Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single crystal growth and structure analysis of type-I (Na/Sr)–(Ga/Si) quaternary clathrates†
Hironao Urushiyamaab,
Haruhiko Morito *c and
Hisanori Yamane
*c and
Hisanori Yamane a
a
aInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
bDepartment of Metallurgy, Materials Science and Materials Processing, Graduate School of Engineering, Tohoku University, 6-6-04 Aramaki Aza Aoba, Aoba-ku, Sendai 980-8579, Japan
cInstitute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan. E-mail: morito@imr.tohoku.ac.jp
First published on 9th May 2019
Abstract
Single crystals of (Na/Sr)–(Ga/Si) quaternary type-I clathrates, Na8−ySryGaxSi46−x, were synthesized by evaporating Na from a mixture of Na–Sr–Ga–Si–Sn in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 773 K for 12 h in an Ar atmosphere. Electron-probe microanalysis and single-crystal X-ray diffraction revealed that three crystals from the same product were Na8−ySryGaxSi46−x with x and y values of 7.6, 2.96; 8.4, 3.80; and 9.1, 4.08. It was also shown that increasing the Sr and Ga contents increased the electrical resistivity of the crystal from 0.34 to 1.05 mΩ cm at 300 K.
1 molar ratio at 773 K for 12 h in an Ar atmosphere. Electron-probe microanalysis and single-crystal X-ray diffraction revealed that three crystals from the same product were Na8−ySryGaxSi46−x with x and y values of 7.6, 2.96; 8.4, 3.80; and 9.1, 4.08. It was also shown that increasing the Sr and Ga contents increased the electrical resistivity of the crystal from 0.34 to 1.05 mΩ cm at 300 K.
1. Introduction
Silicon (Si) clathrate compounds are composed of host Si atoms organized in three-dimensional frameworks and guest atoms enclosed in the Si cages of the frameworks. Kasper et al. first synthesized binary Si clathrate, Na8Si46, in 1965.1 Since then, many researchers have studied Si clathrate compounds,2 altering their physical properties by partial or full substitution of different elements for the host and guest atoms. Kawaji et al. synthesized a type-I clathrate, (Na,Ba)8Si46;3 this compound was the first Si clathrate superconductor with a TC value of 4 K derived from the partial substitution of Ba for Na in the Na8Si46 cages. Another Si clathrate, Ba8Si46, which was synthesized at high pressure (3 GPa) and 1073 K, exhibited the highest TC value 8 K among the various Si clathrate compounds.4 The framework Si atoms can also be partially replaced by Ga atoms in some type-I clathrate compounds, such as A8Ga8Si38 (A = K, Rb, Cs), as described by Sui et al.5 A8Ga8Si38 powder was sintered by spark plasma sintering to obtain bulk polycrystalline samples, which exhibited band gaps in the range of 1.14–1.18 eV.5 Another clathrate composed of Ga/Si cages and Ba atoms, Ba7.94Ga15.33Si30.67, was shown to have a relatively high thermoelectric dimensionless figure of merit, ZT, of 0.87 at 870 K.6,7 Sr8Ga11Si35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and Sr8Ga13.6Si32.4,9 which contain Sr guest atoms in Ga/Si cages, exhibit electrical resistivities of approximately 0.2 and 0.26 mΩ cm, respectively, at 280 K.
8 and Sr8Ga13.6Si32.4,9 which contain Sr guest atoms in Ga/Si cages, exhibit electrical resistivities of approximately 0.2 and 0.26 mΩ cm, respectively, at 280 K.
Some ternary silicon clathrates could be synthesized via solid-state reactions between each elements at high temperature5 or melting method.6,8,9 However, the silicon clathrates containing a Na atom could not be synthesized by simple reaction because these clathrates have been regarded as metastable or intermediate phases. These clathrates were generally prepared by thermal decomposition of the precursor compounds. For example, the binary silicon clathrates containing Na, Na8Si46 and Na24Si136, were synthesized by thermal decomposition of Na4Si4 Zintl compound.1,2 The clathrate samples obtained by this method were powdery due to the solid state of the precursor compounds. Therefore, it is difficult to prepare the bulk crystal of the silicon clathrates containing Na. In our previous study, the single crystals of the Na–Si binary clathrate were successfully grown by using Sn flux.10,11 Single crystals of type-I Na8Si46 and type-II Na24Si136 clathrates were selectively grown in Na–Sn rich Na–Sn–Si ternary melt by Na evaporation.11 Single crystals of a ternary type-I clathrate, Na8Ga5.70Si40.30, could also be prepared by a self-flux method using Ga as a flux.12 Furthermore, the crystal growth of Na8GaxSi46−x (x = 4.94–5.52) clathrates was achieved by adding a Sn flux to the starting melt. We could measure the electrical resistivity of the single crystals for these clathrates containing Na. The clathrates exhibited metallic conduction, and their electrical resistivity decreased as the Ga content decreased (e.g., the resistivities of Na8Ga5.70Si40.30 and Na8Ga4.94Si41.06 were 1.40 and 0.72 mΩ cm, respectively, at 300 K).
