Open Access Article
Open Access ArticleHigh resolution tracking of macrophage cells in deep organs and lymphatics using fluorescent polymer dots†
Shiyi
Tang
a,
Yixiao
Guo
a,
Yidian
Yang
ab,
Yao
Li
a,
Yanhong
Gao
c,
Chunfu
Zhang
 a and
Liqin
Xiong
a and
Liqin
Xiong
 *a
*a
aShanghai Med-X Engineering Center for Medical Equipment and Technology, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai 200030, P. R. China. E-mail: xiongliqin@sjtu.edu.cn
bThe Key Laboratory of Resource Chemistry of Ministry of Education, Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Shanghai Normal University, Shanghai 200234, P. R. China
cDepartment of Geriatrics, Xinhua Hospital of Shanghai Jiao Tong University, School of Medicine, Shanghai 200092, P. R. China
First published on 9th April 2019
Abstract
In vivo cell tracking can provide information on cell migration and accumulation in the organs. Here, both folate and amino modified polymer dots were synthesized and screened for in vitro and in vivo tracking of macrophage Ana-1 cells. Flow cytometry analysis demonstrated that prepared polymer dots showed cellular uptake of approximately 98% within a short incubation time of 2 h, and these polymer dots maintained a cell labeling rate over 97% after 2 d. Moreover, a CCK-8 assay suggested that these polymer dots increased Ana-1 cell viabilities up to 110% at concentrations from 5 to 50 μg mL−1. Furthermore, the in vivo real time imaging of labelled Ana-1 cells in the alveolus of lung and lymph nodes were clearly detected by probe-based confocal laser endomicroscopy (pCLE). This study demonstrates a unique approach using polymer dots for real-time high resolution tracking of macrophage cells in deep organs and the lymphatic system.
Introduction
Cell tracking has attracted increasing attention for imaging and cellular therapeutics to assess the tumor immune microenvironment.1 Galon et al. found that cytotoxic T lymphocytes are associated with the immune surveillance of tumors.2 Anguille et al. found that dendritic cells traffic to locoregional lymphatics after activation to present tumor antigens and can become targets for vaccine therapy.3 Macrophages have an innate targeting ability to recognize and accumulate into pathological sites and thus play an important role in inflammation and tumor progression.4–11 In addition, tumor-associated macrophages (TAMs) influence tumor progression related to prognosis and anti-cancer therapies.12,13 Therefore, the detection, quantification, and localization of macrophages labeled with imaging agents can be utilized in delivery systems for therapeutic and imaging applications.14,15Recently, fluorescence imaging has become a powerful modality due to its advantageous features, such as real-time tracking16–19 capacity, and has been used for guiding surgery in cancer patients. However, limited penetration depth of optical wavelength photons and high autofluorescence from living tissues that significantly compromises imaging sensitivity and specificity.20–22 These issues can be tacked by using imaging probes that emit in the near-infrared (NIR) wavelength range.23–27 Semiconducting polymer dots, as an attractive fluorescent nanoprobe, have gained growing interest for their attractive optical properties, such as bright fluorescent intensity, long-time photostability, high emission rate, and low cytotoxicity.17,19,28–38 In addition, the colors of polymer dots can be widely regulated by modifying their structures. And NIR emission can be realized based on the fluorescence resonance energy transfer (FRET) effect.30 In this study, NIR775-doped poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV) polymer dots39 were used as NIR fluorescence imaging probes for in vitro and in vivo cell tracking macrophage Ana-1 cells. In addition, by combining probe-based confocal laser endomicroscopy (pCLE), real-time imaging macrophage cell in deep organs and lymphatics were obtained.
Experimental
Materials
Polystyrene graft EO functionalized with carboxy (PS-PEG-COOH) and amino terminated poly(methyl methacrylate) (MMA-NH2; Mn = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were purchased from Polymer Source Inc. Folate Cap PE (PE-FA) was purchased from Avanti, Polar Lipids, Inc. Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV; MW: 150
000 g mol−1) were purchased from Polymer Source Inc. Folate Cap PE (PE-FA) was purchased from Avanti, Polar Lipids, Inc. Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV; MW: 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–250
000–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) was purchased from J&K, Inc. Silicon 2,3-naphthalocyanine bis(trihexylsilyloxide) (NIR775) was purchased from Sigma Aldrich, Inc. Anti-F4/80 antibody [CI:A3-1] (ab6640) and Donkey Anti-Rat IgG H&L (Alexa Fluor® 488) preadsorbed (ab150153) were purchased from Abcam Plc. Other chemicals were used without purification.
000 Da) was purchased from J&K, Inc. Silicon 2,3-naphthalocyanine bis(trihexylsilyloxide) (NIR775) was purchased from Sigma Aldrich, Inc. Anti-F4/80 antibody [CI:A3-1] (ab6640) and Donkey Anti-Rat IgG H&L (Alexa Fluor® 488) preadsorbed (ab150153) were purchased from Abcam Plc. Other chemicals were used without purification.
