Open Access Article
Open Access ArticleAntibiotic removal by agricultural waste biochars with different forms of iron oxide
Yue Chena,
Jing Shi *ab,
Qiong Dua,
Haowen Zhanga and
Yixin Cuia
*ab,
Qiong Dua,
Haowen Zhanga and
Yixin Cuia
aSchool of Engineering, China Pharmaceutical University, Nanjing 211198, People's Republic of China. E-mail: shijing_cpu@163.com
bDepartment of Civil Engineering, McMaster University, Hamilton L8S 4L7, Canada
First published on 7th May 2019
Abstract
Pollution by antibiotics has become a serious threat to public health. In this study, agricultural waste, corn husk, in the form of biochar, was utilized for antibiotic removal from wastewater. Two kinds of iron-loaded biochars, impregnation–pyrolysis biochar (IP) and pyrolysis–impregnation biochar (PI), were synthesized to adsorb the typical antibiotics tetracycline (TC) and levofloxacin (LEV). PI contained amorphous hydrated iron oxide, whereas the major component of IP was γ-Fe2O3. Compared with IP, PI had a much higher adsorption capacity for both TC and LEV. This was because PI could provide more –OH, especially –OHads, to serve as the adsorption sites. In comparison with TC, –OH was prone to combine with LEV. FT-IR and XPS results indicated that the mechanisms of LEV adsorption included hydrogen bonding, F-replacement, electrostatic attraction and bridging bidentate complexation. TC adsorption may involve complexation, hydrogen bonding and electrostatic attraction.
1 Introduction
Antibiotics are extensively used in treating infective diseases for both humans and animals. Levofloxacin (LEV) and tetracycline (TC) (Fig. 1) are two typical and commonly used antibiotics. LEV is a fluoroquinolone antibiotic, which is generally utilized to treat gastrointestinal tract, genitourinary and respiratory infections.1 TC is used for the treatment of inflammatory bowel disease and acne.2 However, owing to the improper treatment of pharmaceutical wastewater, its abuse in food additives used for livestock breeding or animal husbandry, and incomplete metabolism in human and animal bodies, excessive antibiotics are discharged into the environment.3 As a result, they have been frequently detected in different environments and even in drinking water. Animals and humans could absorb excess environmental antibiotics through the water and food chains, resulting in antibiotic resistance.4,5 On the other hand, the conventional biological wastewater treatment was not appropriate for antibiotic removal, due to their toxic effects on microorganisms.4 Consequently, cost-effective approaches for LEV and TC removal from wastewater are urgently needed.Adsorption is considered as one of the most applicable technologies because it is safety, economical and simple. Several adsorbents have been tested to adsorb LEV or TC, such as chitosan, montmorillonite, graphene-based materials.5,6 To achieve the aims of low-cost, corn husks, the agricultural waste, were selected as the major part of the adsorbent. It could not only reduce the antibiotics pollution but also supply an additional method for corn husks reuse. In addition, corn is a worldwide and plentiful crop, which makes it cheap and easy to obtain. For adsorption capacity enhancement and recovery improvement, the corn husks were transformed into biochar and Fe, which was magnetic and non-toxic, was employed for the biochar modification. In summary, iron modified-biochars advocate the concept of disposal waste with waste. The corn husks are easy to obtain and cheap and they are harmless to the environment. In addition, they can remove antibiotics high-efficiently from aqueous solution. Furthermore, they can be recycled and reuse, which still maintains high adsorption capacities.
In this study, two kinds of Fe-loaded biochars with different forms of Fe oxide were prepared for LEV and TC removal. The adsorption performances were investigated and compared. The possible adsorption mechanisms were analyzed based on X-ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS) and Fourier Transform Infrared Spectroscopy (FTIR) results.
2 Material and methods
2.1 Materials
LEV (>98.5%) and TC (>98.5%) were purchased from TCI company. Corn husks were acquired from Shandong Province, China. The type of corn was Liangyu 99. Ferric chloride hexahydrate (FeCl3·6H2O) and other chemicals were analytical grades and purchased from Aladdin company.2.2 The preparation of adsorbents
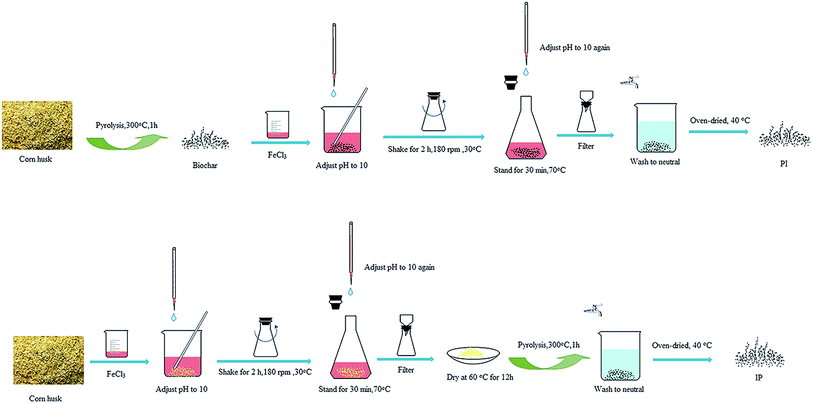
The raw corn husks were washed with deionized water, and then dried in oven at 100 ± 5 °C for 24 h. After cooling down to room temperature, the corn husks were ground to 60–100 mesh with a disintegrator.The modification for the two types of Fe-biochars both included the following three operations, but in a different order. (1) Impregnation: 40.545 g FeCl3·6H2O was dissolved into 500 mL deionized water, and put 8.377 g biomass into the solution (the mass ratio of total iron to biomass was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then, the pH value was adjusted to 10. The mixture was placed in a shaker for 2 h with 180 rpm at 30 °C, and next, pH was adjusted to 10 again. The obtained mixture was deposited at 70 °C for 0.5 h. After cooling to room temperature, the solid–liquid mixture was filtered and the obtained solid was dried at 60 °C for 12 h. (2) Pyrolysis: the pyrolysis temperature was raised to 300 °C at a rate of 5 °C min−1 and maintained for 1 h. During this process, nitrogen gas was passed over to isolate oxygen with the rate of 200 mL min−1 in the muffle furnace. After that, it was cooled to room temperature. (3) Drying: the solid residues were washed with distilled water and dried at 40 °C.