To extend the variation of clathrate compounds and their field of properties and applications, doping or partial substitution of other atoms at the Na atom site is also designed. Recently, quaternary Ga/Si and Zn/Si clathrates having Na and Rb or Cs guest atoms, such as Cs6Na2Ga8.25Si37.75, Rb6.34Na1.66Ga8.02Si37.98, and Rb8Na16Zn8.4Si127.6, have been synthesized using a Ga or Zn flux.13 However, the typical size of the single crystals was below 0.1 mm, and the properties of the crystals could not be characterized. So, synthesis of quaternary Na and Si based clathrate single crystals with a size enough for characterization is still challenging. In the present study, we succeeded in growing the single crystals of quaternary Ga–Si cage clathrate compounds encapsulating Na and Sr guest atoms by the Sn flux method. The compounds are the first examples of the Ga/Si clathrates containing Na (1+) and other guest cations with a different formal ionic charge (2+). The crystal structures and electrical properties were investigated for the single crystals of the new clathrates.
2. Experimental methods
The experiments were conducted as described in the previous studies.10–12 Metal Na pieces (Nippon Soda Co. Ltd., 99.95%), Si powder (Kojundo Chemical Laboratory Co. Ltd., 4N), Ga grains (Dowa Electronics Co. Ltd., 6N), and Sn granules (Mitsuwa Chemicals Co. Ltd., 5N) were combined by weight at a Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn molar ratio of 6
Sn molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (total 8.70 mmol) in a glove box with an Ar atmosphere. The raw material mixture was then put in a boron nitride (BN) crucible (Showa Denko KK; inner diameter of 6.5 mm and depth of 18 mm) and sealed in a stainless steel (SUS) container (SUS316, outer diameter of 12.7 mm, inner diameter of 10.7 mm, and height of 80 mm) with Ar gas. The SUS container was heated in an electric furnace at 1173 K for 12 h then the furnace was cooled to room temperature. The BN crucible was then taken from the SUS container in the glove box and Sr grains (Alfa Aeser, 4N) were added to the Na–Ga–Si–Sn mixture in the BN crucible to make the Na
1 (total 8.70 mmol) in a glove box with an Ar atmosphere. The raw material mixture was then put in a boron nitride (BN) crucible (Showa Denko KK; inner diameter of 6.5 mm and depth of 18 mm) and sealed in a stainless steel (SUS) container (SUS316, outer diameter of 12.7 mm, inner diameter of 10.7 mm, and height of 80 mm) with Ar gas. The SUS container was heated in an electric furnace at 1173 K for 12 h then the furnace was cooled to room temperature. The BN crucible was then taken from the SUS container in the glove box and Sr grains (Alfa Aeser, 4N) were added to the Na–Ga–Si–Sn mixture in the BN crucible to make the Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr
Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn molar ratio 6
Sn molar ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Next, the BN crucible was sealed in the upper part of another long SUS container (outer diameter of 12.7 mm, inner diameter of 10.7 mm, and height of 300 mm) with Ar gas. The upper part of the container was heated at 773 K for 12 h, and the lower part was cooled using a fan to keep the temperature almost the same with the room temperature. By generating a temperature gradient in the container, the Na was evaporated from the mixture in the crucible, and condensed on the inner wall in the lower cooler part of the container.
1. Next, the BN crucible was sealed in the upper part of another long SUS container (outer diameter of 12.7 mm, inner diameter of 10.7 mm, and height of 300 mm) with Ar gas. The upper part of the container was heated at 773 K for 12 h, and the lower part was cooled using a fan to keep the temperature almost the same with the room temperature. By generating a temperature gradient in the container, the Na was evaporated from the mixture in the crucible, and condensed on the inner wall in the lower cooler part of the container.
After heating, the crucible was taken out in the glove box, and the weight loss from the sample was measured to calculate the amount of evaporated Na against the amount of Na in the starting mixture. The sample in the crucible was subjected to an alcohol treatment by which any excess Na and Na–Sn and Na–Ga compounds in the sample were completely reacted with 2-propanol followed by ethanol, and the reaction products of Na were removed from the samples by washing with water. A mixture of Ga and Sn remained after the decomposition of Na–Sn and Na–Ga compounds by the alcohol treatment and a Sr–Ga–Si ternary compound in the sample were then subjected to a hydrochloric acid treatment by dissolving in an aqueous hydrochloric acid (35.0–37.0 mass% HCl) and rinsing the residue with water.