Preparation of polymer dots
The FA-MEH-PPV-COOH, MEH-PPV-COOH, FA-MEH-PPV-NH2 and MEH-PPV-NH2 polymer dots were prepared by the coprecipitation according to our previous study,39–43 with some modifications. In a typical procedure, a solution of 2 mL of THF containing 250 μg MEH-PPV and 250 μg PS-PEG-COOH or 250 μg MMA-NH2 and 5 μg NIR775 with/without 50 μg PE-FA was mixed in stock. Then, the mixture was quickly dispersed into 10 mL of purified water under water ultrasound. Extra THF was evaporated at 45 °C under the protection of nitrogen for 30 min. Finally, the prepared polymer dots were passed through a 0.45 μm filter with a PVDF membrane.Characterization of polymer dots
The particle size and zeta potential of the polymer dots were measured in aqueous solution using a Dynamic Light Scattering (DLS) instrument (Brookhaven 90 Plus Nanoparticle Size Analyzer). The absorption spectrum (360–750 nm) was obtained with a Shimadzu UV-2550 ultraviolet-visible spectrometer. The fluorescence spectrum (562–800 nm) was measured with an excitation wavelength at 537 nm with SpectraMax i3x (MOLECULAR DEVICES). pH was measured by FiveEasy Plus (METTLER TOLEDO). The fluorescence quantum yield (QY) of the polymer dots was measured with a UV-NIR absolute PL QY spectrometer (Hamamatsu, Japan) with 510 nm excitation for polymer dots from a xenon lamp.42 In the size and fluorescence stability test, polymer dots were dispersed in DMEM supplemented with 10% FBS at 37 °C for 96 h.In vitro cell culture, cytotoxicity and cell imaging
The Ana-1 cell line was obtained from the cell bank at the Chinese Academy of Sciences (Shanghai, China). QBC, GBC-SD, SGC-996 and RBE cell lines were kindly provided from Dr Fei Ma of Xinhua Hospital of Shanghai Jiao Tong University. The Ana-1, RBE and NCI-H292 cell lines were grown in RPMI 1640 medium. The QBC, SGC and GBC-SD cell lines were grown in DMEM. The media mentioned above were supplemented with 10% FBS and 1% penicillium streptomycin. Cultures were maintained at 37 °C under a humidified atmosphere containing 5% CO2.The in vitro cytotoxicity test was measured using the CCK-8 assay in Ana-1, NCl-H292, QBC, SGC, GBC-SD and RBE cell lines. Cells growing in log phase were seeded onto a 96-well cell-culture plate for approximately 5 × 103 cells per well and then incubated for 12 h at 37 °C under 5% CO2. Then, corresponding dots were added at different concentrations (0, 5, 25, 50, and 100 μg mL−1), and 100 μL per well DMEM/1640 was added to the negative control group and incubated for 24 h.
Subsequently, 10 μL of CCK-8 was added to each well of the 96-well plate and incubated for an additional 2 h at 37 °C under 5% CO2. A Tecan microplate reader was used to measure the OD450 (A value) of each well. Cell viability = (mean of absorbance value of treatment group/mean of absorbance value of control) × 100%.
For cell imaging, 5 × 104 Ana-1 cells grown on 6-well cell culture plates were cultured in RPMI 1640 medium for 12 h and then incubated with polymer dots (∼20 μg) at 37 °C for 2 h. After washing, the cells were imaged under a fluorescence microscope, LEICA DM I 3000B (Leica, Germany).
To analyze the specific protein markers F4/80 of macrophages, Ana-1 cells were investigated by immunofluorescence staining and laser confocal imaging.44 A total of 1 × 106 Ana-1 cells grown on confocal dishes were cultured in RPMI 1640 medium. After incubating for 24 h, the cells were fixed with 4% paraformaldehyde for 15 min. After washing in PBS for 5 min, the cells were permeabilized in 0.3% Triton for 15 min. After washing three times, the cells were blocked with 10% serum for 30 min at room temperature. The Ana-1 cells were then incubated with the macrophage-specific protein F4/80 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) for 24 h at 4 °C. After washing with PBS, the secondary antibody (1
300) for 24 h at 4 °C. After washing with PBS, the secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) was applied and incubated for 2 h at room temperature in the dark. After washing with PBS, the cells were stained with DAPI for 10 min at room temperature in the dark. Subsequently, the laser confocal imaging was analyzed.
300) was applied and incubated for 2 h at room temperature in the dark. After washing with PBS, the cells were stained with DAPI for 10 min at room temperature in the dark. Subsequently, the laser confocal imaging was analyzed.
For laser scanning confocal imaging, Ana-1 cells were incubated with polymer dots for 12 h. After washing, the cells were immobilized by paraformaldehyde. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) staining solution at room temperature. Images revealing DAPI (excitation: 405 nm, emission: 430–520 nm), MEH-PPV (excitation: 561 nm, emission: 585–650 nm), NIR775 (excitation: 561 nm, emission: 750–790 nm) and F4/80 (excitation: 488 nm, emission: 508–530 nm) fluorescence were captured using a laser scanning confocal microscope Leica TCS SP5 (Leica, Germany). The confocal images were analyzed using LAS AF Lite software.