1). Then, the pH value was adjusted to 10. The mixture was placed in a shaker for 2 h with 180 rpm at 30 °C, and next, pH was adjusted to 10 again. The obtained mixture was deposited at 70 °C for 0.5 h. After cooling to room temperature, the solid–liquid mixture was filtered and the obtained solid was dried at 60 °C for 12 h. (2) Pyrolysis: the pyrolysis temperature was raised to 300 °C at a rate of 5 °C min−1 and maintained for 1 h. During this process, nitrogen gas was passed over to isolate oxygen with the rate of 200 mL min−1 in the muffle furnace. After that, it was cooled to room temperature. (3) Drying: the solid residues were washed with distilled water and dried at 40 °C.
If the corn husks were modified as the order above, it was defined as the impregnation–pyrolysis biochar (IP). If it was in the sequence of (2) (1) (3), the product was the pyrolysis–impregnation biochar (PI). The operation process was shown in Fig. 2.
2.3 Adsorption experiments
0.04 g PI or IP was added to 50 mL 0.55 mol L−1 LEV (200 mg L−1) or TC (266 mg L−1) solutions in a shaking water bath for 24 h at 120 rpm and 30 °C, respectively. pH values were adjusted by HCl and NaOH solution. The adsorption kinetic and isotherm experiments were carried out under optimal initial pH values. In the kinetic experiments, samples were taken at different time intervals. Next, the supernatant was filtered through 0.22 μm filter. Then the absorbance of the supernatant was measured using ultraviolet-visible spectrophotometer at 357 nm and 287 nm for TC and LEV, respectively.7–10To facilitate description, the adsorbed samples would be represented by abbreviations. For example, IP–LEV is the IP sample after LEV adsorption. The others were abbreviated similarly.
2.4 Regeneration and reuse
Five reuse cycles were performed to evaluate the regenerability of two modified biochars. 50 mL 0.55 mol L−1 LEV (200 mg L−1) or TC (266 mg L−1) solution was adsorbed first by 0.04 g PI or IP in a shaking water bath for 24 h at 120 rpm and 30 °C, respectively. Afterward, they were regenerated by 25 mL 0.01 mol L−1 NaOH solution and shaken at 120 rpm under 30 °C for 24 h. Next, the regenerated adsorbents was washed with deionized water and dried at 105 °C. The adsorbents were reused to remove TC or LEV for the next four cycle as described above.2.5 Materials characterization
BET (Quantachrome, Autosorb-IQ-AG-MP) was used to explore the specific surface area and the micropore properties. The specific surface area of the modified biochars was determined by nitrogen adsorption method.11 The structure of the compounds was detected by XPS (Thermo Fisher Scientific, Escalab 250Xi) using mono-chromatized Al Kα X-ray source with a power of 100 W and pass energy of 30 eV.12,13 XRD (Bruker-Axs, D8 Advance) was utilized to character the components of Fe-loaded biochar. The samples were scanned in the range of 5–90 °C using Cu-Kα radiation at 40 kV.12,14 The modified-biochar was dissolved by nitric and hydrochloric acid, and the iron content was determined by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES). The surface functional groups of sample were investigated by FTIR spectroscopy (Thermo Fisher Scientific, Nicolet-46). The powder sample was mixed with KBr, then pressed into pellets with a radius of 0.65 cm. The spectra were recorded in the mid-infrared from 4000 to 400 cm−1 with 4 cm−1 resolution.15–172.6 Data analysis
The amount of TC and LEV absorbed on Fe-loaded biochar could be calculated by the following formula.
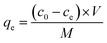 | (1) |
The pseudo-first-order kinetic model, the pseudo-second-order kinetic model and intra-particle diffusion model were used to fit the kinetics data. The equations were written as follows.
| qt = qe(1 − exp(−k1t)) | (2) |
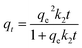 | (3) |
| qt = kit0.5 + C | (4) |
Freundlich, Langmuir and Temkin isotherm models were utilized to depict the adsorption performance.
| qe = KFCe1/n | (5) |
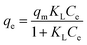 | (6) |
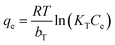 | (7) |
The difference between original adsorption amount and regenerated adsorption amount in different cycles can be calculated by the following formula.