The morphologies of the obtained single crystals were observed with an optical microscope (Olympus, SZX16) and a scanning electron microscope (SEM; JEOL, JXA-8200) at an accelerating voltage of 15 kV. The single crystals were cut to about 100–150 μm in size and subjected to X-ray diffraction (XRD) measurements (Bruker, D8 QUEST). APEX3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 was used to collect the diffraction data and refine the unit cells. X-ray absorption correction was performed by SADABS installed in APEX3.14 SHELEXL-97 software15 was used to refine the occupancies, coordinates, and displacement parameters of the atoms. The crystal structure was drawn by VESTA.16 The compositions of the obtained single crystals were analyzed with an electron-probe microanalyzer (EPMA, JEOL, JXA-8200). The electrical characteristics of the single crystals were measured in the range of 8–300 K by the four-terminal method using Ag paste as electrodes.
14 was used to collect the diffraction data and refine the unit cells. X-ray absorption correction was performed by SADABS installed in APEX3.14 SHELEXL-97 software15 was used to refine the occupancies, coordinates, and displacement parameters of the atoms. The crystal structure was drawn by VESTA.16 The compositions of the obtained single crystals were analyzed with an electron-probe microanalyzer (EPMA, JEOL, JXA-8200). The electrical characteristics of the single crystals were measured in the range of 8–300 K by the four-terminal method using Ag paste as electrodes.
3. Results and discussion
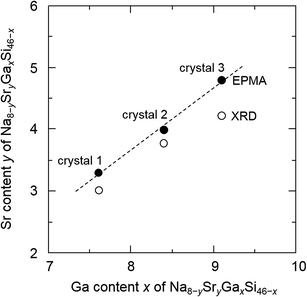
When Sr was heated with other starting materials, at 1173 K for 12 h, a SrGaSi ternary compound was crystallized.17 Once this compound was formed, it did not melt or dissolve into a liquid phase at 773 K and Sr was not provided to the crystal growth of clathrate. Thus, Sr was added to the Na–Ga–Si–Sn mixture prepared in advance. By heating the Na–Ga–Si–Sn mixture and Sr at 773 K for 12 h, 46% of Na was evaporated. The residual excess Na and Na of Na–Sn and Na–Ga compounds in the sample were removed by the alcohol treatment. After hydrochloric acid treatment for removal of Sn and Ga by decomposition of the Na–Sn and Na–Ga compounds and a SrGaSi compound contained in the product, the black single crystals of clathrate were clearly separated. Fig. 1 shows optical and SEM micrographs of the crystals picked up from the obtained sample. Quantitative EPMA analyses were performed on the flat surfaces of the three black single crystals with sizes of 0.96 mm (crystal 1), 0.93 mm (crystal 2), and 0.83 mm (crystal 3) which were taken from the same sample. The Na, Sr, Ga, and Si contents of crystals 1, 2, and 3 are summarized in Table 1. The chemical formulas of crystals 1, 2, and 3 were calculated by setting the total number of Si and Ga atoms to 46 (based on the general formula of the type-I clathrate, Na8−ySryGaxSi46−x) as Na4.9(2)Sr3.3(2)Ga7.6(2)Si38.4(2), Na3.8(5)Sr4.0(3)Ga8.4(2)Si37.3(2), and Na3.2(1)Sr4.8(1)Ga9.1(1)Si36.9(2), respectively. The sum of the Na and Sr numbers was close to 8. As shown in Fig. 2, the Sr content, y, linearly increased as the Ga content, x, increased. The largest crystal, crystal 1, had the smallest Sr and Ga contents among the three crystals.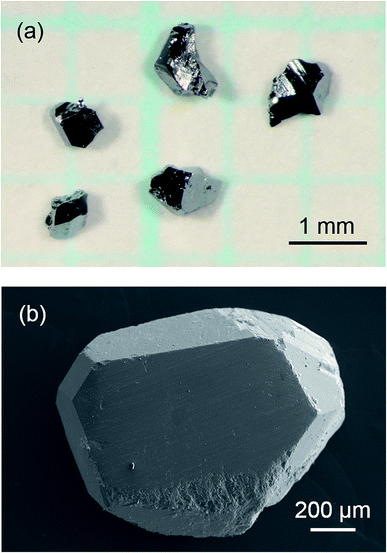 | ||
| Fig. 1 Optical micrograph of the sample after the hydrochloric acid treatment (a) and an SEM image of a Na–Sr–Ga–Si quaternary type-I clathrate single crystal (b). | ||
| Crystal | Composition (EPMA) | ||||
|---|---|---|---|---|---|
| Na (at%) | Sr (at%) | Ga (at%) | Si (at%) | Chemical formual (Ga + Si = 46) | |
| 1 | 9.08(44) | 6.06(33) | 14.01(45) | 70.85(31) | Na4.9(2)Sr3.3(2)Ga7.6(2)Si38.4(2) |
| 2 | 7.03(91) | 7.48(47) | 15.66(32) | 69.83(32) | Na3.8(5)Sr4.0(3)Ga8.4(1)Si37.6(2) |
| 3 | 5.88(3) | 8.89(13) | 16.93(18) | 68.30(27) | Na3.2(1)Sr4.8(1)Ga9.1(1)Si36.9(2) |
In addition, cross sections of the crystals were also analyzed by EPMA; the results are shown in Fig. S1.† In crystals 2 and 3, Na, Sr, Ga, and Si were homogeneously distributed. In contrast, in crystal 1, the surface was Na4.9(2)Sr3.3(2)Ga7.6(2)Si38.4(2), but its composition changed sharply at one region that did not contain Sr and had a composition of Na8.3(2)Ga4.3(2)Si41.7(2). The crystal 1 containing the Na–Ga–Si ternary clathrate part which were surrounded with the low Sr content Na4.9(2)Sr3.3(2)Ga7.6(2)Si38.4(2) was probably grown at the early stage of the crystal formation. This may indicate that Sr which was added to the Na–Ga–Si–Sn mixture was gradually provided to the melt during heating at 773 K. Homogeneous and high Sr concentrations in the crystals 2 and 3 suggest the crystal growth from Sr-rich melts at later stages. Further studies are needed to clarify the process by which crystals with different Sr and Ga contents are grown from the same starting mixture.
The results of the X-ray crystal structure analyses of pieces from the surface of crystals 1–3 are listed in Tables 2–4. The crystal structures were analyzed based on the model of a type-I clathrate (space group, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n). The occupancies of Si/Ga1(24k), Si/Ga2(16i), and Si/Ga3(6c) were refined under the constraint that the total Ga content was equal to that measured by EPMA. The reliability factor, R1(all), was in the range of 1.13–1.34% for all analyses. The chemical formulas of crystals 1, 2, and 3 were calculated from the refined occupancies as Na5.04Sr2.96(2)Ga7.6Si38.4, Na4.20Sr3.80(3)Ga8.4Si37.6, and Na3.92Sr4.08(2)Ga9.1Si36.9, respectively. The Sr contents were relatively consistent with those derived by EPMA (3.3(2), 4.0(3), 4.8(1)). The unit cell constants increased with the increase of the Ga and Sr contents (10.3645(3), 10.3747(3), and 10.3804(4) Å for crystals 1, 2, and 3, respectively).
n). The occupancies of Si/Ga1(24k), Si/Ga2(16i), and Si/Ga3(6c) were refined under the constraint that the total Ga content was equal to that measured by EPMA. The reliability factor, R1(all), was in the range of 1.13–1.34% for all analyses. The chemical formulas of crystals 1, 2, and 3 were calculated from the refined occupancies as Na5.04Sr2.96(2)Ga7.6Si38.4, Na4.20Sr3.80(3)Ga8.4Si37.6, and Na3.92Sr4.08(2)Ga9.1Si36.9, respectively. The Sr contents were relatively consistent with those derived by EPMA (3.3(2), 4.0(3), 4.8(1)). The unit cell constants increased with the increase of the Ga and Sr contents (10.3645(3), 10.3747(3), and 10.3804(4) Å for crystals 1, 2, and 3, respectively).
| Crystal 1 | Crystal 2 | Crystal 3 | |
|---|---|---|---|
| a R1 = Σ||Fo| − |Fc||/Σ|Fo|. wR2 = [Σw(Fo2 − Fc2)2/Σ(wFo2)2]1/2, w = 1/[σ2(Fo2) + (aP)2 + bP], where Fo is the observed structure factor, Fc is the calculated structure factor, σ is the standard deviation of Fc2, and P = (Fo2 + 2Fc2)/3. S = [Σw(Fo2 − Fc2)2/(n − p)]1/2, where n is the number of reflections and p is the total number of parameters refined. | |||
| Chemical formula | Na5.04Sr2.96(2)Ga7.6Si38.4 | Na4.20Sr3.80(3)Ga8.4Si37.6 | Na3.92Sr4.08(2)Ga9.1Si36.9 |
| Formula weight, Mr (g mol−1), Z | 1983.75, 1 | 2071.35, 1 | 2118.58, 1 |
| Temperature, T (K) | 302(2) | 302(2) | 302(2) |
| Crystal system, space group | Cubic, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
Cubic, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
Cubic, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
| Unit-cell dimension, a (Å) | 10.3645(3) | 10.3747(3) | 10.3804(4) |
| Unit-cell volume, V (Å3) | 1113.38(10) | 1116.67(10) | 1118.52(13) |
| Calculated density, Dcal (Mg m−3) | 2.958(5) | 3.080(6) | 3.146(6) |
| Radiation wavelength, l (Å) | 0.71073 | 0.71073 | 0.71073 |
| Size (mm3) | 0.216 × 0.160 × 0.152 | 0.141 × 0.174 × 0.183 | 0.142 × 0.152 × 0.149 |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan |
| absorption coefficient, μ (mm−1) | 9.139 | 10.544 | 11.248 |
| Limiting indices | −11 ≤ h ≤ 14 | −10 ≤ h ≤ 14 | −13 ≤ h ≤ 9 |
| −10 ≤ k ≤ 13 | −12 ≤ k ≤ 12 | −10 ≤ k ≤ 14 | |
| −12 ≤ l ≤ 10 | −14 ≤ l ≤ 11 | −11 ≤ l ≤ 13 | |
| F000 | 941 | 977 | 997 |
| θ range for date colletion (°) | 2.779–28.686 | 2.777–28.656 | 2.775–28.638 |
| Reflections collected/unique | 3300/284 | 3777/284 | 2901/284 |
| Rint | 0.0246 | 0.0310 | 0.0389 |
| Date/restraints/parameters | 284/1/21 | 284/1/21 | 284/1/21 |
| Weight parameters, a, b | 0.0101, 0.2875 | 0.0129, 0.2629 | 0.0101, 0.2209 |
| Goodness-of-fit on F2, S | 1.223, 1.220 | 1.191, 1.189 | 1.223, 1.220 |
| R1, wR2 (I > 2σ(I)) | 0.0113, 0.0254 | 0.0113, 0.0283 | 0.0134, 0.0311 |
| R1, wR2 (all date) | 0.0124, 0.0258 | 0.0125, 0.0287 | 0.0143, 0.0314 |
| Largest diff. park and hole, Δρ (eÅ−3) | 0.326, −0.242 | 0.261, −0.239 | 0.329, −0.244 |
| Atom | Site | Occupancy | x | y | z | Ueq |
|---|---|---|---|---|---|---|
| Crystal 1 (Na5.04Sr2.96(2)Ga7.6Si38.4) | ||||||
| Na/Sr 1 | 6d | 0.709/0.291(3) | 1/4 | 1/2 | 0 | 0.0440(4) |
| Na/Sr 2 | 2a | 0.395/0.605(3) | 0 | 0 | 0 | 0.0113(2) |
| Si/Ga 1 | 24k | 0.8995/0.1005(7) | 0 | 0.30576(3) | 0.11653(3) | 0.00852(12) |
| Si/Ga 2 | 16i | 0.9656/0.0344(10) | 0.18442(3) | x | x | 0.00811(15) |
| Si/Ga 3 | 6c | 0.227/0.773(2) | 1/4 | 0 | 1/2 | 0.00876(13) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Crystal 2 (Na4.20Sr3.80(3)Ga8.4Si37.6) | ||||||
| Na/Sr 1 | 6d | 0.600/0.400(3) | 1/4 | 1/2 | 0 | 0.0420(4) |
| Na/Sr 2 | 2a | 0.300/0.700(4) | 0 | 0 | 0 | 0.0104(2) |
| Si/Ga 1 | 24k | 0.8775/0.1225(7) | 0 | 0.30587(3) | 0.11661(3) | 0.00834(13) |
| Si/Ga 2 | 16i | 0.9521/0.0479(10) | 0.18454(3) | x | x | 0.00800(16) |
| Si/Ga 3 | 6c | 0.218/0.782(2) | 1/4 | 0 | 1/2 | 0.00853(15) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Crystal 3 (Na3.92Sr4.08(2)Ga9.1Si36.9) | ||||||
| Na/Sr 1 | 6d | 0.565/0.435(3) | 1/4 | 1/2 | 0 | 0.0423(4) |
| Na/Sr 2 | 2a | 0.266/0.734(3) | 0 | 0 | 0 | 0.0114(2) |
| Si/Ga 1 | 24k | 0.8613/0.1387(8) | 0 | 0.30578(4) | 0.11662(4) | 0.00884(14) |
| Si/Ga 2 | 16i | 0.9387/0.0613(11) | 0.18452(3) | x | x | 0.00851(17) |
| Si/Ga 3 | 6c | 0.202/0.798(2) | 1/4 | 0 | 1/2 | 0.00879(15) |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| Crystal 1 (Na5.04Sr2.96(2)Ga7.6Si38.4) | ||||||
| Na/Sr1 | 0.0272(5) | 0.0524(5) | =U11 | 0 | 0 | 0 |
| Na/Sr2 | 0.0113(2) | =U11 | =U11 | 0 | 0 | 0 |
| Si/Ga1 | 0.00866(18) | 0.00866(18) | 0.00825(18) | −0.00038(12) | 0 | 0 |
| Si/Ga2 | 0.00811(15) | =U11 | =U11 | −0.00057(9) | =U23 | =U23 |
| Si/Ga3 | 0.0099(2) | 0.00819(15) | =U22 | 0 | 0 | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Crystal 2 (Na4.20Sr3.80(3)Ga8.4Si37.6) | ||||||
| Na/Sr1 | 0.0244(5) | 0.0508(5) | =U11 | 0 | 0 | 0 |
| Na/Sr2 | 0.0104(2) | =U11 | =U11 | 0 | 0 | 0 |
| Si/Ga1 | 0.00860(18) | 0.00827(18) | 0.00814(19) | −0.00028(12) | 0 | 0 |
| Si/Ga2 | 0.00800(16) | =U11 | =U11 | −0.00058(10) | =U23 | =U23 |
| Si/Ga3 | 0.0097(2) | 0.00819(16) | =U22 | 0 | 0 | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Crystal 3 (Na3.92Sr4.08(2)Ga9.1Si36.9) | ||||||
| Na/Sr1 | 0.0244(5) | 0.0512(5) | =U11 | 0 | 0 | 0 |
| Na/Sr2 | 0.0114(2) | =U11 | =U11 | 0 | 0 | 0 |
| Si/Ga1 | 0.0090(2) | 0.0089(2) | 0.0086(2) | −0.00038(13) | 0 | 0 |
| Si/Ga2 | 0.00851(17) | =U11 | =U11 | −0.00049(11) | =U23 | =U23 |
| Si/Ga3 | 0.0101(2) | 0.00815(17) | =U22 | 0 | 0 | 0 |
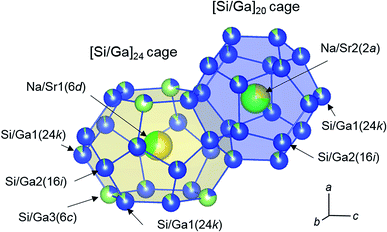
The pentagonal dodecahedral ([Si/Ga]20) and tetrakaidecahedral ([Si/Ga]24) cages of Na3.92Sr4.08(2)Ga9.1Si36.9 (crystal 3) are depicted in Fig. 3. In crystals 1, 2, and 3, the Ga occupancies for the Si/Ga1(24k), Si/Ga2(16i), and Si/Ga3(6c) sites ranged from 0.1005(7) to 0.1387(8), 0.0344(10) to 0.0613(11), and 0.773(2) to 0.798(2), respectively, and Ga atoms preferentially occupied the Si/Ga3(6c) sites in the [Si/Ga]24 cages. Similar preferential occupation of Ga atoms in the Si/Ga3(6c) sites has been previously reported for other type-I clathrates, Rb6.34Na1.66(2)Ga8.02Si37.98(3) and Cs6Na2Ga8.25Si37.75(3).13 The occupancies of the Sr atoms for the Na/Sr2(2a) sites in the [Si/Ga]20 cages (0.605(3)–0.734(3)) were larger than those for the Na/Sr1(6d) sites in the [Si/Ga]24 cages (0.291(3)–0.435(3)). Further, the volumes of the [Si/Ga]20 cages (107.2–107.8 Å3) were smaller than those of the [Si/Ga]24 cages (142.8–143.4 Å3). In the Cs6Na2Ga8.25Si37.75 and Rb6.34Na1.66Ga8.02Si37.98 crystals, Cs and Rb fully occupy the 6d sites inside the larger cage of [Si/Ga]24, which indicates selective occupation of larger cations in larger cages.13 In the present study, Sr atom preferred to occupy the 2a sites in the smaller [Si/Ga]20 cage despite the atomic size of Sr larger than that of Na. This result suggests that the cation size is not related to the site preference in the Na8−ySryGaxSi46−x clathrate. Since the electronegativities (Pauling scale18) of Rb (χRbP = 0.82) and Cs (χCsP = 0.79) in the Cs6Na2Ga8.25Si37.75 and Rb6.34Na1.66Ga8.02Si37.98 were smaller than that of Na (χNaP = 0.93), the outermost electrons of Rb and Cs atoms could effectively be transferred to the more electronegative Ga atoms (χGaP = 1.81). In the case of Na8−ySryGaxSi46−x, the electronegativities of Sr (χSrP = 0.95) and Na (χNaP = 0.93) were similar, but the first ionization energy of Na (5.139 eV) was smaller than that of Sr (5.695 eV).19 Thus, Na atoms may preferentially occupy the Ga-rich [Si/Ga]24 cages of Na8−ySryGaxSi46−x clathrates.
The crystal structure of Na3.92Sr4.08(2)Ga9.1Si36.9 (crystal 3) is drawn with displacement ellipsoids representing the 99% probability region in Fig. S2.† The U22 = U33 parameters of the Na/Sr1(6d) sites in crystals 1, 2, and 3 were 0.0508(4), 0.0524(5), and 0.0512(5) Å2, respectively, which were larger than the atomic displacement parameters of U11 (0.0272(5), 0.0244(5), and 0.0244(5) Å2) and much larger than those of Uij at other sites (≤0.0114(2) Å2) (Table 4). This probably corresponds to the large, distorted shape of the [Si/Ga]24 cages. Such large anisotropic atomic displacement parameters of U22 = U33 for the 6d sites in [Si/Ga]24 cages have similarly been reported for other type-I clathrates including Na8GaxSi46−x (4.94(6) ≤ x ≤ 5.70(7)),12 A8Ga8Si38 (A = K, Rb, Cs),5 Rb6.34Na1.66(2)Ga8.02Si37.98(3), and Cs6Na2Ga8.25Si37.75(3).13
Fig. 4 shows the temperature dependence of electrical resistivity, ρ, measured for the three crystals and the type-I clathrate single crystal, Na8Ga5.7Si40.3, synthesized by our group in a previous study.12 The ρ values for crystals 1, 2, and 3 increased with increasing temperature, reaching 0.34, 0.55, and 1.05 mΩ cm, respectively, at room temperature (300 K). The previously reported electrical resistivities at 280–300 K for other type-I clathrates, Na8Si46,20 Na8GaxSi46−x,12 and Sr8GaxSi46−x,8,9 are compared to those in the Na8−ySryGaxSi46−x sample (crystal 1: Na5.0Sr3.0Ga7.6Si38.4, crystal 2: Na4.2Sr3.8Ga8.4Si37.6, and crystal 3: Na3.9Sr4.1Ga9.1Si36.9) with respect to their respective Ga contents, x, in Fig. 5. The electrical resistivity of crystal 1 was plotted at x = 7.6 in Fig. 5 even though one region in the Na4.9(2)Sr3.3(2)Ga7.6(2)Si38.4(2) crystal had a composition of Na8.3(2)Ga4.3(2)Si41.7(2) (as shown in Fig. S1†).
 | ||
| Fig. 4 Temperature dependence of the electrical resistivity of crystal 1 (Na5.0Sr3.0Ga7.6Si38.4), crystal 2 (Na4.2Sr3.8Ga8.4Si37.6), crystal 3 (Na3.9Sr4.1Ga9.1Si36.9), and Na8Ga5.7Si40.3.12 | ||
 | ||
| Fig. 5 Electrical resistivity at room temperature versus Ga content of type-I clathrates: Na8Si46,20 Na8GaxSi46−x,12 Sr8GaxSi46−x,8,9 and Na8−ySryGaxSi46−x crystals 1 (Na5.0Sr3.0Ga7.6Si38.4), 2 (Na4.2Sr3.8Ga8.4Si37.6), and 3 (Na3.9Sr4.1Ga9.1Si36.9). | ||
The resistivity of Na8GaxSi46−x was greater than that of Na8Si46 (0.098 mΩ cm) as measured previously by Stefanoski at 300 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and increased with increasing x.12 Eight electrons are formally transferred from Na to the Si framework to form Na8Si46; these electrons enter into the conduction band. Since the valences of Si and Ga are 4 and 3, respectively, the number of the electrons (or carriers) in the conduction band should decrease with increasing Ga content in the [Si/Ga] framework, thus reducing the conductivities and changing the structure to a semiconducting Zintl clathrate with x = 8 like A8Ga8Si38 (A = K, Rb, Cs).5 The ρ values of Na8−ySryGaxSi46−x and Sr8GaxSi46−x followed trends similar to that shown in Fig. 5. Na5.04Sr2.96(2)Ga7.6Si38.4 (crystal 1), Na4.20Sr3.80(3)Ga8.4Si37.6 (crystal 2), and Na3.92Sr4.08(2)Ga9.1Si36.9 (crystal 3) have valence electron numbers of 3.36, 3.40, and 2.98, respectively, and crystal 3 exhibited the highest resistivity among Na8−ySryGaxSi46−x. The resistivity of crystal 2 is higher than crystal 1 although the numbers are almost the same. The resistivity may also be related to the content of Ga atoms which scatter conduction carrier electrons and holes on the clathrate frame. The Ga content of crystal 2 (x = 8.40) is higher than crystal 1 (x = 7.6).
20 and increased with increasing x.12 Eight electrons are formally transferred from Na to the Si framework to form Na8Si46; these electrons enter into the conduction band. Since the valences of Si and Ga are 4 and 3, respectively, the number of the electrons (or carriers) in the conduction band should decrease with increasing Ga content in the [Si/Ga] framework, thus reducing the conductivities and changing the structure to a semiconducting Zintl clathrate with x = 8 like A8Ga8Si38 (A = K, Rb, Cs).5 The ρ values of Na8−ySryGaxSi46−x and Sr8GaxSi46−x followed trends similar to that shown in Fig. 5. Na5.04Sr2.96(2)Ga7.6Si38.4 (crystal 1), Na4.20Sr3.80(3)Ga8.4Si37.6 (crystal 2), and Na3.92Sr4.08(2)Ga9.1Si36.9 (crystal 3) have valence electron numbers of 3.36, 3.40, and 2.98, respectively, and crystal 3 exhibited the highest resistivity among Na8−ySryGaxSi46−x. The resistivity of crystal 2 is higher than crystal 1 although the numbers are almost the same. The resistivity may also be related to the content of Ga atoms which scatter conduction carrier electrons and holes on the clathrate frame. The Ga content of crystal 2 (x = 8.40) is higher than crystal 1 (x = 7.6).
4. Summary
Here, single crystals of quaternary type-I clathrates, Na8−ySryGaxSi46−x, were grown by heating a Na–Sr–Ga–Si melt at 773 K and evaporating the Na from the melt in an Ar atmosphere. The compositions and crystal structures of the single crystals were analyzed by EPMA and XRD. The Ga and Sr contents (x and y) of the obtained crystals were 7.6–9.1 and 2.96(2)–4.08(2), respectively. Na atoms preferentially occupied the Na/Si1(6d) sites, whereas Ga atoms preferentially occupied the Si/Ga3(6c) sites in the [Si/Ga]24 cages. The electrical resistivities of the Na8−ySryGaxSi46−x single crystals were found to increase from 0.34 to 1.05 mΩ cm with increasing x and y at 300 K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank T. Kamaya for the EPMA analysis. This work was supported by JSPS KAKENHI grants (JP16H06123 and JP18H01887).References
- J. S. Kasper, P. Hagenmuller, M. Pouchard and C. Cros, Science, 1965, 150, 1713 CrossRef CAS PubMed.
- C. Cros and M. Pouchard, C. R. Chim., 2009, 12, 1014 CrossRef CAS.
- H. Kawaji, H. Horie, S. Yamanaka and M. Ishikawa, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 74, 8 Search PubMed.
- S. Yamanaka, E. Enishi, H. Fukuoka and M. Yasukawa, Inorg. Chem., 2000, 39, 56 CrossRef CAS PubMed.
- F. Sui, H. He, S. Bobev, J. Zhao, F. E. Osterloh and S. M. Kauzlarich, Chem. Mater., 2015, 27, 2812 CrossRef CAS.
- J. L. Cohn, G. S. Nolas, V. Fessatidis, T. H. Metcalf and G. A. Slack, Phys. Rev. Lett., 1999, 82, 779 CrossRef CAS.
- V. L. Kuznetsov, L. A. Kuznetsova, A. E. Kaliazin and D. M. Rowe, J. Appl. Phys., 2000, 87, 7871 CrossRef CAS.
- M. Imai, K. Nishida, T. Kimura and K. Yamada, J. Alloys Compd., 2002, 335, 270 CrossRef CAS.
- K. Suekuni, M. A. Avila, K. Umeo and T. Takabatake, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 195210 CrossRef.
- H. Morito, M. Shimoda and H. Yamane, J. Cryst. Growth, 2016, 450, 164 CrossRef CAS.
- H. Morito, M. Shimoda, H. Yamane and K. Fujiwara, Cryst. Growth Des., 2018, 18, 351 CrossRef CAS.
- H. Urushiyama, M. Morito, H. Yamane and M. Terauchi, RSC Adv., 2018, 8, 40505 RSC.
- M. C. Schäfer and S. Bobev, Inorganics, 2014, 2, 79 CrossRef.
- Bruker AXS, Instrument Service v6.2.3, APEX3 v2016.9-0, SADABS, SAINT v8.37A, XT v2014/5, Bruker AXS Inc., Madison, Wisconsin, USA, 2014 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2015, C71, 3 CrossRef PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272 CrossRef CAS.
- R. L. Meng, B. Lorenz, Y. S. Wang, J. Cmaidalka, Y. Y. Sun, Y. Y. Xue, J. K. Meen and C. W. Chu, Physica C, 2002, 382, 113 CrossRef CAS.
- L. Pauling, Nature of the Chemical Bond, Cornell University Press, 1960, pp. 88–107 Search PubMed.
- CRC Handbook Chemistry and Physics, ed. W. M. Haynes, CRC Press, 97th edn, 2016–2017, pp. 10–204 Search PubMed.
- S. Stefanoski, J. Martin and G. S. Nolas, J. Phys.: Condens. Matter, 2010, 22, 485404 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1898606–1898608. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra01489f |
| This journal is © The Royal Society of Chemistry 2019 |