Flow cytometry assay
Ana-1 cells (1 × 106 cells) were incubated with polymer dots (∼20 μg) at 37 °C for 2, 6, and 10 h. After washing, the cells were resuspended in PBS and analyzed with a flow cytometer Accuri C6 (BD, USA) by counting 104 events. The collected data were analyzed using BD Accuri C6 software. The fluorescence emission channel setting was 610 ± 10 nm.Biological distribution in balb/c mice
Balb/c mice (∼25 g, n = 12) were anesthetized with 200 μL of pentobarbital sodium (1%) by intraperitoneal injection. Then 50 μg four typical MEH-PPV polymer dots were intravenously injected to the mice. After 2 d, mice were euthanized by cervical dislocation. The lymph nodes (cervical, axillary, inguinal, popliteal, and medial iliac lymph nodes), major organs (heart, liver, spleen, lungs, and kidneys), interscapular brown adipose tissue (iBAT),42 muscle and bone were isolated. Ex vivo fluorescence imaging was performed with the IVIS Lumina XRMS Series III Imaging System by using a 520 nm excitation filter and a 790 nm emission filter (bin = 8).Long-time cell labeling rate and fluorescence intensity
Ana-1 cells (1 × 106 cells) were incubated with FA-MEH-PPV-NH2 and FA-MEH-PPV-COOH polymer dots (∼20 μg) at 37 °C for 24 h (n = 3), respectively. After washing with PBS, half of the cells were resuspended in PBS and analyzed with a flow cytometer Accuri C6 (BD, USA) by counting 104 events. The collected data were analyzed using BD Accuri C6 software. The fluorescence emission channel setting was 610 ± 10 nm. The cell labeling rate and fluorescence intensity were analyzed. The other half of the cells was resuspended in RPMI 1640 medium for cell culture. The same steps were repeated after one and two days.In vivo fluorescence imaging
For in vivo imaging, Ana-1 cells (1 × 106 cells) were initially labeled with 20 μg FA-MEH-PPV-NH2 polymer dots for 12 h. After washing, the cells were resuspended in PBS. The labeled Ana-1 cells were subcutaneously injected at 2 × 106 cells per hind leg footpad (right) or in the caudal vein. In vivo fluorescence imaging was performed with the IVIS Lumina XRMS Series III Imaging System by using a 520 nm excitation filter and a 790 nm emission filter. In vivo fluorescence imaging was obtained under anesthesia and analyzed at 20 min, 80 min, 2 h and 2 d by using Living Image software.Probe-based confocal laser endomicroscopy (pCLE) was conducted using the Cellvizio Dual Band System (Mauna Kea Technologies, Paris, France). In this study, a Z1800 scanning probe was used (diameter = 1.8 mm, lateral resolution = 3.5 μm, working distance = 100 μm, max field of view = 600 μm, λexcitation = 488 nm), and the spectral detection = 505–700 nm. Balb/c mice were anesthetized with 200 μL of pentobarbital sodium (1%) by intraperitoneal injection, and Ana-1 cells (∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) labeled with FA-MEH-PPV-NH2 polymer dots were injected via the tail vein or the right footpad. Polymer dots were excited at 488 nm, and emission was collected from 505 to 700 nm. The imaging and video of organs and lymph nodes were collected after 20 min, 120 min and 2 d. The data were analyzed by matching software (IC viewer, Mauna Kea Technologies, Paris, France).
000) labeled with FA-MEH-PPV-NH2 polymer dots were injected via the tail vein or the right footpad. Polymer dots were excited at 488 nm, and emission was collected from 505 to 700 nm. The imaging and video of organs and lymph nodes were collected after 20 min, 120 min and 2 d. The data were analyzed by matching software (IC viewer, Mauna Kea Technologies, Paris, France).
Ex vivo imaging and analysis of the tissues
After in vivo fluorescence imaging, the tissues were removed for ex vivo fluorescence imaging by using the IVIS Lumina XRMS Series III Imaging System by using a 520 nm excitation filter and a 790 nm emission filter. Then, the lymph nodes and organs were immediately fixed using 10% neutral buffered formalin and stored at −80 °C. Subsequently, the tissues were extracted and embedded in OCT compound and cryosectioned by microtome at −20 °C into slices of 10 μm thicknesses. The sections were analyzed under a fluorescence microscope.Results and discussion
Preparation and characterization of the polymer dots
To discuss the influence of ligands on the cellular uptake in the Ana-1 cells, four typical MEH-PPV polymer dots were designed and prepared, including (1) MEH-PPV dots modified with folate and carboxyl (FA-MEH-PPV-COOH) had the hydrodynamic diameter of 50.38 ± 5.43 nm; (2) MEH-PPV dots modified with carboxyl (MEH-PPV-COOH) had the hydrodynamic diameter of 47.93 ± 3.60 nm; (3) MEH-PPV dots modified with folate and amino (FA-MEH-PPV-NH2) had the hydrodynamic diameter of 54.94 ± 4.04 nm; and (4) MEH-PPV dots modified with amino (MEH-PPV-NH2) had the hydrodynamic diameter of 54.06 ± 2.72 nm. Table 1 and Scheme S1† describes the synthesis of these polymer dots. The high percentages of ligands improved the hydrophilicity and stability of the MEH-PPV polymer dots. The zeta potential of synthesized polymer dots was in the range of −34.2 mV to −39.8 mV in water. Due to the long negatively charged PMMA chain in MMA-NH2, the electropositive amino termination playing a negligible role, the particulate MMA-NH2 having a negative zeta potential of −23.8 ± 3 mV.45 And MMA-NH2 modified FA-MEH-PPV-NH2 and MEH-PPV-NH2 polymer dots also exhibited a negative zeta potential, which was consistent with our pervious results.42 The pH of the prepared polymer dots was between 6.38 and 6.60. And Z-average sizes versus pH change (Fig. S1†) illustrated good colloidal stability of the polymer dots.| Sample | MEH-PPV (μg) | NIR775 (μg) | PS-PEG-COOH (μg) | MMA-NH2 (μg) | PE-FA (μg) | Size (nm) | Zeta (mV) | pH |
|---|---|---|---|---|---|---|---|---|
| FA-MEH-PPV-COOH | 250 | 5 | 250 | 0 | 50 | 50.38 ± 5.43 | −37.0 ± 0.9 | 6.40 |
| MEH-PPV-COOH | 100 | 3 | 100 | 0 | 0 | 47.93 ± 3.60 | −34.2 ± 1.6 | 6.38 |
| FA-MEH-PPV-NH2 | 250 | 5 | 0 | 250 | 50 | 54.94 ± 4.04 | −39.8 ± 2.2 | 6.43 |
| MEH-PPV-NH2 | 100 | 3 | 0 | 100 | 0 | 54.06 ± 2.72 | −39.5 ± 1.7 | 6.60 |
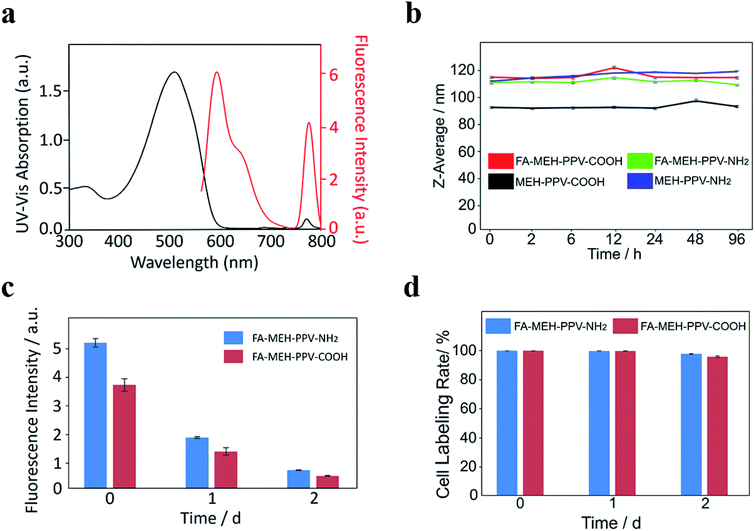
The prepared polymer dots in water exhibited a broad peak at 505–510 nm and a weak NIR peak at 771 nm. The absorption peaks represented the shift of MEH-PPV from 505 nm to 510 nm with increased diameters of the polymer dots. The redshift may be due to the fluorescence mechanism of conjugated polymer nanoparticles reported previously.46 The absorbance and fluorescence spectra of the prepared polymer dots were similar, and the spectra of FA-MEH-PPV-NH2 were shown in Fig. 1a. Under excitation at 537 nm, the polymer dots exhibited emission at 595 and 778 nm. And the conversion rate from MEH-PPV to NIR775 of the four typical polymer dots was in the range of 30–40%. The FRET efficiency was defined by the ratio of the integrated total emission (750–800 nm) from the NIR775 to the integrated total emission (562–750 nm) from the MEH-PPV.47 Although the spectral overlap between MEH-PPV fluorescence and NIR775 absorbance was poor.48 The result showed an efficient fluorescence resonance energy transfer ratio, which displays bright fluorescence in the near-infrared region. The high fluorescence resonance energy transfer (FRET) exhibited a large Stokes shift between the excitation and emission, providing the possibility for the experiment in vivo. Furthermore, the fluorescence yield (QY) test reflected the high light-harvesting efficiency of polymer dots (Table S1†). The emission of MEH-PPV was above 12% and the emission of NIR775 was above 1%. In addition, the physical stability of the MEH-PPV polymer dots was detected by dispersing these particles in DMEM supplemented with 10% FBS and in different pH. The Z-average size of these polymer dots showed no significant variation (Fig. 1b and S1†), indicating their good colloidal stability.
Cytotoxicity assay
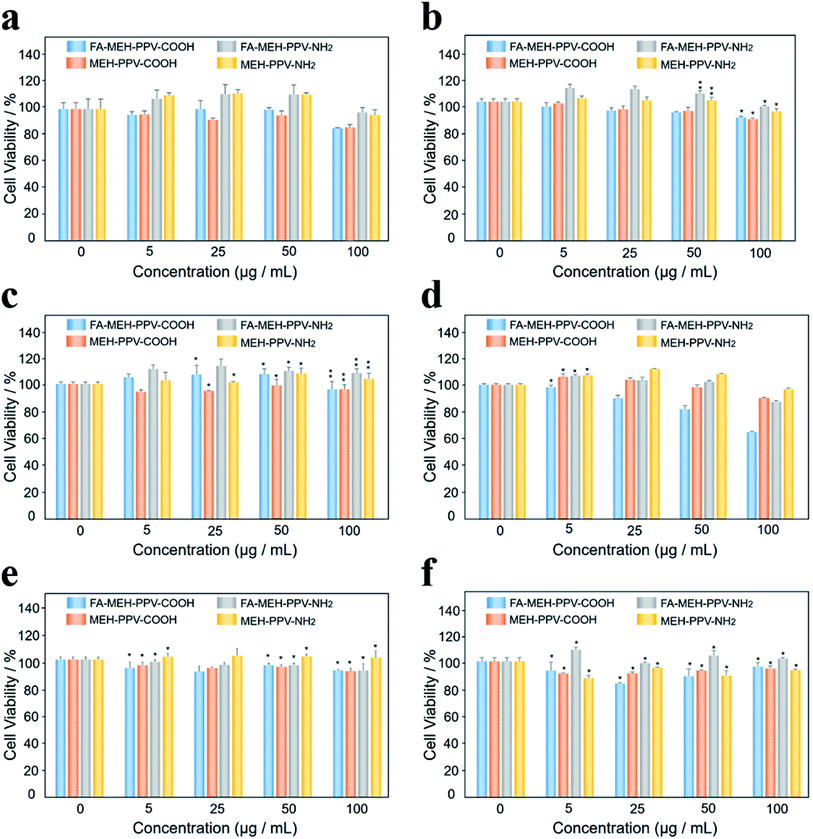
As shown in Fig. 2, different concentrations (0, 5, 25, 50, and 100 μg mL−1) of the polymer dots were added to the Ana-1 cell line. After 24 h of incubation with the four kinds of polymer dots, the cells maintained greater than 85%, 85%, 97%, and 95% cell viabilities for Ana-1 cells at 100 μg mL−1, respectively (Fig. 2a). At low concentrations, the cell viabilities showed a slight increase for the polymer dots modified with amino. Such as, the cellular viability was estimated to be more than 110% for the Ana-1 cell line at a concentration of 50 μg mL−1, indicating the growth promoting effect of amino modified polymer dots on the cells at low concentrations. While for the polymer dots modified with carboxyl, the cellular viability decreased slowly with the sample concentration increased. Compared with the results reported by Eleonore Fröhlich,49 polymer dots with positive charge exhibited a higher cytotoxicity. While our results displayed the weak cytotoxicity of the prepared polymer dots under these conditions even for the polymer dots modified with MMA-NH2.To further compare the cytotoxicity of polymer dots on different cells, five kinds of cancer cells were selected: lung cancer cell line NCI-H292, cholangiocarcinoma cell line QBC, gallbladder cancer cell line GBC-SD, SGC-996 cells and bile duct carcinoma cell line RBE. The viability was estimated to be higher than 90% for H292, QBC, SGC and RBE cell lines at 100 μg mL−1, displaying that the prepared polymer dots showed minimal cytotoxicity within 24 h for these cancer cells. However, FA-MEH-PPV-COOH revealed low cytotoxicity at a concentration of 100 μg mL−1 for the GBC-SD cell line (Fig. 2d), with the cell viability was 64%. This result may be due to the special spindle morphology of GBC-SD cells, making cells sensitive to the environment, especially to the polymer dots modified by PE-FA and carboxyl. Besides, for the polymer dots modified with carboxyl, the viability of the tumor cells was slightly decreased with increasing concentration from 5 to 100 μg mL−1, but for QBC cells, the cellular viability showed an increase from 5 to 50 μg mL−1 and decreased at 100 μg mL−1. For the polymer dots modified with amino, tumor cellular viability exhibited the same trend as macrophages.
Biological distribution in balb/c mice
To explore the biological distribution of MEH-PPV polymer dots in mice, the lymph nodes (cervical, axillary, inguinal, popliteal, and medial iliac lymph nodes), major organs (heart, liver, spleen, lungs, and kidneys), interscapular brown adipose tissue (iBAT), muscle and bone were removed and imaged. As shown in Fig. S2,† all the polymer dots exhibited high accumulation in the liver and spleen. While a few signals of MEH-PPV-NH2 polymer dots were observed in the lungs. Interestingly, the signals of MEH-PPV-COOH polymer dots were detected in the iBAT, cervical, axillary and medial iliac lymph nodes, indicating the long circulation time of polymer dots in the lymphatic vascular system.Flow cytometry assay and in vitro cell imaging
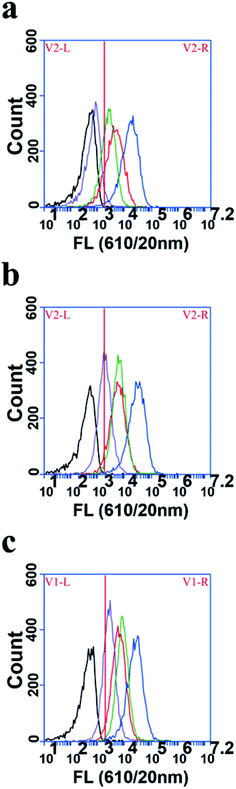
To explore the cellular uptake rate of polymer dots in the Ana-1 cells, the flow cytometric profiles experiment was conducted. Polymer dots were incubated with Ana-1 cells in serum-containing medium at 2, 6, and 10 h (Fig. 3). After 2 h incubation, as shown in Fig. 3a, FA-MEH-PPV-NH2 polymer dots showed the highest cellular uptake of 98.94%, followed by MEH-PPV-NH2 polymer dots with 75.88%, and FA-MEH-PPV-COOH polymer dots showed cellular uptake of 87.67%. While MEH-PPV-COOH polymer dots showed lowest cellular uptake of 4.91%. The results exhibited that Ana-1 cells are more likely to uptake polymer dots modified by amino in a short incubation time. As time increased to 6 h, the cell labeling rate of polymer dots increased gradually. And MEH-PPV-COOH polymer dots showed a rapid increase in the cellular uptake from 4.91% to 53.20%.After 10 h incubation, the labeling rate of FA-MEH-PPV-NH2 and MEH-PPV-NH2 polymer dots was over 98%, and the FA-MEH-PPV-COOH exhibited cell labeling rate over 81%, while the labeling rate of MEH-PPV-COOH polymer dots was only 57.17%. This experiment showed that Ana-1 cells were likely to absorb polymer dots with surface modification of amino and folate ligands. Our results were not consistent with the report of the uptake of NH2 (PEG) QDs.50 It may due to the structure of MMA-NH2 used in this work and the different cell lines or different uptake mechanism.51
The cellular uptake and location of polymer dots were further studied by cell imaging. The emission was collected from 620–710 nm with excitation at 546 nm, and the cells were incubated with four typical MEH-PPV polymer dots for 2, 6, and 10 h (Fig. S3 and S4†). The signals were detected uniformly in the cytoplasm. In particular, FA-MEH-PPV-NH2 polymer dots exhibited strong fluorescence in the cells under the microscope and the cell labeling rate was above 97% in 2 h. In contrast, MEH-PPV-COOH polymer dots showed much less fluorescence in the cells. Moreover, both the fluorescence intensity and cellular uptake rate increased over time. The results of cell imaging were consistent with the flow cytometry analysis, suggesting that FA-MEH-PPV-NH2 showed highest cellular uptake among the prepared polymer dots.
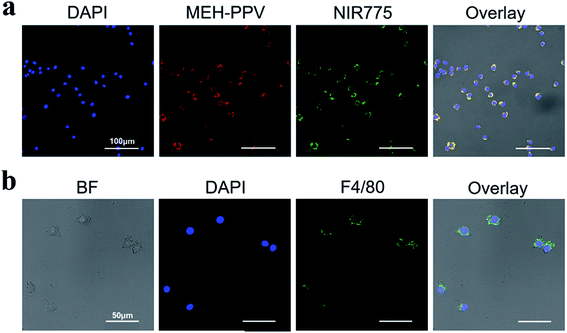
Laser confocal imaging was used to further study the location of polymer dots in the Ana-1 cells. As shown in Fig. 4a, the cells are evenly distributed and in a good state. FA-MEH-PPV-NH2 polymer dots exhibited strong fluorescence in both the visible and near-infrared regions. The signals were distributed mainly in the cytoplasm around the nucleus. The MEH-PPV and NIR775 channels displayed an efficient FRET ratio. The high FRET efficiency and strong NIR fluorescence indicated that the FA-MEH-PPV-NH2 polymer dots were suitable for in vivo study.
To analyze the specific protein markers F4/80 of macrophages, Ana-1 cells were investigated by immunofluorescence staining and laser confocal imaging. As shown in Fig. 4b, the macrophage surface antigen F4/80 emission was obtained from 508 to 530 nm with excitation at 488 nm. The cell nuclei were regularly distributed in the center of the cell, and the specific protein F4/80 was evenly distributed on the surface of the cell membrane. The positive result of immunofluorescence staining indicated that Ana-1 cells are a type of phagocyte.
To explore the long-term labeling rate and fluorescence stability of polymer dots, the labeled Ana-1 cells were incubated for 2 d. As shown in Fig. 1c, the fluorescence intensity of Ana-1 cells labeled with FA-MEH-PPV-NH2 decreased to 35% after 1 d and to 13% after 2 d, and the fluorescence intensity of Ana-1 cells labeled with FA-MEH-PPV-COOH decreased to 36% after 1 d and to 13% after 2 d. The cell labeling rate of these polymer dots in Ana-1 cells was still over 95% at 2 d (Fig. 1d), providing the basis for the in vivo cell tracking experiment.
In vivo NIR fluorescence imaging
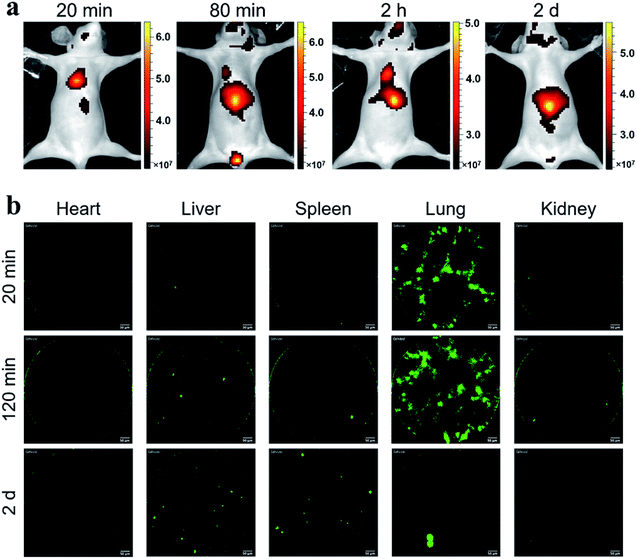
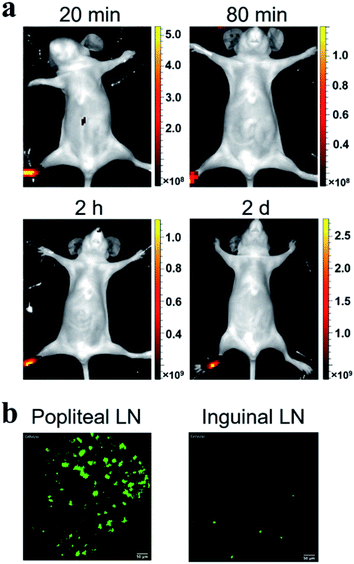
Considering the fluorescence intensity and high cell labeling rate, the FA-MEH-PPV-NH2 polymer dots were used in in vivo experiments. To explore the cell migration in blood circulation and signal distribution in organs, Ana-1 cells labeled by FA-MEH-PPV-NH2 polymer dots were injected into the caudal vein of mice. NIR fluorescence imaging was carried out at 20 min, 80 min, 120 min and 2 d post injection (Fig. 5a). At 20 min, intense NIR signals in the lung were observed with weak signals in the liver and face. At 80 and 120 min, the NIR signals in the liver became intense. And NIR signals in the neck region and urinary bladder were visualized. The signals in the bladder were produced by the autofluorescence of the mice.52 At 2 d, intense NIR signals in the liver were observed, while no obvious signals in the lungs were visualized. From 20 min to 2 d, the signals gradually migrated from the lungs to the liver. The results were similar to those reported for other cell lines.43,53 In addition, organs were dissected at 20 min and 2 h post injection. As shown in Fig. S5a and b,†ex vivo imaging revealed that the ingestion of probes increased in the spleen and liver. While the region-of-interest measurements showed that the fluorescence intensity in the lung decreased to 50% from 20 min to 2 h. Furthermore, frozen sections of the organs demonstrated that strong signals were visualized in the edge of the alveolus of lung as well as in the spleen and liver (Fig. S6†). These results were consistent with the in vivo imaging results, suggesting the migration of labelled Ana-1 cells in the deep organs and the endothelial reticular system.To further explore the cell migration in lymphatic system, the labeled Ana-1 cells were injected into the right footpads of mice. As shown in Fig. 6a, most fluorescent signals were still concentrated on the footpad after 2 d,54 and no obvious signals were detected in the draining lymph nodes. Therefore, the bilateral inguinal, sciatic, and popliteal lymph nodes were isolated, and ex vivo imaging confirmed that fluorescence signal was detected in the popliteal lymph node at the experimental side (Fig. S5c†), indicating that the migration of labelled Ana-1 cells in the lymphatic system.
In vivo real-time pCLE
Probe-based confocal laser endomicroscopy (pCLE) was further used to demonstrate the distribution of labeled Ana-1 cells in mice. As shown in Fig. 5b, after the intravenous injection of Ana-1 cells labeled with FA-MEH-PPV-NH2 for 20 min, intense signals were visualized in the lungs with weak signals in the heart, liver, spleen and kidney. As shown in the ESI movie S1,† the signals of Ana-1 cells were round with clear boundaries between cells. Notably, the signals of cells in the alveolus of lung were observed, and there were more than three alveolus were simultaneously visualized in the field of vision with high signal-noise-ratio. After 2 h, the most signals were still observed in the lungs (ESI movie S2†), and with a slight increase in the liver. After 2 d, the signal in the lungs decreased while the signal increased in the liver and spleen. From 20 min to 2 d, the signals gradually migrated from the lungs to the liver. The pCLE provided real-time high resolution visualizing labelled cells in the alveolus of lung, this result was consistent with in vivo NIR imaging. Besides, because the in vivo fluorescence imaging involves the superposition of two-dimensional images, providing the fluorescence signal of the whole tissue, while the pCLE provided the signals at the focal plane of the ROI (region of interest). Therefore, the fluorescence signals in the liver were much higher detected by in vivo fluorescence imaging than that detected by pCLE.As shown in Fig. 6b, after 2 d intradermal injection of Ana-1 cells labeled with FA-MEH-PPV-NH2 at the footpad of mice, massive signals were visualized in the popliteal lymph node, and scattered signals were also observed in the inguinal lymph node. This result indicated that the phagocytes can reach the popliteal and inguinal lymph node from the injection site. Compared with in vivo fluorescence imaging, pCLE displayed higher sensitivity to detect the migration of labelled cells in the draining lymph nodes.
Conclusions
In summary, we designed and compared four typical polymer dots with different ligands for tracking macrophage cells. The in vitro cytotoxicity experiment demonstrated low cytotoxicity of the synthesized polymer dots in the macrophage Ana-1 cells and tumor cells. Moreover, flow cytometry and cell imaging analysis showed that both folate and amino modification increased uptake of polymer dots in the Ana-1 cells. In addition, the in vivo NIR fluorescence imaging demonstrated that labeled Ana-1 cells showed strong and rapid uptake in the lungs of mice. Furthermore, the real-time high-resolution imaging of labelled Ana-1 cells in the alveolus of lung and lymph nodes were recorded by pCLE. This result provides a new strategy for combination polymer dots and pCLE for in vivo high resolution tracking the fate of macrophage cells in organs.Live subject statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shanghai Jiao Tong University and experiments were approved by the Animal Ethics Committee of Shanghai Jiao Tong University (Shanghai, China).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2016YFC1303100), the National Natural Science Foundation of China (81671738, 81301261, and 21374059), the Shanghai Pujiang Project (13PJ1405000), and the Medicine-Engineering Cross Project of Shanghai Jiao Tong University (YG2015MS67).Notes and references
- D. M. Kurtz and S. S. Gambhir, Adv. Cancer Res., 2014, 124, 257–296 CrossRef CAS.
- J. Galon, Science, 2006, 313, 1960–1964 CrossRef CAS.
- S. Anguille, E. L. Smits, E. Lion, V. F. Van Tendeloo and Z. N. Berneman, Lancet Oncol., 2014, 15, 257–267 CrossRef.
- T. J. Curiel, G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J. R. Conejo-Garcia, L. Zhang and M. Burow, Nat. Med., 2004, 10, 942–949 CrossRef CAS.
- R. Medzhitov, Nature, 2008, 454, 428–435 CrossRef CAS.
- C. N. Serhan and J. Savill, Nat. Immunol., 2005, 6, 1191 CrossRef CAS.
- A. L. Doedens, C. Stockmann, M. P. Rubinstein, D. Liao, N. Zhang, D. G. DeNardo, L. M. Coussens, M. Karin, A. W. Goldrath and R. S. Johnson, Cancer Res., 2010, 70, 7465–7475 CrossRef CAS.
- B. Z. Qian and J. W. Pollard, Cell, 2010, 141, 39–51 CrossRef CAS.
- R. Weissleder, M. Nahrendorf and M. J. Pittet, Nat. Mater., 2014, 13, 125–138 CrossRef CAS.
- C. N. Lumeng and A. R. Saltiel, J. Clin. Invest., 2011, 121, 2111–2117 CrossRef CAS.
- I. Tabas, Nat. Rev. Immunol., 2010, 10, 36–46 CrossRef CAS.
- B. Ruffell, N. I. Affara and L. M. Coussens, Trends Immunol., 2012, 33, 119–126 CrossRef CAS.
- A. P. Anselmo, S. Pilotti and A. Mantovani, Cancer Cell, 2013, 23, 249–262 CrossRef.
- J. Choi, H. Y. Kim, E. J. Ju, J. Jung, J. Park, H. K. Chung, J. S. Lee, J. S. Lee, H. J. Park and S. Y. Song, Biomaterials, 2012, 33, 4195–4203 CrossRef CAS.
- S. K. Patel and J. M. Janjic, Theranostics, 2015, 5, 150–172 CrossRef CAS.
- S. Kim, C. K. Lim, J. Na, Y. D. Lee, K. Kim, K. Choi, J. F. Leary and I. C. Kwon, Chem. Commun., 2010, 46, 1617 RSC.
- C. Zhu, L. Liu, Q. Yang, F. Lv and S. Wang, Chem. Rev., 2012, 112, 4687–4735 CrossRef CAS.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570 RSC.
- C. Wu, B. Bull, C. Szymanski, K. Christensen and J. McNeill, ACS Nano, 2008, 2, 2415–2423 CrossRef CAS.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS.
- V. Ntziachristos, C. Bremer and R. Weissleder, Eur. Radiol., 2003, 13, 195–208 Search PubMed.
- J. C. Rasmussen, I. C. Tan, M. V. Marshall, C. E. Fife and E. M. Sevick-Muraca, Curr. Opin. Biotechnol., 2009, 20, 74–82 CrossRef CAS PubMed.
- A. Wagh, S. Y. Qian and B. Law, Bioconjug. Chem., 2012, 23, 981–992 CrossRef CAS.
- T. Kitai, T. Inomoto, M. Miwa and T. Shikayama, Breast Cancer, 2005, 12, 211–215 CrossRef.
- N. Unno, M. Nishiyama, M. Suzuki, N. Yamamoto, K. Inuzuka, D. Sagara, H. Tanaka and H. Konno, Eur. J. Vasc. Endovasc. Surg., 2008, 36, 230–236 CrossRef CAS.
- J. Pecher and S. Mecking, Chem. Rev., 2010, 110, 6260–6279 CrossRef CAS.
- Y. Jiang, P. K. Upputuri, C. Xie, Y. Lyu, L. Zhang, Q. Xiong, M. Pramanik and K. Pu, Nano Lett., 2017, 17, 4964–4969 CrossRef CAS.
- S. Santra and A. Malhotra, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 501–510 CAS.
- C. Wu and D. T. Chiu, ChemInform, 2013, 52, 3086–3109 CAS.
- Y. Jin, F. Ye, M. Zeigler, C. Wu and D. T. Chiu, ACS Nano, 2011, 5, 1468 CrossRef CAS.
- Y. Lyu and K. Pu, Adv. Sci., 2017, 4, 1600481 CrossRef.
- Y. Lyu, J. Zeng, Y. Jiang and K. Pu, ACS Nano, 2018, 12, 1801–1810 CrossRef CAS PubMed.
- C. Xie, X. Zhen, Q. Miao and K. Pu, Adv. Mater., 2018, 30, 1801331 CrossRef PubMed.
- Q. Miao, C. Xie, X. Zhen and K. Pu, Nat. Biotechnol., 2017, 35, 1102–1110 CrossRef CAS.
- X. Xu, R. Liu and L. Li, Chem. Commun., 2015, 51, 16733–16749 RSC.
- F. Tang, C. Wang and L. Li, ACS Appl. Mater. Interfaces, 2015, 7, 25077–25083 CrossRef CAS PubMed.
- Y. Lv, M. Liu and Z. Tian, ACS Nano, 2018, 12, 1350–1358 CrossRef CAS.
- S. Chen, S. Cui and Y. Zhang, J. Mater. Chem. B, 2018, 6, 7871–7876 RSC.
- F. Cao and L. Xiong, Chin. J. Chem., 2016, 34, 570–575 CrossRef CAS.
- L. Xiong, A. J. Shuhendler and J. Rao, Nat. Commun., 2012, 3, 1193 CrossRef.
- F. Cao, Y. Guo, Y. Li, S. Tang, Y. Yang, H. Yang and L. Xiong, Adv. Funct. Mater., 2018, 1707174 CrossRef.
- Y. Guo, Y. Li and Y. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 20884 CrossRef CAS.
- L. Xiong, F. Cao, X. Cao, Y. Guo, Y. Zhang and X. Cai, Bioconjug. Chem., 2015, 26, 817–821 CrossRef CAS PubMed.
- J. Qin, Z. Peng and B. Li, Nanoscale, 2015, 7, 13991–14001 RSC.
- M. Khademi, W. M. Wang and W. Reitinger, Langmuir, 2017, 33, 10473–10482 CrossRef CAS.
- R. Potai and R. Traiphol, J. Colloid Interface Sci., 2013, 403, 58–66 CrossRef CAS.
- M. K. So, A. M. Loening and S. S. Gambhir, Nat. Protoc., 2006, 1, 1160–1164 CrossRef CAS.
- D. Chen, Q. Li, Z. Meng, L. Guo, Y. Tang, Z. Liu, S. Yin, W. Qin, Z. Yuan and X. Zhang, Theranostics, 2017, 7, 1820–1834 CrossRef CAS.
- F. Eleonore, Int. J. Nanomed., 2012, 7, 5577–5591 Search PubMed.
- M. J. D. Clift, B. Rothen-Rutishauser and D. M. Brown, Toxicol. Appl. Pharmacol., 2008, 232, 418–427 CrossRef CAS PubMed.
- O. Lunov, T. Syrovets and C. Loos, ACS Nano, 2011, 5, 1657–1669 CrossRef CAS.
- M. C. Jacobson, R. D. White and S. G. Demos, J. Biomed. Opt., 2012, 17, 036011 CrossRef.
- Z. Zhang, Y. Yuan, Z. Liu, H. Chen, D. Chen, X. Fang, J. Zheng, W. Qin and C. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 26928 CrossRef CAS.
- N. L. Trevaskis, L. M. Kaminskas and C. J. H. Porter, Nat. Rev. Drug Discovery, 2015, 14, 781 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence QY, and fluorescence imaging data. See DOI: 10.1039/c9ra00954j |
| This journal is © The Royal Society of Chemistry 2019 |