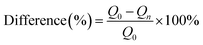 | (8) |
3 Results and discussion
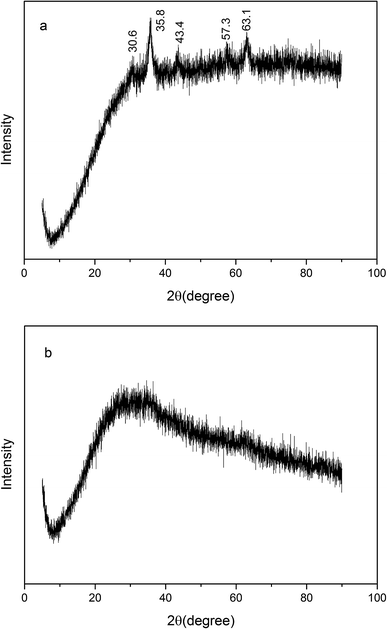
3.1 Characterization of biochar
3.2 Effects of pH
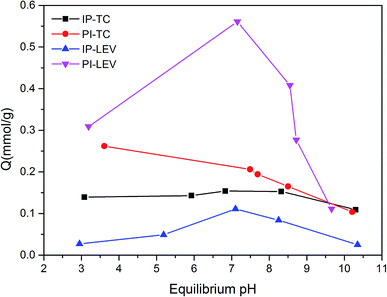
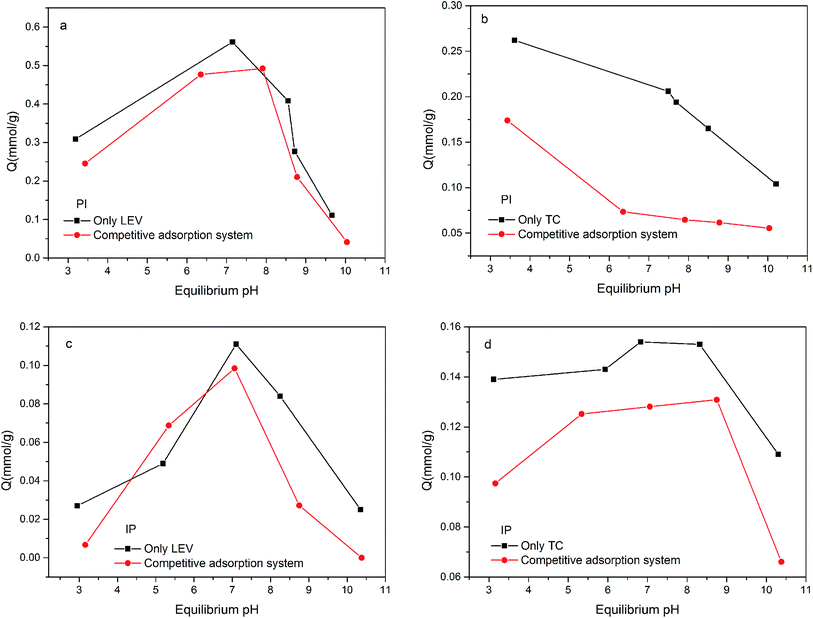
Fig. 4 illustrated the antibiotics adsorption at different equilibrium pH values. In the PI system, the amount of TC adsorption decreased as pH increased. Because the pHpzc was lower than 2.5, PI surface was negatively charged during the whole experiment. While TC existed as a cationic under lower pH, which was easy to combine with the negative sites on the PI surface.23 When pH increased, TC transformed into an anion, at which time the electrostatic repulsion enhanced, and the adsorption capacity reduced. Compared with PI, the IP adsorption capacity was much lower, especially under acid condition. This may due to the limitation of the active adsorption sites on IP.For LEV, the optimal adsorption capacity appeared at around the neutral condition in both the PI and IP adsorption systems. This was probably related to synthetical effect of the deprotonated carboxyl group, which was favorable for the complexation with ferric oxide,24 and the electrostatic repulsion also. Under acidic conditions, the percentage of deprotonated carboxyl group declined, resulting in the reduced adsorption amount. During alkaline conditions, electrostatic repulsion inhibited the contact of LEV molecule with biochar, leading to the adsorption decrement.
Although PI (24.9%, 94.9 m2 g−1) contained lower Fe content and surface area than IP (54.10%, 112.45 m2 g−1), it showed a larger adsorption capacity for both TC and LEV. This implied that the Fe and surface area were not the main adsorption active sites. So other functional group may play a key role during the adsorption.
3.3 Adsorption kinetics
Adsorption kinetic was a widely used method to explore the adsorption rate. The results of the relevant parameters were listed in the Table 1. It could be seen that the pseudo-second-order model were more accurate than the pseudo-first-order model for all adsorptions, which suggested that the antibiotics adsorption on PI or IP was mainly controlled by chemical process.25 Intra-particle diffusion model described the IP removal results well, which indicated that intra-particle was the main control steps during adsorption of two antibiotics on IP.26,27| Model | Parameters | IP–LEV | IP–TC | PI–LEV | PI–TC |
|---|---|---|---|---|---|
| Pseudo-first-order | qe (mmol g−1) | 0.0964 | 0.1572 | 0.5451 | 0.2494 |
| k1 (h−1) | 0.2081 | 0.1613 | 5.576 | 2.822 | |
| R2 | 0.8991 | 0.9865 | 0.9492 | 0.9050 | |
| Pseudo-second-order | qe (mmol g−1) | 0.1119 | 0.1948 | 0.5686 | 0.2620 |
| k2 (g (mmol h)−1) | 2.439 | 0.870 | 15.878 | 15.50 | |
| R2 | 0.90417 | 0.9909 | 0.9577 | 0.9175 | |
| Intra-particle diffusion | ki (mmol h0.5 g−1) | 0.01957 | 0.03382 | 0.04312 | 0.02992 |
| C | 0.01230 | 7.102 × 10−4 | 0.4010 | 0.1405 | |
| R2 | 0.9871 | 0.9926 | 0.5687 | 0.8254 |
3.4 Adsorption isotherms
The adsorption isotherm could explain the functional relationship between adsorption capacity and concentration. Table 2 listed the fitting results of two isotherm models. The qmax values of PI were 0.310 mmol g−1 (149.1 mg g−1) for TC and 0.757 mmol g−1 (273.7 mg g−1) for LEV respectively. Both were much higher than those of IP. In addition, compared with some other adsorbents (Table 3), PI and IP also showed the outstanding adsorption capacity.| Model | Parameters | IP–LEV | IP–TC | PI–LEV | PI–TC |
|---|---|---|---|---|---|
| Langmuir | KL (L mmol−1) | 5.361 | 7.893 | 10.26 | 27.319 |
| qm (mmol g−1) | 0.1567 | 0.2120 | 0.7573 | 0.3097 | |
| R2 | 0.7993 | 0.8797 | 0.8211 | 0.8385 | |
| Freundlich | KF (mmol(1−1/n) L1/n g−1) | 0.1532 | 0.2122 | 0.7086 | 0.3234 |
| 1/n | 0.4181 | 0.3471 | 0.1738 | 0.1409 | |
| R2 | 0.9330 | 0.9253 | 0.8688 | 0.9436 | |
| Temkin | KT (L mmol−1) | 0.1448 | 0.2096 | 0.4105 | 0.1855 |
| bT (×103) | 52.69 | 29.22 | 4.990 | 11.15 | |
| R2 | 0.9902 | 0.9395 | 0.8963 | 0.9823 |
| Adsorbents | Adsorbate | qm (mg g−1) | References |
|---|---|---|---|
| Chitosan | TC | 13.3 | 28 |
| Montmorillonite | TC | 54.0 | 28 |
| Bamboo charcoal | TC | 22.7 | 29 |
| Magnetic porous carbon with γ-Fe2O3 particles | TC | 25.4 | 30 |
| Carbon disulfide-modified magnetic ion-imprinted chitosan-Fe(III) | TC | 516.3 | 5 |
| Graphene-based materials | TC | 70.0 | 31 |
| PI | TC | 149.1 (0.310 mmol g−1) | This work |
| IP | TC | 102.0 (0.212 mmol g−1) | This work |
| Iron-pillared montmorillonite | LEV | 48.6 | 4 |
| Zr-modified corn biochar | LEV | 73.1 | 32 |
| Wood chip biochars | LEV | 7.7 | 33 |
| Porous nano-cerium oxide wood chip biochar composites | LEV | 73.0 | 4 |
| PI | LEV | 273.7 (0.757 mmol g−1) | This work |
| IP | LEV | 56.6 (0.157 mmol g−1) | This work |
Freundlich model was more appropriate for the TC and LEV adsorption behaviors on the two adsorbents, indicating that the adsorption took place on heterogeneous surface, which was probably due to the different kinds of active sites.6 The n values were all greater than 2, indicating the strong interaction between the antibiotics and the modified biochars.34 In addition, the difference of LEV adsorption capacity between PI and IP was much more significant than that of TC. The Temkin isotherm model fitted the adsorption data better. This result indicated that electrostatic interaction had an effect on the interaction between modified-biochar and the two antibiotics.35,36
3.5 Adsorption mechanism
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O or C–O from LEV. Moreover, as can be seen from Table 5, deconvolved N1s of TC into two peaks at 399.4 eV and 401.7 eV were assigned to –NH– or –NH2 and C–N–C.6,40 After adsorption, the binding energy shifted upward to 399.8 eV and 403.2 eV, respectively, due to the protonated amines or the formation of hydrogen bonds.41,42 The F1s signal of LEV presented at 686.7 eV and was transferred to 687.3 eV after adsorption. The increase in binding energy may be attributed to the replacement of –OH and the interaction between metal and F.43
C–O or C–O from LEV. Moreover, as can be seen from Table 5, deconvolved N1s of TC into two peaks at 399.4 eV and 401.7 eV were assigned to –NH– or –NH2 and C–N–C.6,40 After adsorption, the binding energy shifted upward to 399.8 eV and 403.2 eV, respectively, due to the protonated amines or the formation of hydrogen bonds.41,42 The F1s signal of LEV presented at 686.7 eV and was transferred to 687.3 eV after adsorption. The increase in binding energy may be attributed to the replacement of –OH and the interaction between metal and F.43
| Peak position (eV) | Group | Peak area percentage (%) | |
|---|---|---|---|
| IP | 530.1 | Lattice-O2− | 65.62 |
| 531.4 | –OHlat | 34.38 | |
| IP–TC | 530.1 | Lattice-O2− | 62.22 |
| 531.4 | –OHlat/–OH from TC | 27.52 | |
| 532.8 | C–O from TC | 10.26 | |
| IP–LEV | 530.0 | Lattice-O2− | 67.55 |
| 531.3 | –OHlat/–OH from LEV | 26.62 | |
| 533.0 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O/C–O from LEV C–O/C–O from LEV |
5.830 | |
| PI | 530.1 | Lattice-O2− | 6.442 |
| 531.1 | –OHlat | 7.372 | |
| 532.3 | –OHads | 58.65 | |
| 533.6 | H2O | 27.54 | |
| PI–TC | 530.2 | Lattice-O2− | 8.755 |
| 531.6 | –OHlat/–OH from TC | 18.00 | |
| 532.3 | –OHads | 43.41 | |
| 533.5 | H2O | 29.84 | |
| PI–LEV | 530.0 | Lattice-O2− | 9.012 |
| 531.2 | –OHlat/–OH from LEV | 18.94 | |
| 532.2 | –OHads | 44.69 | |
| 533.5 | H2O | 27.36 |
| Element | Sample | Binding energy (eV) |
|---|---|---|
| F | LEV | 686.7 |
| IP–LEV | 687.3 | |
| PI–LEV | 687.2 | |
| N | TC | 399.4, 401.7 |
| IP–TC | 399.8, 403.2 | |
| PI–TC | 399.9, 402.8 |
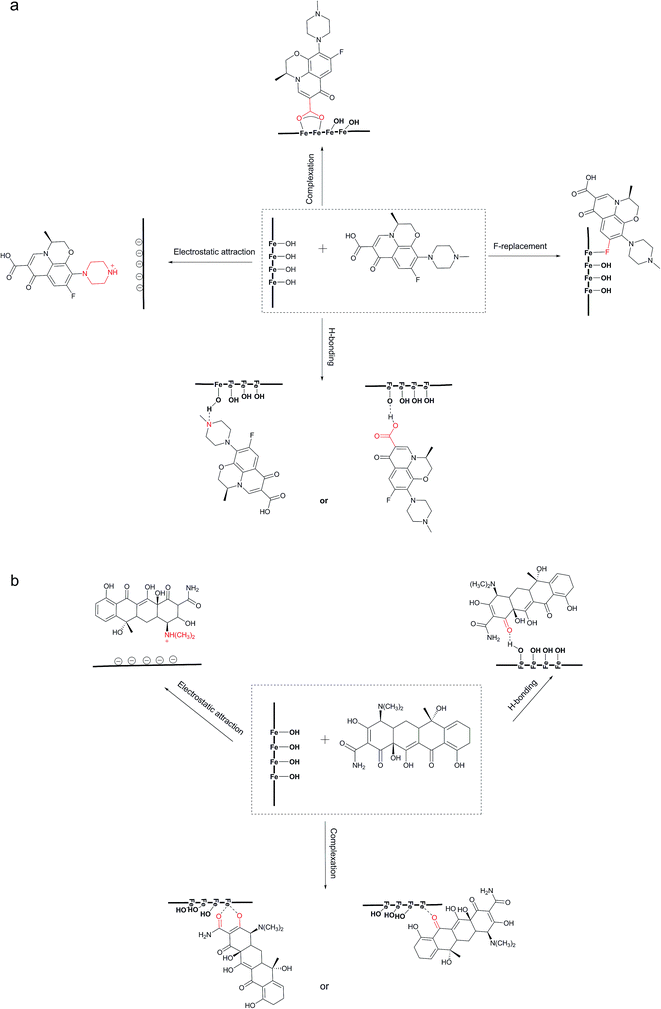
As for the PI, the O1s were divided into three peaks, –OHlat, –OHads, and adsorbed H2O. Compared with the IP, the lattice-O2- and the –OHlat portions on PI were much lower. PI contained a large amount of –OHads. Previous studies had also stated that amorphous materials could offer more (or higher density) hydroxyl groups.44 The –OHads content reduced from 58.65% to 43.41% and 44.69% after TC and LEV adsorption, which indicated the key role of –OHads in the adsorption. The –OHlat was also thought to be one of the adsorption functional groups despite its percentage increase after adsorption, on account of overlap of the –OH from LEV or TC. Similarly as IP, the F and N binding energy values of PI both increased after adsorption.
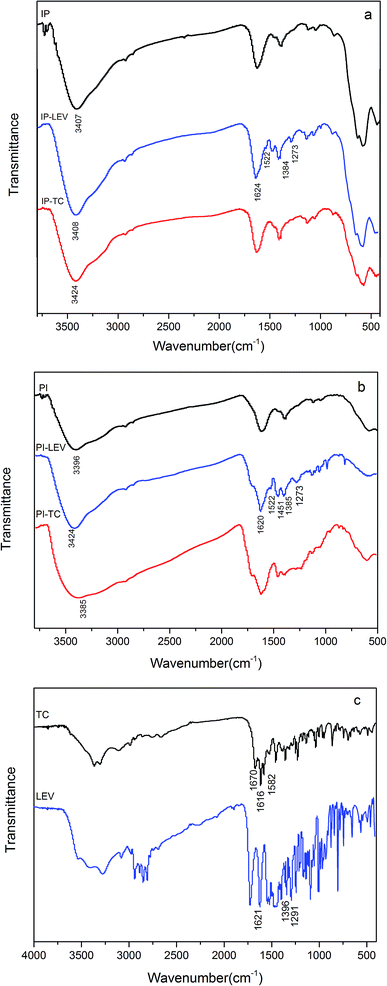 | ||
| Fig. 5 FT-IR spectra of IP (a), PI (b) before and after adsorption, FT-IR spectra of LEV and TC (c). | ||
Additionally, the frequency at 1291 cm−1 (Fig. 5c), assigned to the coupling of carboxylic acid C–O stretching and O–H deformation, changed to 1273 cm−1 (Fig. 5b), because Fe combined with the O atom of carboxyl. This could be confirmed by the shift of Fe–O stretching peak after LEV adsorption. The peak at 1621 cm−1 (Fig. 5c), representing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in LEV molecular, transferred to 1522 cm−1 (Fig. 5b) after adsorption, illustrating that C
O in LEV molecular, transferred to 1522 cm−1 (Fig. 5b) after adsorption, illustrating that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was involved in the reaction. In addition, the bands in PI–LEV at 1624 cm−1 and 1384 cm−1 corresponded to the antisymmetric and symmetric stretching vibrations of the carboxylate group.52,53 And the Δν value was 240 cm−1. It was comparable to the free LEV (Δνfree), which was 245 cm−1, implying that the complex was the bridging-type.54,55 As for the PI–LEV FTIR spectra, the changes were very similar as the above.
O was involved in the reaction. In addition, the bands in PI–LEV at 1624 cm−1 and 1384 cm−1 corresponded to the antisymmetric and symmetric stretching vibrations of the carboxylate group.52,53 And the Δν value was 240 cm−1. It was comparable to the free LEV (Δνfree), which was 245 cm−1, implying that the complex was the bridging-type.54,55 As for the PI–LEV FTIR spectra, the changes were very similar as the above.
For the TC spectra (Fig. 5c), the 1670 cm−1 bands were ascribed to the carbonyl of the amide in ring A of TC. The frequencies at 1616 cm−1 and 1582 cm−1 corresponded to the carbonyl groups in A and C rings, respectively. After adsorption on IP, the two peaks vanished (Fig. 5b). The FTIR spectrum of PI–TC was similar as the above. This phenomenon revealed that interaction between carbonyl (C-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-3 in ring A, C-11 in ring C, amide group in ring A) and Fe.6
C-3 in ring A, C-11 in ring C, amide group in ring A) and Fe.6
To understand the adsorption behavior and mechanism further, competitive adsorption was investigated (Fig. 6). In the PI binary adsorption systems, LEV maintained the high adsorption capacity or only decreased slightly. It could be deduced that –OH would give priority to LEV molecular for combination rather than TC. On the other hand, TC decreased significantly in the presence of LEV, but it still remained some adsorption, demonstrating that –OH was not the only or not the most important reason for TC adsorption. Moreover, in the IP binary adsorption systems, LEV's priority was not so obvious due to less –OH in IP.
PI with amorphous ferric oxide could provide more –OH groups, mainly in the form of –OHads, enhancing both the LEV and TC adsorption capacities. –OH groups preferred to combine with LEV molecular rather than TC. And it played the leading role in LEV adsorption, however it was not the only or not the most important reason for TC adsorption, especially at the near-neutral condition.
3.6 Regeneration and reuse analysis
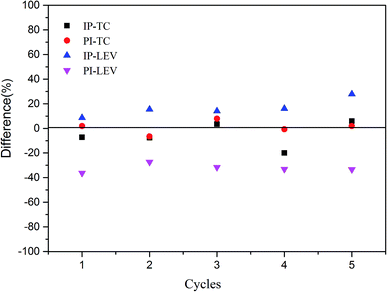
Fig. 8 showed the results in five adsorption cycles. Compared with the adsorption capacity in the first cycle, except for PI–LEV, other adsorption amounts were maintained high and stable. Compared with the original adsorption amount, the differences were less than ±20% in the reuse cycle experiments. The adsorption amount of PI–LEV decreased, but it can also be kept more than 64% of the original adsorption amount. This phenomenon manifested that the IP and PI could be reused and recycled efficiently.4 Conclusions
In this study, an agricultural waste, corn husk, was used as carrier to prepare adsorbents with two different forms of iron oxides. IP biochar showed lower adsorption capacity. PI, mainly covered with amorphous hydrated iron oxide, could provide more –OH, especially –OHads, as the reactive sites. Moreover, –OH was inclined to integrate with LEV in comparison with TC. FT-IR and XPS results indicated that the mechanisms of LEV adsorption were the hydrogen bonding, F-replacement, electrostatic attraction and bridging bidentate complexation. TC adsorption may involve complexation, hydrogen bonding, electrostatic attraction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities (2632019FY02), the Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents, the Qing Lan Project, Natural Science Foundation of Jiangsu Province (BK20160744), National Natural Science Foundation of China (21707166) and the College Students Innovation Project for the R&D of Novel Drugs (J1310032).References
- I. Sousa, V. Claro, J. L. Pereira, A. L. Amaral, S. L. Cunha, B. Castro, M. J. Feio, E. Pereira and P. Gameiro, Synthesis, characterization and antibacterial studies of a copper (II) levofloxacin ternary complex, J. Inorg. Nucl. Chem., 2012, 110, 64 CAS.
- D. J. Margolis, M. Fanelli, O. Hoffstad and J. D. Lewis, Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease, Am. J. Gastroenterol., 2010, 105, 2610 CrossRef CAS PubMed.
- Y. Y. Zhou, X. C. Liu, Y. J. Xiang and L. Tang, Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling, Bioresour. Technol., 2017, 245, 266 CrossRef CAS PubMed.
- Y. N. Liu, C. X. Dong, H. Wei, W. H. Yuan and K. B. Li, Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): kinetics, equilibrium and mechanism, Appl. Clay Sci., 2015, 118, 301 CrossRef CAS.
- A. W. Chen, C. Shang, J. H. Shao, Y. Q. Lin, S. Luo, J. C. Zhang, H. L. Huang, M. Lei and Q. R. Zeng, Carbon disulfide-modified magnetic ion-imprinted chitosan-Fe(III): A novel adsorbent for simultaneous removal of tetracycline and cadmium, Carbohydr. Polym., 2017, 155, 19 CrossRef CAS PubMed.
- Z. Y. Zhang, H. J. Liu, L. Y. Wu, H. C. Lan and J. H. Qu, Preparation of amino-Fe(III) functionalized mesoporous silica for synergistic adsorption of tetracycline and copper, Chemosphere, 2015, 138, 625 CrossRef CAS PubMed.
- A. Vazquez, D. B. H. Uresti and S. Obregon, Electrophoretic deposition of CdS coatings and their photocatalytic activities in the degradation of tetracycline antibiotic, Appl. Surf. Sci., 2016, 386, 412 CrossRef CAS.
- J. H. Li, M. S. Han, Y. Guo, F. Wang, L. J. Meng, D. J. Mao, S. S. Ding and C. Sun, Hydrothermal synthesis of novel flower-like BiVO4/Bi2Ti2O7 with superior photocatalytic activity toward tetracycline removal, Appl. Catal., A, 2016, 524, 105 CrossRef CAS.
- M. F. Nazar, W. Azeem, U. A. Rana, M. Ashfaq, A. Lashin, N. Arifi, H. M. A. U. Rahman, A. M. Lazim and A. Mahmood, pH-dependent probing of levofloxacin assimilated in surfactant mediated assemblies: Insights from photoluminescent and chromatographic measurements, J. Mol. Liq., 2016, 220, 26 CrossRef CAS.
- Z. L. Song, Y. J. Ma, G. G. Xia, Y. Wang, W. Kapadia, Z. Y. Sun, W. Wu, H. C. Gu, W. G. Cui and X. Y. Huang, In vitro and in vivo combined antibacterial effect of levofloxacin/silver co-loaded electrospun fibrous membranes, J. Mater. Chem. B, 2017, 5, 7632 RSC.
- K. S. Walton and R. Q. Snurr, Applicability of the BET Method for Determining Surface Areas of Microporous Metal-Organic Frameworks, J. Am. Chem. Soc., 2007, 27, 8552 CrossRef PubMed.
- H. W. Wei, Q. Y. Feng, H. Yang, E. Alam, B. Gao and D. Gu, Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms, Colloids Surf., A, 2017, 517, 63 CrossRef.
- Y. Yuan, T. Yuan, D. M. Wang, J. H. Tang and S. G. Zhou, Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell, Bioresour. Technol., 2013, 144, 115 CrossRef CAS PubMed.
- Z. H. Ruan, J. H. Wu, J. F. Huang, Z. T. Lin, Y. F. Li, Y. L. Liu, P. Y. Cao, Y. P. Fang, J. Xie and G. B. Jiang, Facile preparation of rosin-based biochar coated bentonite for supporting α-Fe2O3 nanoparticles and its application for Cr(VI) adsorption, J. Mater. Chem. A, 2015, 3, 4595 RSC.
- S. Kloss, F. Zehetner, A. Dellantonio, R. Hamid, F. Ottner, V. Liedtke, M. Schwanninger, M. H. Gerzabek and G. Soja, Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties, J. Environ. Qual., 2012, 41, 990 CrossRef CAS PubMed.
- B. M. Richveisova, V. Fristak, M. Pipiska, L. Duriska, E. M. Jimenez and G. Soja, Iron-impregnated biochars as effective phosphate sorption materials, Environ. Sci. Pollut. Res., 2017, 24, 463 CrossRef PubMed.
- X. Hu, Z. H. Ding, A. R. Zimmerman, S. S. Wang and B. Gao, Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis, Water Res., 2015, 68, 206 CrossRef CAS PubMed.
- H. Wang, C. R. Fang, Q. Wang, Y. X. Chu, Y. L. Song, Y. M. Chen and X. D. Xue, Sorption of tetracycline on biochar derived from rice straw and swine manure, RSC Adv., 2018, 8, 16260 RSC.
- S. S. Wang, B. Gao, A. R. Zimmerman, Y. C. Li, L. N. Ma, W. G. Harris and K. W. Migliaccioac, Removal of arsenic by magnetic biochar prepared from pinewood and natural hematit, Bioresour. Technol., 2015, 175, 391 CrossRef CAS PubMed.
- M. Zhang, B. Gao, S. Varnoosfaderani, A. Hebard, Y. Yao and M. Inyang, Preparation and characterization of a novel magnetic biochar for arsenic removal, Bioresour. Technol., 2013, 130, 457 CrossRef CAS PubMed.
- L. Han, S. Xue, S. C. Zhao, J. C. Yan, L. B. Qian, M. F. Chen and M. S. Yao, Biochar supported nanoscale iron particles for the efficient removal of methyl orange dye in queous solutions, PLoS One, 2015, 10, e0132067 CrossRef PubMed.
- H. G. Zhou, Z. M. Jiang and S. Q. Wei, A novel absorbent of nano-Fe loaded biomass char and its enhanced adsorption capacity for phosphate in water, J. Chem., 2013, 2013, 1 Search PubMed.
- G. Caminati and M. Puggelli, Europium in phospholipid nanoscaffolds for the photophysical detection of antibiotic traces in solution, ResearchGate, 2011, vol. 6, p. 203 Search PubMed.
- H. Wei, D. Hu, J. Su and K. B. Li, Intensification of levofloxacin sono-degradation in a US/H2O2 system with Fe3O4 magnetic nanoparticles, Chin. J. Chem. Eng., 2015, 23, 296 CrossRef CAS.
- B. H. Huang, Y. G. Liu, B. Li, S. B. Liu, G. M. Zeng, Z. W. Zeng, X. H. Wang, Q. M. Ning, B. H. Zheng and C. P. Yang, Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite, Carbohydr. Polym., 2017, 157, 576 CrossRef CAS PubMed.
- S. S. Fan, Y. Wang, Z. Wang, J. Tang, J. Tang and X. D. Li, Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism, J. Environ. Chem. Eng., 2017, 5, 601 CrossRef CAS.
- F. M. Pellera, A. Giannis, D. Kalderis, K. Anastasiadou, R. Stegmann, J. Y. Wang and E. Gidarakos, Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products, J. Environ. Manage., 2012, 96, 35 CrossRef CAS PubMed.
- L. Peng, Y. Q. Ren, J. D. Gu, P. F. Qin, Q. R. Zeng, J. H. Shao, M. Lei and L. Y. Chai, Iron improving bio-char derived from microalgae on removal of tetracycline from aqueous system, Environ. Sci. Pollut. Res., 2014, 21, 7631 CrossRef CAS PubMed.
- B. Kakavandia, A. Takdastana, N. Jaafarzadeh, M. Azizi, A. Mirzaei and A. Azari, Application of Fe3O4@C catalyzing heterogeneous UV-Fenton system for tetracycline removal with a focus on optimization by a response surface method, J. Photochem. Photobiol., A, 2016, 314, 178 CrossRef.
- X. D. Zhu, Y. C. Liu, F. Qian, C. Zhou, S. C. Zhang and J. M. Chen, Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal, Bioresour. Technol., 2014, 154, 209 CrossRef CAS PubMed.
- X. T. Zhang, J. C. Shen, N. Zhuo and W. B. Yang, Interactions between antibiotics and graphene-based materials in water: A comparative experimental and theoretical investigation, ACS Appl. Mater. Interfaces, 2016, 8, 24273 CrossRef CAS PubMed.
- Y. Yu, W. Wang, J. Shi, S. Y. Zhu and Y. C. Yan, Enhanced levofloxacin removal from water using zirconium (IV) loaded corn bracts, Environ. Sci. Pollut. Res., 2017, 24, 10685 CrossRef CAS PubMed.
- S. Z. Yi, B. Gao, Y. Y. Sun and X. Hu, Removal of levofloxacin from aqueous solution using rice-husk and wood-chip biochars, Chemosphere, 2016, 150, 694 CrossRef CAS PubMed.
- B. H. Huang, Y. G. Liu, B. Li, S. B. Liu, G. M. Zeng, Z. W. Zeng, X. H. Wang, Q. M. Ning, B. H. Zheng and C. P. Yang, Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite, Carbohydr. Polym., 2017, 157, 576 CrossRef CAS PubMed.
- Y. Gao, Y. Li, L. Zhang, H. Huang, J. J. Hu, S. M. Shah and X. G. Su, Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide, J. Colloid Interface Sci., 2012, 368, 540 CrossRef CAS PubMed.
- A. C. Martins, O. Pezoti, A. L. Cazetta, K. C. Bedin, D. A. S. Yamazaki, G. F. G. Bandoch, T. Asefa, J. V. Visentainer and V. C. Almeida, Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies, Chem. Eng. J., 2015, 260, 2–91 CrossRef.
- R. Pai, A. Singh, S. Simotwo and V. Kalra, In situ grown iron oxides on carbon nanofibers as reestanding anodes in aqueous supercapacitors, Adv. Eng. Mater., 2018, 20, 1 CrossRef.
- A. P. Grosvenor, B. A. Kobe and N. S. Mcityre, Studies of the oxidation of iron by air after being xposed to water vapour using angle-resolved x-ray hotoelectron spectroscopy and QUASES, Surf. Interface Anal., 2004, 36, 1637 CrossRef CAS.
- H. Y. Hou, Z. P. Dai, X. X. Liu, Y. Yao, Q. S. Liao, C. Y. Yu and D. D. Li, Reutilization of the expired tetracycline for lithium ion battery anode, Sci. Total Environ., 2018, 630, 495 CrossRef CAS PubMed.
- C. Ling, F. Q. Liu, C. Xu, T. P. Chen and A. M. Li, An integrative technique based on synergistic coremoval and sequential recovery of copper and tetracycline with dual functional chelating resin: Roles of amine and carboxyl groups, ACS Appl. Mater. Interfaces, 2013, 5, 11808 CrossRef CAS PubMed.
- E. T. Vandenberg, L. Bertilsson, B. Liedberg, K. Uvdal, R. Erlandsson, H. Elwing and I. Lundström, Structure of 3-aminopropyl triethoxy silane on silicon oxide, J. Colloid Interface Sci., 1991, 147, 103 CrossRef CAS.
- S. Kerber, J. J. Bruckner, K. Wozniak and T. L. Barr, The nature of hydrogen in x-ray photoelectron spectroscopy: General patterns from hydroxides to hydrogen bonding, J. Vac. Sci. Technol., A, 1996, 14, 1314 CrossRef CAS.
- R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Organic fluorine compounds: a great opportunity for enhanced materials properties, Chem. Soc. Rev., 2011, 40, 3496 RSC.
- J. E. Schaff and J. T. Roberts, Structure sensitivity in the surface chemistry of ice: Acetone adsorption on amorphous and crystalline ice films, J. Phys. Chem., 1994, 98, 6900 CrossRef CAS.
- M. A. Henderson, S. A. Joyce and J. Rustad, Interaction of water with the (1×1) and (2×1) surfaces of α-Fe2O3(012), Surf. Sci., 1988, 417, 66 CrossRef.
- T. Iwasita and F. C. Nart, In situ infrared spectroscopy at electrochemecal interfaces, Prog. Surf. Sci., 1997, 55, 271 CrossRef CAS.
- K. Kandori, J. Sakai and T. Ishikawa, Definitive effects of chloride ions on the formation of spherical hematite particles in a forced hydrolysis reaction, Phys. Chem. Chem. Phys., 2000, 2, 3293 RSC.
- C. Song, X. F. Sun, S. F. Xing, P. F. Xia, Y. J. Shi and S. G. Wang, Characterization of the interactions between tetracycline antibiotics and microbial extracellular polymeric substances with spectroscopic approaches, Environ. Sci. Pollut. Res. Int., 2014, 21, 1786 CrossRef CAS PubMed.
- Z. F. Jiang, J. M. Xie, D. L. Jiang, Z. X. Yan, J. J. Jing and D. Liu, Enhanced adsorption of hydroxyl contained/anionic dyes on non functionalized Ni@SiO2 core-shell nanoparticles: Kinetic and thermodynamic profile, Appl. Surf. Sci., 2014, 292, 301 CrossRef CAS.
- P. Liu, W. J. Liu, H. Jiang, J. J. Chen, W. W. Li and H. Q. Yu, Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution, Bioresour. Technol., 2012, 121, 235 CrossRef CAS PubMed.
- J. Kang, H. J. Liu, Y. M. Zheng, J. Qu and J. P. Chen, Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan, J. Colloid Interface Sci., 2010, 344, 117 CrossRef CAS PubMed.
- E. K. Efthimiadou, N. Katsaros, A. Karaliota and G. Psomas, Synthesis, characterization, antibacterial activity, and interaction with DNA of the vanadyl-enrofloxacin complex, Bioorg. Med. Chem. Lett., 2007, 17, 1238 CrossRef CAS PubMed.
- Q. Liu, L. B. Zhong, Q. B. Zhao, C. Frear and Y. M. Zheng, Synthesis of Fe3O4/Polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline, ACS Appl. Mater. Interfaces, 2015, 7, 14573 CrossRef CAS PubMed.
- C. Gu and K. G. Karthkeyan, Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides, Environ. Sci. Technol., 2005, 39, 9166 CrossRef CAS PubMed.
- P. Trivedi and D. Vasudevan, Spectroscopic investigation of ciprofloxacin speciation at the goethite-water interface, Environ. Sci. Technol., 2007, 41, 3153 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |