
Intensive efforts have been devoted to introduce the key amino group on M5B.17,25–27 Among them, a typical Gabriel reaction using potassium phthalimide (KPI) as ammonia resource has a promising commercial availability, however, only a moderate M5P yield of 59% was achieved at 110 °C for 12 h.27 In this work, optimizing the experimental conditions of Gabriel reaction including reaction time, temperature, the amount of solvent and the molar ratio of KPI to M5B (Tables S3 and S4†) was subsequently conducted, and a maximum M5P yield of 88% was obtained at 40 °C for only 4 h (detected by GC-MS, Fig. S4†). This significant improvement is no doubt accelerate the practical application of 5-ALA. Finally, an acid hydrolysis process was applied with 6 M HCl (Fig. S2†). The obtained products were concentrated in vacuum at 40 °C to avoid the polymerization of 5-ALA at high temperatures,13 affording a satisfied 5-ALA yield of 85% (Fig. S11 and 12†) (determined by HPLC, Fig. S5†).
In summary, we have developed a new efficient bromination method for the conversion of biomass derived ML to 5-ALA, a key chemical that has been widely applied in medical and agricultural areas. CuBr2 was applied as both catalyst and bromine atom donor and was demonstrated to be of higher selectivity and activity than the conventional hazardous Br2 in ML bromination. Each stage proceeds in high (∼85%) yield and affords 5-ALA in 95% purity, giving a process that could be commercially viable.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank for the financial support from the National Natural Science Foundation of China (Grant No. 21506177; 21676223), the Fundamental Research Funds for the Central Universities (Grant No. 20720160087; 20720160077; 20720170062), and the Energy Development Foundation of the College of Energy, Xiamen University (No. 2017NYFZ02).Notes and references
- K. Sasaki, M. Watanabe, T. Tanaka and T. Tanaka, Appl. Microbiol. Biotechnol., 2002, 58, 23–29 CrossRef CAS PubMed.
- M. C. Tetard, M. Vermandel, S. Mordon and J. P. Lejeune, Photodiagn. Photodyn. Ther., 2014, 11, 319–330 CrossRef CAS PubMed.
- T. Ishikawa, Y. Kajimoto, Y. Inoue, Y. Ikegami and T. Kuroiwa, Adv. Cancer Res., 2015, 125, 197 CrossRef PubMed.
- H. Fukuda, A. Casas and A. Batlle, Int. J. Biochem. Cell Biol., 2005, 37, 272–276 CrossRef CAS PubMed.
- Z. J. Zhang, H. Z. Li, W. J. Zhou, Y. Takeuchi and K. Yoneyama, Plant Growth Regul., 2006, 49, 27–34 CAS.
- S. L. Liu, G. M. Zhang, X. K. Li and J. Zhang, Appl. Microbiol. Biotechnol., 2014, 98, 7349–7357 CrossRef CAS PubMed.
- H. Kawakami, T. Ebata and H. Matsushita, Agric. Biol. Chem., 1991, 55, 1687–1688 CAS.
- H. Takeya, H. Ueki, S. Miyanari, T. Shimizu and M. Kojima, J. Photochem. Photobiol., A, 1996, 94, 167–171 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- M. Mascal and S. Dutta, Green Chem., 2010, 13, 40–41 RSC.
- L. Cottier, G. Descotes, L. Eymard and K. Rapp, Cheminform, 1995, 303–306 CAS.
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- M. Mascal, ChemSusChem, 2015, 8, 3391–3395 CrossRef CAS PubMed.
- S. G. Wettstein, D. M. Alonso, E. I. Gurbuz and J. A. Dumesic, Curr. Opin. Chem. Eng., 2012, 1, 218–224 CrossRef CAS.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- D. J. Hayes, S. Fitzpatrick, M. H. B. Hayes and J. R. H. Ross, The Biofine Process – Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks, Wiley-VCH Verlag GmbH & Co., 2008, pp. 139–164 Search PubMed.
- H. J. Ha, S. K. Lee, Y. J. Ha and J. W. Park, Synth. Commun., 1994, 24, 2557–2562 CrossRef CAS.
- V. Z. Shirinian, D. V. Lonshakov, V. V. Kachala, I. V. Zavarzin, A. A. Shimkin, A. G. Lvov and M. K. Mikhail, Regio- and chemoselective bromination of 2,3-diarylcyclopent-2-en-1-ones, J. Org. Chem., 2012, 77, 8112–8123 CrossRef CAS PubMed.
- R. H. Vekariya and H. D. Patel, Synthesis of a-bromocarbonyl compounds: recent advances, Tetrahedron, 2014, 70, 3949–3961 CrossRef CAS.
- L. Moens, Synthesis of δ-Aminolevulinic Acid, ACS Symp. Ser., 2001, 784, 37–50 CrossRef CAS.
- D. R. Lane, M. Mascal and P. Stroeve, Renewable Energy, 2016, 85, 994–1001 CrossRef CAS.
- T. Liu, J. Sun, Q. Wang, L. Li and M. D. Zhou, Eur. J. Org. Chem., 2017, 2017, 1915–1921 CrossRef CAS.
- M. Y. Zhou, S. S. Kong, L. Q. Zhang, M. Zhao, J. A. Duan, Z. Ou-Yang and M. Wang, Tetrahedron Lett., 2013, 54, 3962–3964 CrossRef CAS.
- R. W. Evans, J. R. Zbieg, S. Zhu, W. Li and D. W. C. Macmillan, J. Am. Chem. Soc., 2013, 135, 16074–16077 CrossRef CAS PubMed.
- L. Moens, US005907058A, Lakewood and Colo, 1999.
- L. Moens, US006583317B1, Lakewood and Colo, 2003.
- J. J. Bozell, L. Moens, D. C. Elliott, Y. Wang, G. G. Neuenscwander, S. W. Fitzpatrick, R. J. Bilski and J. L. Jarnefeld, Resour., Conserv. Recycl., 2000, 28, 227–239 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01517e |
| This journal is © The Royal Society of Chemistry 2019 |