Open Access Article
Open Access ArticleAdamantyl and homoadamantyl derivatives from Garcinia multiflora fruits†
Yu Chen‡
a,
Ziyu Ma‡a,
Haida Tenga,
Fei Gana,
Hui Xiongb,
Zhinan Mei*b and
Guangzhong Yang *bc
*bc
aCollege of Chemistry and Material Sciences, South-Central University for Nationalities, Wuhan 430074, P. R. China
bSchool of Pharmaceutical Sciences, South-Central University for Nationalities, Wuhan 430074, P. R. China. E-mail: meizhinan@163.com; yanggz888@126.com; Fax: +86 27 6784 1196; Tel: +86 27 6784 1196
cNational Demonstration Center for Experimental Ethnopharmacology Education (South-Central University for Nationalities), Wuhan 430074, P. R. China
First published on 17th April 2019
Abstract
Nine undescribed caged polycyclic polyprenylated acylphloroglucinols (PPAPs), including adamantane type PPAPs (1–2), and homoadamantane type PPAPs (3–9), were isolated from the fruits of Garcinia multiflora, along with three known analogues. A new epimeric pair of isohypersampsonone B (5) and epi-isohypersampsonone B (6), featuring an unusual hexahydrofuro[2,3-b]furan-diepoxy ring system fused in a homoadamantane skeleton, was not separated due to the rapid equilibration between the two isomeric forms. All new caged PPAPs (1–9), sharing a common isogeranyl group, were determined on the basis of comprehensive NMR and MS spectroscopic data. Their cytotoxicity against three human tumor cell lines (SGC-7901, HepG2, HCT-116) and the nitric oxide production inhibitory activity of lipopolysaccharides-stimulated RAW 264.7 cells were tested. Compounds 8 and 12 displayed mild cytotoxicity against three human cancer cell lines with IC50 values of 10–20 μM. Furthermore, compounds 8 and 12 also exhibited NO production inhibitory effect with an IC50 value of 18.24 and 12.50 μM respectively.
1 Introduction
Fruits, fruit rinds, flowers, leaves, barks and stems originating from the genus Garcinia plant have been used as traditional medicine.1 They have received considerable attention from the scientific community for their powerful capacities to produce structurally fascinating and pharmacologically active polycyclic polyprenylated acylphloroglucinols (PPAPs), such as garcinol,2 xanthochymol,3 isoxanthochymol,3 cycloxanthochymol,3 etc. PPAPs with highly oxygenated acylphloroglucinol-derived cores are substituted with one or more prenyl or geranyl side chains. Up to now, more than 400 PPAPs have been isolated from nature, of which the majority are bicyclic polyprenylated acylphloroglucinols (BPAPs) with a bicyclo[3.3.1]nonane-2,4,9-trione core. Caged PPAPs are a subclass of PPAPs containing the adamantane and homoadamantane skeleton, most of which are isolated from Hypericum species, especially from H. sampsonii. However, caged PPAPs are rarely reported from Garcinia plants.4 Garcinia multiflora Champ. is a traditional Zhuang medicine widely distributed in the south of China. Its fruits are edible consisting of vitamins, proteins, and minerals, but may also provide pharmacologically active compounds.5 Previous phytochemical investigations on the fruits led to isolation of seven new PPAPs with anti-inflammatory activity, including two caged PPAPs garcimultiflorone D and G.6–8Taken together, these results prompted us to investigate the isolation of further caged PPAPs form G. multiflora and evaluate their biological activities. As a result, nine undescribed caged polycyclic polyprenylated acylphloroglucinols (PPAPs), including adamantane type PPAPs (1–2) and homoadamantane type PPAPs (3–9) were isolated from the fruits of G. multiflora, along with three known analogues. Herein, we report the isolation, structural elucidation, and biological activities of these isolated compounds.
2 Result and discussion
2.1 Structural elucidation of isolated compounds
Compound 1 was isolated as colorless, amorphous powder. Its molecular formula was determined to be C38H48O5 on the basis of the negative HR-ESI-MS ion at m/z 583.34296 [M − H]− (calcd 583.34290). The 1H NMR spectrum of 1 revealed the presence of an unsubstituted benzoyl group [δH 7.23 (2H, d, J = 7.2 Hz), 7.49 (1H, t, J = 7.2 Hz) and 7.32 (2H, t, J = 7.2 Hz)], two olefinic protons [δH 4.98 (1H, t, J = 7.2 Hz) and 5.03 (1H, t, J = 7.2 Hz)], one terminal double bonds [δH 4.64 (1H, s) and 4.59 (1H, s)] and nine singlet methyl groups (δH 1.30–1.69). The 13C NMR data, aided by a HSQC experiment, disclosed the presence of 38 carbon signals, including nine methyl groups, five methylene groups, 11 methine groups (7 olefinic carbons), and 13 quaternary carbons. The characteristic resonances of three nonconjugated carbonyl groups at δC 203.0 (C-2), 204.4 (C-4), 203.7 (C-9), four quaternary carbons at δC 83.7 (C-1), 74.3 (C-3), 69.7 (C-5), 56.7 (C-8), two methine groups at δC 47.1 (C-7) and 57.9 (C-32), and a methylene group at δC 44.9 (C-6) indicated that the molecule consists of an adamantane skeleton. In comparison of its 1H and 13C NMR data with those of garcimultiflorone D,6 an adamantane type PPAPs from the same plant, suggested that their structures were closely related, except that the diagnostic difference of chemical shifts at δC 57.9 (C-32), 62.8 (C-33), and 58.9 (C-34) for 1 and δC 51.1 (C-32), 60.7 (C-33), and 61.1 (C-34) for garcimultiflorone D. The data indicated that 1 was 33-epimer of garcimultiflorone D. According to the literature,9 the chemical shift of C-34 was about 58 ppm in (33S)-configured 33,34-epoxide adamantane type PPAPs while in the spectrum of (33R)-configured derivatives the chemical shift of C-34 was about 62 ppm. This rule has been used to determine a number of the configurations of C-33 of 33,34-epoxide adamantane type PPAPs, which was supported by the chemical transformations and calculated ECDs. Thus, the configuration of C-33 of 1 was determined as to be S. The relative configurations at the chiral centers C-1, C-3, C-5, and C-7 were obvious for the adamantyl core. Furthermore, H-32 was determined as α-oriented based on NOE correlations of Me-38/H-6a, Me-37/H-32 in the ROESY spectrum. According to the literature,4 the name gracimultiflorone D seems to have been given to two different compounds. Their structures of 1 and sampsonione J were closely related except for the isogeranyl group at C-5 in 1 instead of a geranyl group at C-5 in sampsonione J and the different configuration of C-33. In order avoid to confusion, it is the best way to name 1 as epi-isosampsonione J.Compound 2 was obtained as an amorphous powder. It gave a molecular formula of C38H48O5 according to its HR-ESI-MS at m/z 585.35748 [M + H]+ (calcd. 585.35745). Detailed analysis of the 1D and 2D NMR spectra indicated that compound 2 featured a unique caged tetracyclo-[6.3.1.13,10.03,7]tridecane skeleton which was the same as those of hyperisampsins A–D.10 Comparison of the 13C NMR data of 2 with that of hyperisampsin C revealed that two compounds were similar except for the presence of an isogeranyl group at C-5 in 2 instead of a geranyl group in hyperisampsin C. The constitution of 2 was confirmed by HMBC correlations between H2-22 and C-5 (δC 68.2) and C-9 (δC 201.9). The relative configuration of 2 was established as the same that of hyperisampsin C by ROESY spectrum. Thus, compound 2 was established and named isohyperisampsin C.
Compound 3 was isolated as colorless amorphous powder. Its molecular formula was determined by its 13C NMR and HR-ESI-MS data (m/z 587.37323 [M + H]+, calcd. 587.3731) as C38H50O5. The 13C and DEPT NMR data showed the characteristic resonances of homo-adamantane type PPAPs, including three nonconjugated carbonyl groups at δC 206.6 (C-2), 206.4 (C-4) and 204.5 (C-9), four quaternary carbons at δC 82.4 (C-1), 72.1 (C-3), 66.9 (C-5), and 52.0 (C-8), two methine groups at δC 43.6 (C-7) and 57.9 (C-33), and two methylene groups at δC 48.0 (C-6) and 28.2 (C-32). Comparison of its 1H and 13C NMR data with those of hypersampsonone G,11 a known homo-adamantane type PPAP from H. sampsonii, suggested that they had a same tetrocyclo[7.3.1.13,11.03,7]tetradecane core skeleton. However, the only structural difference was an isogeranyl group at C-5 in 3 instead of a geranyl group in hypersampsonone G. This was further supported by HMBC correlations between H2-22 and C-5 (δC 66.9), C-4 (δC 206.4) and C-9 (δC 204.5). In the ROESY spectrum of 3, NOE correlations of H-7/H3-37, H-33/H3-37, H-33/H3-35 and H-18/H3-35 indicated that these protons were cofacial and designated as α-oriented. Therefore, compound 3 was established and named isohypersampsonone G.
Compound 4 was isolated as amorphous powder. It had the molecular formula C35H44O5 as determined by HR-ESI-MS at (m/z 545.32617 [M + H]+, calcd. 545.32615), with three carbon atoms less than that of 3. In comparison of 13C NMR data of 4 with those of 3, signals of C-17, C-18, and C-34 in 4 appeared at high chemical shift, suggesting that 1-hydroxy-1-methylethyl group at C-18 in 3 was replaced by a hydroxy group in 4. This was further supported by HMBC correlations between H3-35 and H3-36 and C-34 (δC 49.8), C-33 (δC 53.2) and C-18 (δC 82.0). In the ROESY spectrum of 4, NOE correlations between H-33/H3-37, H-33/H3-35 and H-18/H3-36 suggested that H-33 and 18-OH were α-oriented respectively. Thus, compound 4 was assigned as depicted in Fig. 1 and was named garcimultinone A.
Compounds (5) and (6) were isolated as inseparable epimeric mixture and obtained as white amorphous powders. The ratio of 5 and 6 is about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by NMR analysis. Their molecular formula was determined as C35H44O6 by HR-ESI-MS data (m/z 561.32141 [M + H]+, calcd. 561.32107), Firstly, we discussed the structure elucidation of 5. Detailed analysis of the 1D and 2D NMR spectra indicated that compound 5 had an unusual hexahydrofuro[2,3-b]furan-diepoxy ring system fused in homoadamantane skeleton. Comparison of 1H and 13C NMR data of 5 with those of hypersampsonone B11 indicated that the two compounds were closely related, except for an isogeranyl group at C-5 of 5 instead of a geranyl group of hypersampsonone B. The relative configuration of 5 was determined by analyzing its ROESY data. H-33 and 18-OH were determined as α and β-oriented based on the NOE correlations between H-33/H3-35 and H-18/H3-35. Except for the 13C-NMR signals of 5 mentioned above, the remaining 35 carbon signals were attributed to 6. In comparison of 1H and 13C NMR data of 6 with those of 5, it was found that NMR data of 6 were almost identical with those of 5, except for the chemical shift of C-18 (δC 98.7 in 5 and δC 100.0 in 6) and coupling constants between and CH2-17 and H-18 [δH 5.84 (t, J = 6.6 Hz) in 5 and 5.79 (dd, J = 6.6, 3.6 Hz) in 6], indicating that 6 was a 18-epimer of 5. Thus, compound 5 and 6 were assigned as depicted in Fig. 1 and were named isohypersampsonone B and epi-isohypersampsonone B, respectively. We tried to isolate the epimeric mixture by HPLC. However, it was unsuccessful for the isolation of isohypersampsonone B and epi-isohypersampsonone B due to the rapid equilibration between the two isomeric forms.
1 by NMR analysis. Their molecular formula was determined as C35H44O6 by HR-ESI-MS data (m/z 561.32141 [M + H]+, calcd. 561.32107), Firstly, we discussed the structure elucidation of 5. Detailed analysis of the 1D and 2D NMR spectra indicated that compound 5 had an unusual hexahydrofuro[2,3-b]furan-diepoxy ring system fused in homoadamantane skeleton. Comparison of 1H and 13C NMR data of 5 with those of hypersampsonone B11 indicated that the two compounds were closely related, except for an isogeranyl group at C-5 of 5 instead of a geranyl group of hypersampsonone B. The relative configuration of 5 was determined by analyzing its ROESY data. H-33 and 18-OH were determined as α and β-oriented based on the NOE correlations between H-33/H3-35 and H-18/H3-35. Except for the 13C-NMR signals of 5 mentioned above, the remaining 35 carbon signals were attributed to 6. In comparison of 1H and 13C NMR data of 6 with those of 5, it was found that NMR data of 6 were almost identical with those of 5, except for the chemical shift of C-18 (δC 98.7 in 5 and δC 100.0 in 6) and coupling constants between and CH2-17 and H-18 [δH 5.84 (t, J = 6.6 Hz) in 5 and 5.79 (dd, J = 6.6, 3.6 Hz) in 6], indicating that 6 was a 18-epimer of 5. Thus, compound 5 and 6 were assigned as depicted in Fig. 1 and were named isohypersampsonone B and epi-isohypersampsonone B, respectively. We tried to isolate the epimeric mixture by HPLC. However, it was unsuccessful for the isolation of isohypersampsonone B and epi-isohypersampsonone B due to the rapid equilibration between the two isomeric forms.
Compound 7 was obtained white amorphous powder. It gave the molecular formula of C38H50O7 on the basis of HR-ESI-MS data (m/z 619.36310 [M + H]+, calcd. 619.36293). Furthermore, the NMR data of 7 were similar to those of hypersampsonone C,11 indicating that 7 is also a homoadamantane derivative with an unique tetrahydrofuro[3,4-b]furan moiety. Extensive comparison of NMR data of 7 with those of hypersampsonone C revealed that the geranyl attached to C-5 in hypersampsonone C was replaced by an isogeranyl in 7. The constitution structure of 7 was also confirmed by 2D-NMR spectroscopy. The relative configuration of 7 was assigned to be the same as that of hypersampsonone C by comparison of its 1D NMR and ROESY data with those of hypersampsonone C. Thus, the structure of 7 was deduced completely as showed in Fig. 1 and was named isohypersampsonone C.
Compound 8 was isolated as amorphous powder. Its molecular formula was determined by its 13C NMR and HR-ESI-MS data (m/z 635.35791 [M + H]+, calcd. 635.35784) as C38H50O8, with one more O-atom than that of garcimultiflorone G.8 Furthermore, these NMR data showed high degrees of similarity to those of hyperisampsin O,12 which suggested that 8 is also a homoadamantane PPAP with an 1,2-dioxepane functionality. In comparison with those of hyperisampsin O indicated that 8 possessed an isogeranyl at C-5 instead of a geranyl at C-5 in hyperisampsin O. In the ROESY spectrum of 8, NOE correlations between H-33/H3-35, H-18/H-33 and H3-37/H-33 implied that both H-18 and H-33 were α-oriented. Therefore, compound 8 was assigned as depicted in Fig. 1 and was named isohyperisampsin O.
Compound 9 was isolated as amorphous powder. It had the molecular formula C35H44O7 as determined by HR-ESI-MS at (m/z 577.31610 [M + H]+, calcd. 577.31598), with three carbon atoms less than that of 8. In comparison with 8, signals of C-17 and C-18 in 9 appeared at high chemical shift, suggesting that 1-hydroperoxy-1-methylethyl group at C-18 in 8 was replaced by a hydroxy group in 9. This was further supported by HMBC correlations from H2-17 to C-3 (δC 66.6), C-18 (δC 99.8), C-2 (δC 208.4) and C-4 (δC 205.4). 18-OH configuration was established as β-oriented based on the NOE correlations between H-18/H-33, H-33/H3-35 in the ROESY spectrum of 9. Thus, compound 9 was assigned as depicted and was named garcimultinone B (Fig. 2–4).
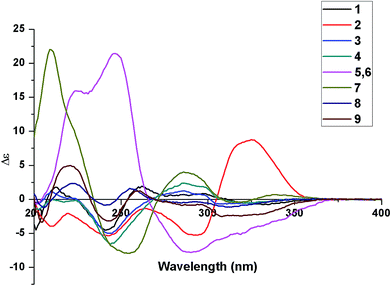
The absolute stereochemistry of 1–9 were assigned by CD analysis. Nine new isolates were elucidated to possess adamantyl and homoadamantyl skeleton with an isogeranyl group. Considering the isogeranyl group away from the chromophoric system, the absolute configuration of C-23 has an insignificant effect on the CD spectrum.13 Thus, the absolute configurations of compounds 1–9 except C-23 can be determined by comparison of their CD curves with those of known compounds. Compounds 1 (adamantane type) and 3–9 (homoadamantyl type) displayed the negative Cotton effect around 330 nm, indicating 1R configuration based on the CD benchmark summarized by Zhang et al.10 Furthermore, the ECD spectra of 1, 3 and 5–8 matched well those of known compounds hyperisampsin G,10 hypersampsonone G,11 hypersampsonone B,11 hypersampsonone C11 and hyperisampsins O12 respectively, in which the main differences in their structure are that the former have an isogeranyl group attached to C-5 position, while the latter have a geranyl group. Hence, the absolute configuration of 1 and 3–9 was assigned as depicted in Fig. 1. The absolute configuration of C-1 for most of naturally occurring adamantyl and homoadamantyl PPAPs appeared as 1R, which showed the negative CE at 333 nm.10 However, a positive Cotton effect at 325 nm and a negative Cotton effect at 243 and 294 nm were observed in ECD spectrum of 2, which was the opposite to those of hyperisampsin C.10 Consequently, the absolute configuration of 2 was established as depicted in Fig. 1.
The known compounds garcimultiflorone D (10),6 sampsonione B (11)14 and hyphenrone M (12)15 were identified by comparison of their NMR data with those in the literature.
All the isolated compounds were assessed for their cytotoxic effects against three human tumor cell lines (SGC-7901, HepG2, HCT-116) by CCK-8 method. In comparison with the positive control cisplatin against SGC-7901, HepG2 and HCT-116 with IC50 values 7.35, 4.58 and 8.23 μM respectively, compounds 8 showed mild cytotoxicity against SGC-7901 and HepG2 with an IC50 values of 13.05 and 18.05 μM, and compounds 12 also displayed mild cytotoxicity against three tested human cancer cells with an IC50 values of 17.63, 19.64, and 18.93 μM respectively. The other compounds showed no obvious cytotoxicity against three tested human cancer cells (IC50 > 20 μM). Additionally, the NO production inhibitory activity of all isolated compounds on LPS-activated RAW 264.7 cells was also tested. The cell viability was first confirmed by the CCK-8 method to determine whether the cytotoxicity of the tested compounds resulted in the inhibition of NO production. Compounds 8 and 12 also exhibited NO production inhibitory effect with IC50 values 18.24 and 12.50 μM respectively, while did not obviously affect cell viability up to 20 μM and the others compounds had no inhibitory activity (IC50 > 20 μM).
3 Materials and methods
3.1 General experimental procedures
Optical rotations were determined in MeOH on an Autopol IV polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). UV spectra were obtained on a UH5300 UV-VIS Double Beam spectrophotometer (Hitachi Co., Tokyo, Japan). ECD spectra were recorded on a Chirascan Plus spectrometer (Applied Photophysics Ltd, London, England). 1D and 2D NMR spectra were recorded on a Bruker AVANCE IIITM 600 MHz spectrometer (Bruker, Ettlingen, Germany) in CDCl3, CD3OD using tetramethylsilane (TMS) as an internal reference standard. Chemical shifts (δ) have been expressed in ppm and the coupling constants (J) have been given in Hz. High-resolution electrospray mass spectroscopy was performed on an Thermo Scientific Q Exactive Orbitrap LC-MS/MS System (HR-ESI-MS) (Thermo Scientific, Waltham, MA, USA). High-performance liquid chromatography (HPLC) was conducted on an Ultimate 3000 HPLC system (Dionex Co., Sunnyvale, CA, USA) equipped with an Ultimate 3000 pump and Ultimate 3000 Variable Wavelength detector, as well as a semi-preparative YMC-Pack ODS-A column (250 × 10 mm, 5 μm) and a preparative YMC-Pack ODS-A column (250 × 20 mm, 5 μm) from YMC Co., Ltd (Kyoto, Japan), column chromatography (CC) was conducted over silica gel (200–300 mesh and 300–400 mesh, Qingdao Haiyang Chemical Industry Co., Ltd., Qingdao, China). Chromatographic grade acetonitrile was purchased from Chang Tech Enterprise Co., Ltd (Taiwan, China). RAW 264.7 murine macrophages and three human tumor cell lines (SGC-7901, HepG2, HCT-116) were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). Cisplatin was purchased from Sigma Chemical Co. Ltd. (St. Louis, MO, USA). Dexamethasone and lipopolysaccharides (LPS) were purchased from Sigma Chemical Co. Ltd. (St. Louis, MO, USA). Cell Counting Kit (CCK-8) was purchased from Beyotime Biotechnology (shanghai, China). Dulbecco modified Eagle medium (DMEM) and Penicillin–Streptomycin solution were purchased from GE healthcare life science (Logan, UT, USA). Fetal bovine serum (FBS) was purchased from Gibco, Life technologies (Grand Island, NY, USA). Reagent grade dimethyl sulfoxide (DMSO) was purchased from Vetec, Sigma Chemical Co. (St. Louis, MO, USA). The absorbance was read on a Multiskan GO microplate reader (Thermo Fisher Scientific Inc. Waltham, MA, USA).3.2 Plant material
The fruits of G. multiflora were purchased from Nanning, Guangxi Zhuang Autonomous Region, P. R. China and identified by Prof. Hongli Teng, Guangxi Zhuang medicine international hospital. The voucher specimen (2014091201) was deposited in the herbarium of School of Pharmaceutical Sciences, South Central University for Nationalities.3.3 Extraction and isolation
The dried fruits of G. multiflora Champ (5.2 kg) were powdered and extracted with 95% EtOH at room temperature for three times (each time for 24 h) to obtain EtOH extract 2.21 kg, and then successively partitioned with petroleum ether (PE), EtOAc and n-BuOH to get PE extract 125 g, EtOAc extract 166 g, n-BuOH extract 80 g. The PE extract (125 g) was chromatographed on a silica gel column (200–300 mesh) eluted successively with PE-acetone gradient (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 25
1, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0
1, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 6 fractions (Fr. 1–Fr. 6). Fr. 2 (42.5 g) was divided into 11 fractions (Fr. 2.1–Fr. 2.11) via silica gel CC (PE-CH2Cl2, 10
1) to obtain 6 fractions (Fr. 1–Fr. 6). Fr. 2 (42.5 g) was divided into 11 fractions (Fr. 2.1–Fr. 2.11) via silica gel CC (PE-CH2Cl2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0
1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fr. 2.9 (2.3 g) was further separated by silica gel CC (PE/CH2Cl2/MeOH, 10
1). Fr. 2.9 (2.3 g) was further separated by silica gel CC (PE/CH2Cl2/MeOH, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 0
0.1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) and repeated semi-preparative HPLC to give compounds 2 (1.2 mg; CH3CN–H2O, 90
0.1) and repeated semi-preparative HPLC to give compounds 2 (1.2 mg; CH3CN–H2O, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; tR 19.6 min); 4 (1.1 mg; CH3CN–H2O, 90
10; tR 19.6 min); 4 (1.1 mg; CH3CN–H2O, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, tR 14.8 min); 8 (3.2 mg, CH3CN–H2O, 95
10, tR 14.8 min); 8 (3.2 mg, CH3CN–H2O, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, tR 10.3 min); 9 (9.6 mg, CH3CN–H2O, 94
5, tR 10.3 min); 9 (9.6 mg, CH3CN–H2O, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, tR 8.4 min) and 10 (1.2 mg; MeOH–H2O, 95
6, tR 8.4 min) and 10 (1.2 mg; MeOH–H2O, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; tR 16.2 min). In the same way, Fr. 3 (31.0 g) was subjected to repeated silica gel CC with PE-CH2Cl2 (50
5; tR 16.2 min). In the same way, Fr. 3 (31.0 g) was subjected to repeated silica gel CC with PE-CH2Cl2 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0
1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ODS CC with H2O–MeOH (7
1), ODS CC with H2O–MeOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 0
3 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and semi-preparative HPLC to afford 1 (3.2 mg; CH3CN–H2O, 96
1) and semi-preparative HPLC to afford 1 (3.2 mg; CH3CN–H2O, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, tR 10.3 min); 3 (1.0 mg, MeOH–H2O, 90
4, tR 10.3 min); 3 (1.0 mg, MeOH–H2O, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, tR 19.6 min); the mixture of 5 and 6 (6.5 mg, MeOH–H2O, 87
10, tR 19.6 min); the mixture of 5 and 6 (6.5 mg, MeOH–H2O, 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, tR 16.4 min); 7 (1.8 mg, MeOH–H2O, 90
13, tR 16.4 min); 7 (1.8 mg, MeOH–H2O, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, tR 16.2 min); 11 (3.1 mg, CH3CN–H2O, 79
10, tR 16.2 min); 11 (3.1 mg, CH3CN–H2O, 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21, tR 30.9 min); 12 (3.0 mg MeOH–H2O, 85
21, tR 30.9 min); 12 (3.0 mg MeOH–H2O, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, tR 12.9 min).
15, tR 12.9 min).
Epi-isosampsonione J (1), white amorphous powder. [α]D = +55.0° (c = 0.02, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 215 (sh) (3.88), 245 (4.02); ECD (c 3.42 × 10−4 M, MeOH) λ (Δε): 213 (+1.80), 241 (−4.55), 262 (+1.94), 293 (+0.82), 335 (−0.77); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 583.34296 [M − H]− (calcd for C38H47O5−: 583.3429).
ε): 215 (sh) (3.88), 245 (4.02); ECD (c 3.42 × 10−4 M, MeOH) λ (Δε): 213 (+1.80), 241 (−4.55), 262 (+1.94), 293 (+0.82), 335 (−0.77); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 583.34296 [M − H]− (calcd for C38H47O5−: 583.3429).
| No. | 1a | 2b | 3b | 4b |
|---|---|---|---|---|
| a Recorded in CD3OD.b Recorded in CDCl3. | ||||
| 6 | 2.69–2.78 m; 2.41–2.48 m | 2.74 d (14.4); 2.40–2.47 m | 2.55 dd (13.2, 6.0); 1.75–1.82 m | 2.56 dd (13.8, 5.4); 1.76–1.82 m |
| 7 | 1.82–1.88 m | 1.88–1.94 m | 2.13 t (6.6) | 2.13–2.19 m |
| 12/16 | 7.23 d (7.2) | 7.22 d (7.2) | 7.31 m | 7.34 d (7.2) |
| 13/15 | 7.32 t (7.2) | 7.37 t (7.2) | 7.31m | 7.31 t (7.2) |
| 14 | 7.49 t (7.2) | 7.44 t (7.2) | 7.43 m | 7.45 t (7.2) |
| 17 | 2.80 dd (15.0, 7.2); 2.31 dd (15.0, 7.2) | 2.15 dd (14.4, 6.6); 2.38–2.45 m | 2.84 dd (13.2, 12.0); 2.47 dd (13.2, 9.0) | 3.16 dd (15.0, 4.8); 2.13–2.20 m |
| 18 | 4.98 t (7.2) | 2.99–3.06 m | 1.96 dd (12.0, 9.0) | 4.00 t (6.6) |
| 20 | 1.69 s | 1.20 s | 1.33 s | |
| 21 | 1.63 s | 1.36 s | 1.41 s | |
| 22 | 2.03–2.14 m; 1.80–1.88 m | 2.05–2.13 m; 1.87 dd (15.0, 3.0) | 2.23 dd (14.4, 10.2); 1.75–1.82 m | 2.88 dd (14.4, 10.2); 1.78–1.84 m |
| 23 | 2.68–2.79 m | 2.45–2.53 m | 2.56–2.64 m | 2.60–2.67 m |
| 25 | 1.57 s | 1.69 s | 1.45 s | 1.42 s |
| 26 | 4.64 s; 4.59 s | 4.72 s; 4.68 s | 4.53 br s; 4.66 br s | 4.67 br s; 4.47 br s |
| 27 | 2.03–2.13 m | 2.05–2.17 m | 1.97–2.04 m | 1.94–2.03 m |
| 28 | 5.03 t (7.2) | 5.02 t (6.6) | 5.00 t (7.2) | 4.99 t (6.6) |
| 30 | 1.63 s | 1.60 s | 1.60 s | 1.60 s |
| 31 | 1.67 s | 1.66 s | 1.68 s | 1.69 s |
| 32 | 2.42–2.48 m | 3.02–3.09 m | 2.19 dd (13.8, 7.2); 1.43–1.52 m | 2.17–2.24 m; 1.44–1.51 m |
| 33 | 2.74–2.80 m | 2.40–2.48 m | 2.06 dd (12.0, 7.8) | 2.38 dd (13.2, 7.8) |
| 35 | 1.30 s | 1.23 s | 1.11 s | 1.02 s |
| 36 | 1.32 s | 1.30 s | 1.07 s | 0.99 s |
| 37 | 1.36 s | 1.52 s | 1.54 s | 1.59 s |
| 38 | 1.44 s | 1.53 s | 1.41 s | 1.43 s |
| No. | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|
| 6 | 2.61 dd (15.0, 6.6); 2.19 d (16.2) | 2.61 dd (15.0, 6.6); 2.19 d (16.2) | 2.29–2.39 m | 2.68 dd (14.4, 6.6); 1.80 d (13.8) | 2.57–2.64 m; 1.77–1.84 m |
| 7 | 1.73–1.81 m | 1.73–1.81 m | 1.96–2.03 m | 2.05–2.13 m | 2.05–2.10 m |
| 12/16 | 7.56 d (7.2) | 7.56 d (7.2) | 7.58 d (8.4) | 7.31 m | 7.33 m |
| 13/15 | 7.38 t (7.8) | 7.38 t (7.8) | 7.26 t (7.8) | 7.31 m | 7.33 m |
| 14 | 7.43 t (7.8) | 7.43 t (7.8) | 7.39 t (7.8) | 7.43 m | 7.46 m |
| 17 | 2.99 dd (16.2, 6.6); 2.39–2.47 m | 2.89–2.96 m; 2.39–2.47 m | 5.07 d (2.4) | 3.44 dd (14.0, 11.4); 1.64 dd (15.0, 3.0) | 3.30 dd (15.0, 9.0); 1.74–1.82 m |
| 18 | 5.84 t (6.6) | 5.79 dd (6.6, 3.6) | 3.92 d (2.4) | 4.94 dd (11.4, 3.0) | 5.86–5.92 m |
| 20 | 1.26 s | 1.19 s | |||
| 21 | 1.33 s | 1.20 s | |||
| 22 | 2.20–2.29 m; 2.04–2.14 m | 2.20–2.29 m; 2.04–2.14 m | 2.14–2.22 m; 2.04–2.11 m | 2.28 dd (14.4, 9.6); 1.85 dd (14.4, 4.2) | 2.28 dd (14.4, 9.6); 1.77–1.84 m |
| 23 | 2.36–2.44 m | 2.36–2.44 m | 2.50–2.57 m | 2.57–2.65 m | 2.58–2.66 m |
| 25 | 1.69 s | 1.70 s | 1.72 s | 1.56 s | 1.50 s |
| 26 | 4.73 s | 4.73 s | 4.79 s; 4.81 s | 4.70 s; 4.62 s | 4.57 br s; 4.70 br s |
| 27 | 2.00–2.07 m; 1.87–1.95 m | 2.00–2.07 m; 1.87–1.95 m | 2.10–2.16 m; 2.01–2.07 m | 2.04–2.14 m | 2.01–2.07 m |
| 28 | 5.07 t (6.6) | 5.07 t (6.6) | 5.03–5.09 m | 5.03 t (6.6) | 5.00 t (6.6) |
| 30 | 1.55 s | 1.56 s | 1.59 s | 1.62 s | 1.61 s |
| 31 | 1.64 s | 1.64 s | 1.65 s | 1.69 s | 1.68 s |
| 32 | 1.87–2.02 m | 1.87–2.02 m | 2.04–2.10 m; 1.97–2.04 m | 2.31–2.40 m; 1.45–1.55 m | 2.33 dd (13.8, 7.2); 1.45–1.52 m |
| 33 | 2.41–2.48 m | 2.50–2.55 m | 2.46–2.53 m | 2.72 t (10.2) | 2.58–2.66 m |
| 35 | 1.58 s | 1.70 s | 1.37 s | 1.33 s | 1.32 s |
| 36 | 1.46 s | 1.50 s | 1.34 s | 1.20 s | 1.25 s |
| 37 | 1.21 s | 1.19 s | 1.44 s | 1.35 s | 1.39 s |
| 38 | 1.47 s | 1.47 s | 1.39 s | 1.48 s | 1.44 s |
| OH | 4.75 s |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 83.7 C | 82.7 C | 82.4 C | 82.1 C | 81.5 C | 82.0 C | 82.6 C | 82.3 C | 82.2 C |
| 2 | 203.0 C | 198.8 C | 206.6 C | 205.3 C | 207.5 C | 207.7 C | 203.5 C | 209.0 C | 208.4 C |
| 3 | 74.3 C | 78.3 C | 72.1 C | 74.7 C | 67.6 C | 70.4 C | 76.3 C | 65.9 C | 66.6 C |
| 4 | 204.4 C | 202.6 C | 206.4 C | 208.3 C | 115.6 C | 118.1 C | 106.5 C | 205.9 C | 205.4 C |
| 5 | 69.7 C | 68.2 C | 66.9 C | 66.3 C | 57.5 C | 57.8 C | 57.5 C | 67.5 C | 67.1 C |
| 6 | 44.9 CH2 | 38.6 CH2 | 48.0 CH2 | 48.8 CH2 | 30.8 CH2 | 30.7 CH2 | 40.6 CH2 | 45.2 CH2 | 47.0 CH2 |
| 7 | 47.1 CH | 44.4 CH | 43.6 CH | 43.6 CH | 43.8 CH | 43.7 CH | 44.6 CH | 44.8 CH | 44.5 CH |
| 8 | 56.7 C | 57.8 C | 52.0 C | 52.6 C | 48.0 C | 48.2 C | 52.0 C | 50.9 C | 51.3 C |
| 9 | 203.7 C | 201.9 C | 204.5 C | 204.0 C | 208.6 C | 208.9 C | 208.0 C | 204.1 C | 203.9 C |
| 10 | 194.6 C | 193.4 C | 194.0 C | 193.5 C | 194.7 C | 194.6 C | 194.7 C | 192.6 C | 192.6 C |
| 11 | 136.4 C | 135.3 C | 136.3 C | 136.1 C | 135.9 C | 136.9 C | 137.1 C | 135.3 C | 135.2 C |
| 12 | 130.7 CH | 129.2 CH | 129.3 CH | 129.4 CH | 129.5 CH | 128.6 CH | 129.7 CH | 129.1 CH | 129.2 CH |
| 13 | 129.1 CH | 128.2 CH | 128.0 CH | 128.0 CH | 128.6 CH | 128.2 CH | 127.9 CH | 128.1 CH | 128.3 CH |
| 14 | 133.7 CH | 132.6 CH | 132.0 CH | 132.2 CH | 132.4 CH | 132.3 CH | 132.0 CH | 132.5 CH | 132.5 CH |
| 15 | 129.1 CH | 128.2 CH | 128.0 CH | 128.0 CH | 128.6 CH | 128.2 CH | 127.9 CH | 128.1 CH | 128.3 CH |
| 16 | 130.7 CH | 129.2 CH | 129.3 CH | 129.4 CH | 129.5 CH | 128.6 CH | 129.7 CH | 129.1 CH | 129.2 CH |
| 17 | 28.1 CH2 | 23.8 CH2 | 32.2 CH2 | 38.7 CH2 | 46.0 CH2 | 45.6 CH2 | 83.3 CH | 31.5 CH | 37.8 CH |
| 18 | 120.7 CH | 52.3 CH | 60.1 CH | 82.0 CH | 98.7 CH | 100.0 CH | 88.7 CH | 85.9 CH | 99.8 CH |
| 19 | 135.6 C | 81.9 C | 73.2 C | 70.0 C | 84.3 C | ||||
| 20 | 18.5 CH3 | 29.1 CH3 | 31.3 CH3 | 27.4 CH3 | 21.8 CH3 | ||||
| 21 | 26.1 CH3 | 33.1 CH3 | 30.5 CH3 | 26.4 CH3 | 21.4 CH3 | ||||
| 22 | 34.0 CH2 | 31.5 CH2 | 35.8 CH2 | 35.9 CH2 | 34.7 CH2 | 34.8 CH2 | 35.1 CH2 | 34.8 CH2 | 35.2 CH2 |
| 23 | 44.7 CH | 43.4 CH | 43.3 CH | 43.3 CH | 43.4 CH | 43.4 CH | 43.2 CH | 43.6 CH | 43.6 CH |
| 24 | 150.2 C | 149.1 C | 149.1 C | 149.2 C | 149.4 C | 149.5 C | 150.4 C | 149.0 C | 148.8 C |
| 25 | 18.6 CH3 | 18.5 CH3 | 18.0 CH3 | 17.9 CH3 | 19.7 CH3 | 19.8 CH3 | 19.3 CH3 | 18.2 CH3 | 18.0 CH3 |
| 26 | 113.5 CH2 | 112.3 CH2 | 112.8 CH2 | 112.9 CH2 | 111.6 CH2 | 111.5 CH2 | 112.2 CH2 | 112.7 CH2 | 113.1 CH2 |
| 27 | 34.7 CH2 | 34.0 CH2 | 33.1 CH2 | 32.9 CH2 | 30.9 CH2 | 30.9 CH2 | 31.9 CH2 | 33.6 CH2 | 33.4 CH2 |
| 28 | 124.3 CH | 122.6 CH | 122.9 CH | 122.7 CH | 122.8 CH | 122.8 CH | 123.2 CH | 122.7 CH | 122.7 CH |
| 29 | 133.2 C | 132.5 C | 132.2 C | 132.4 C | 132.1 C | 132.1 C | 132.3 C | 132.4 C | 132.4 C |
| 30 | 18.3 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 | 18.2 CH3 |
| 31 | 26.6 CH3 | 25.9 CH3 | 26.0 CH3 | 26.0 CH3 | 25.9 CH3 | 25.9 CH3 | 25.9 CH3 | 26.0 CH3 | 26.0 CH3 |
| 32 | 57.9 CH | 59.0 CH | 28.2 CH2 | 28.4 CH2 | 25.3 CH2 | 25.3 CH2 | 29.1 CH2 | 31.7 CH2 | 32.0 CH2 |
| 33 | 62.8 CH | 56.3 CH | 57.9 CH | 53.2 CH | 49.6 CH | 48.9 CH | 50.1 CH | 42.3 CH | 41.8 CH |
| 34 | 58.9 C | 79.4 C | 47.2 C | 49.8 C | 86.5 C | 86.2 C | 86.4 C | 88.8 C | 88.0 C |
| 35 | 19.3 CH3 | 26.3 CH3 | 30.8 CH3 | 23.2 CH3 | 30.6 CH3 | 32.2 CH3 | 31.5 CH3 | 28.9 CH3 | 29.4 CH3 |
| 36 | 25.1 CH3 | 32.0 CH3 | 16.2 CH3 | 19.2 CH3 | 28.0 CH3 | 28.8 CH3 | 24.3 CH3 | 18.2 CH3 | 18.1 CH3 |
| 37 | 23.0 CH3 | 23.5 CH3 | 23.1 CH3 | 23.0 CH3 | 22.6 CH3 | 22.5 CH3 | 23.1 CH3 | 22.8 CH3 | 22.9 CH3 |
| 38 | 23.5 CH3 | 23.9 CH3 | 26.7 CH3 | 27.1 CH3 | 25.4 CH3 | 25.3 CH3 | 27.0 CH3 | 25.4 CH3 | 25.5 CH3 |
Isohyperisampsin C (2), white amorphous powder. [α]D = +93.3° (c = 0.02, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 210 (3.87), 250 (3.87), 295 (3.42), 325 (3.41); ECD (c 3.42 × 10−4 M, MeOH) λ (Δε): 219 (−2.07), 243 (−5.36), 264 (−1.32), 294 (−5.29), 325 (+8.79); 1H- and 13C-NMR see Tables 1 and 3. HR-ESI-MS m/z: 585.35748 [M + H]+ (calcd for C38H49O5+: 585.35745).
ε): 210 (3.87), 250 (3.87), 295 (3.42), 325 (3.41); ECD (c 3.42 × 10−4 M, MeOH) λ (Δε): 219 (−2.07), 243 (−5.36), 264 (−1.32), 294 (−5.29), 325 (+8.79); 1H- and 13C-NMR see Tables 1 and 3. HR-ESI-MS m/z: 585.35748 [M + H]+ (calcd for C38H49O5+: 585.35745).
Isohypersampsonone G (3), white, amorphous powder. [α]D = +34.2° (c = 0.01, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 210 (4.14), 245 (4.12); ECD (c 1.71 × 10−4 M, MeOH) λ (Δε): 210 (−0.03), 244 (−6.56), 286 (+2.40), 321 (−0.55); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 587.37323 [M + H]+ (calcd for C38H51O5+: 587.37310).
ε): 210 (4.14), 245 (4.12); ECD (c 1.71 × 10−4 M, MeOH) λ (Δε): 210 (−0.03), 244 (−6.56), 286 (+2.40), 321 (−0.55); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 587.37323 [M + H]+ (calcd for C38H51O5+: 587.37310).
Garcimultinone A (4), white amorphous powder. [α]D = −25.6° (c = 0.02, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 210 (3.80), 245 (3.79); ECD (c 3.68 × 10−4 M, MeOH) λ (Δε): 210 (+1.06), 244 (−5.00), 286 (+1.24), 307 (−0.71); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 545.32617 [M + H]+ (calcd for C35H45O5+: 545.32615).
ε): 210 (3.80), 245 (3.79); ECD (c 3.68 × 10−4 M, MeOH) λ (Δε): 210 (+1.06), 244 (−5.00), 286 (+1.24), 307 (−0.71); 1H- and 13C-NMR see Tables 1 and 3; HR-ESI-MS m/z: 545.32617 [M + H]+ (calcd for C35H45O5+: 545.32615).
Isohypersampsonone B (5) and epi-isohypersampsonone B (6), white amorphous powders. [α]D = +60.0° (c = 0.01, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 245 (4.32); ECD (c 1.79 × 10−4 M, MeOH) λ (Δε): 224 (+16.0), 246 (+21.46), 290 (−7.81); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 561.32141 [M + H]+ (calcd for C35H45O6+: 561.32107).
ε): 245 (4.32); ECD (c 1.79 × 10−4 M, MeOH) λ (Δε): 224 (+16.0), 246 (+21.46), 290 (−7.81); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 561.32141 [M + H]+ (calcd for C35H45O6+: 561.32107).
Isohypersampsonone C (7), white amorphous powder. [α]D = +81.7° (c = 0.02, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 215 (3.90), 245 (4.03); ECD (c 3.24 × 10−4 M, MeOH) λ (Δε): 209 (+22.01), 255 (−7.96), 289 (+3.90), 319 (−0.48); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 619.36310 [M + H]+ (calcd for C38H51O7+: 619.36293).
ε): 215 (3.90), 245 (4.03); ECD (c 3.24 × 10−4 M, MeOH) λ (Δε): 209 (+22.01), 255 (−7.96), 289 (+3.90), 319 (−0.48); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 619.36310 [M + H]+ (calcd for C38H51O7+: 619.36293).
Isohyperisampsin O (8), white amorphous powder. [α]D = +22.4° (c = 0.06, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 240 (3.60); ECD (c 9.46 × 10−4 M, MeOH) λ (Δε): 221 (+4.99), 243 (−3.20), 261 (+1.24); 319 (−2.46); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 635.35791 [M + H]+ (calcd for C38H51O8+: 635.35784).
ε): 240 (3.60); ECD (c 9.46 × 10−4 M, MeOH) λ (Δε): 221 (+4.99), 243 (−3.20), 261 (+1.24); 319 (−2.46); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 635.35791 [M + H]+ (calcd for C38H51O8+: 635.35784).
Garcimultinone B (9), white amorphous powder. [α]D = +28.3° (c = 0.04, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 210 (3.52), 245 (3.57); ECD (c 6.94 × 10−4 M, MeOH) λ (Δε): 222 (+2.34), 241 (−0.89), 255 (+1.63), 313 (−1.15); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 577.31610 [M + H]+ (calcd for C35H45O7+: 577.31598).
ε): 210 (3.52), 245 (3.57); ECD (c 6.94 × 10−4 M, MeOH) λ (Δε): 222 (+2.34), 241 (−0.89), 255 (+1.63), 313 (−1.15); 1H- and 13C-NMR see Tables 2 and 3; HR-ESI-MS m/z: 577.31610 [M + H]+ (calcd for C35H45O7+: 577.31598).
3.4 Cytotoxicity assay
Cytotoxicity was measured by the CCK-8 method.16 In short, 5 × 103 three human tumor cell lines (SGC-7901, HepG2, HCT-116) per well (in 100 μL of culture medium) were seeded in 96-well plates. Cells were incubated with five concentrations (20 μM, 10 μM, 5 μM, 2.5 μM and 1.25 μM) of each compound in triplicate at 37 °C for 24 h, and cisplatin was used as a positive control. Then, the cell culture medium was taken out and 100 μL cell culture medium containing 10% CCK-8 solution was added to per well for 1 h. The absorbance values of each well at 450 nm were measured using a microplate spectrophotometer. The IC50 values were calculated by the Logit method.173.5 NO production measurement and cell viability assay
The Griess reaction18 was used to measure both the accumulation of nitrite in the culture supernatants and the NO synthase activity. The viability of the microglial cells was evaluated by the CCK-8 method.4 Conclusions
The phytochemical study of the fruits of G. multiflora led to the isolation of nine new caged PPAPs, including adamantane type PPAPs (1–2), and homoadamantane type PPAPs (3–9). A new epimeric pair of isohypersampsonone B (5) and epi-isohypersampsonone B (6) with an unusual hexahydrofuro[2,3-b]furan-diepoxy ring system were not separated due to the rapid equilibration between the two isomeric forms. Cytotoxicities of all isolated compounds against three human cancer cell lines (SGC-7901, HepG2, HCT-116) by CCK-8 method and the nitric oxide production inhibitory activity of lipopolysaccharides-stimulated RAW 264.7 cells were evaluated. Compounds 8 and 12 displayed mild cytotoxicity against three human cancer cell lines and moderate NO inhibitory effects on LPS-induced macrophages. These results indicated that G. multiflora fruits are new rich sources of caged PPAPs with structural diversity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31370379), the National Major New Drugs Innovation and Development (2017ZX09301060), the Special Fund for Basic Scientific Research of Central Colleges, South-Central University for Nationalities (CZP18004) and Hubei Provincial Technical Innovation Program (2017AHB067).References
- L. D. C. Brito, A. L. R. Berenger and M. R. Figueiredo, Food Chem. Toxicol., 2017, 109, 847–862 CrossRef CAS PubMed.
- C. Q. Liu, P. C. Ho, F. C. Wong, G. Sethi, L. Z. Wang and B. C. Goh, Cancer Lett., 2015, 362, 8–14 CrossRef CAS PubMed.
- S. Baggett, P. Protiva, E. P. Mazzola, H. Yang, E. T. Ressler, M. J. Basile, I. B. Weinstein and E. J. Kennelly, J. Nat. Prod., 2005, 68, 354–360 CrossRef CAS PubMed.
- X. W. Yang, R. B. Grossman, E. P. Mazzola and G. Xu, Chem. Rev., 2018, 118, 3508–3558 CrossRef CAS PubMed.
- B. Liu, X. B. Zhang, R. W. Bussmann, R. H. Hart, P. Li, Y. J. Bai and C. L. Long, Econ. Bot., 2017, 70, 416–430 CrossRef.
- C. W. Ting, T. L. Hwang, I. S. Chen, M. H. Yen and J. J. Chen, Chem. Biodiversity, 2012, 9, 99–105 CrossRef CAS PubMed.
- J. J. Chen, C. W. Ting, T. L. Hwang and I. S. Chen, J. Nat. Prod., 2009, 72, 253–258 CrossRef CAS.
- C. W. Ting, T. L. Hwang, I. S. Chen, M. J. Cheng, P. J. Sung and J. J. Chen, Chem. Biodiversity, 2014, 11, 819–824 CrossRef CAS PubMed.
- Y. Ye, X. W. Yang and G. Xu, Tetrahedron, 2016, 72, 3057–3062 CrossRef CAS.
- H. C. Zhu, C. M. Chen, J. Yang, X. N. Li, J. J. Liu, B. Sun, S. X. Huang, D. Y. Li, G. M. Yao, Z. W. Luo, Y. Li, J. W. Zhang, Y. B. Xue and Y. H. Zhang, Org. Lett., 2014, 16, 6322–6325 CrossRef CAS PubMed.
- J. S. Zhang, Y. H. Zou, Y. Q. Guo, Z. Z. Li, G. H. Tang and S. Yin, RSC Adv., 2016, 6, 53469–53476 RSC.
- H. C. Zhu, C. M. Chen, J. W. Zhang, Y. Guo, D. D. Tan, G. Z. Wei, G. Yang, J. P. Wang, Z. W. Luo, Y. B. Xue and Y. H. Zhang, Chin. Chem. Lett., 2017, 28, 986–990 CrossRef CAS.
- G. D. Chen, G. K. Xiao, X. S. Yao and H. Gao, Journal of International Pharmaceutical Research, 2015, 42, 738–743 Search PubMed.
- L. H. Hu and K. Y. Sim, Tetrahedron Lett., 1998, 39, 7999–8002 CrossRef CAS.
- X. W. Yang, M. M. Li, X. Liu, D. Ferreira, Y. Ding, J. J. Zhang, Y. Liao, H. B. Qin and G. Xu, J. Nat. Prod., 2015, 78, 885–895 CrossRef CAS PubMed.
- J. T. Zhang, X. Li, X. Y. Wang, L. L. Mu, M. M. Yuan, B. K. Liu and H. Z. Shi, J. Hazard. Mater., 2018, 359, 1–8 CrossRef CAS.
- W. S. Xiang, J. D. Wang, X. J. Wang and J. Zhang, J. Antibiot., 2009, 62, 229–231 CrossRef CAS PubMed.
- G. Y. Cao, X. W. Yang, W. Xu and F. Li, Food Chem. Toxicol., 2013, 62, 167–171 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01279f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |